The objective of this study was to report our experience with sacral nerve stimulation for the treatment of severe faecal incontinence after the first 10 years with this technique.
Materials and methodsBetween 2001 and 2011, 49 patients with severe faecal incontinence underwent sacral nerve stimulation. Anorectal manometry, endoanal ultrasound and pudendal nerve latency were performed. Bowel habit diary, severity of faecal incontinence and quality of life scales were evaluated preoperatively and at the end of follow-up.
ResultsMorbidity occurred in a third of patients, mostly minor. Four definitive devices were explanted. With a median follow-up of 37 months, severity of faecal incontinence, urge and incontinence episodes significantly improved at the end of follow-up. Patients’ subgroup with a major follow-up of 5 years significantly improved the severity of faecal incontinence but not the parameters of the bowel habit diary. Quality of life showed no significant improvement. Descriptive data of patients with sphincter defects did not show worse results than that of patients with sphincter integrity.
ConclusionSacral nerve stimulation is a safe technique for severe faecal incontinence with good functional medium-term results. In the long term, severity of the faecal incontinence also improves, but studies with larger sample are necessary to show if other clinical parameters and the quality of life support this information. Preliminary results in patients with sphincter defects suggest that this technique could be effective in this group, but future studies will have to confirm these findings.
El objetivo del estudio es presentar nuestra experiencia en el tratamiento de la incontinencia fecal grave mediante neuromodulación sacra tras los primeros 10 años de utilización de la técnica.
Material y métodosCuarenta y nueve pacientes con incontinencia fecal grave se trataron con neuromodulación sacra durante el periodo 2001–2011. Se practicó manometría anorrectal, ecografía endoanal y estudio de latencia pudenda. Se evaluaron diario evacuatorio, escalas de gravedad y calidad de vida de incontinencia fecal en el preoperatorio y al final del seguimiento.
ResultadosUn tercio de los pacientes presentó morbilidad, la mayoría leve. Se explantaron 4 dispositivos definitivos. Con una mediana de seguimiento de 37 meses, la gravedad de la incontinencia fecal, urgencia e incontinencia mejoraron significativamente al cierre del estudio. El subgrupo de pacientes con seguimiento mayor de 5 años mejoró significativamente la gravedad de la incontinencia pero no los parámetros del diario evacuatorio. No hubo diferencias significativas en la calidad de vida. Los datos descriptivos en pacientes con defectos esfinterianos no muestran peores resultados que en la integridad esfinteriana.
ConclusionesLa neuromodulación sacra es una técnica segura para la incontinencia fecal grave con buenos resultados funcionales a medio plazo. A largo plazo, existe también mejoría de la gravedad de la misma pero son necesarios estudios con mayor muestra para objetivar si otros parámetros clínicos y la calidad de vida avalan este dato. Los resultados preliminares en pacientes con defectos esfinterianos sugieren que la técnica puede ser eficaz en este grupo pero futuros estudios deberán confirmar estos hallazgos.
The treatment of choice for faecal incontinence (FI) caused by sphincter defects has traditionally been sphincteroplasty of the external anal sphincter (EAS).1 Until a few years ago, the alternatives for management of severe FI included principally dynamic graciloplasty (DG), an artificial anal sphincter implant (AAS) or permanent stoma.2 Sacral nerve stimulation (SNS) is currently considered a new option. Initially it was performed only in patients with sphincter integrity, but its use has increased over time.3
The aim of this study was to present our experience in the treatment of severe FI with SNS after 10 years of using this technique.
Materials and MethodsA transversal prospective study of 49 patients (43 women and 6 men), with a median age of 63 (range [r]: 22-80) years, presenting severe FI was carried out and the patients were treated with SNS in our hospital between February 2001 and March 2011.
Initial inclusion criteria were severe FI (bowel movement urgency, urge incontinence or passive/soiling incontinence, at least twice a week for 2 consecutive months associated with social impact), intact EAS verified by endoanal ultrasound and the existence of nerve conduction in at least one pudendal branch via electro-stimulation. Since 2006 patients with defects in both anal sphincters regardless of size have been included in the study.
Exclusion criteria were as follows: heart disease requiring a pace-maker or defibrillator, pregnancy, rectal prolapse and diseases causing diarrhoea.
A preoperative assessment was made of all patients for inclusion in the study, with regard to their bowel function, using a bowel movement diary for 21 days, and assessing the severity of the FI and quality of life (QOL) with the Jorge-Wexner4 and FIQL5 scales. A functional study was also performed using anorectal manometry, EAS (B & K Medical, Gentofte, Denmark) and a pudendal nerve terminal motor latency test.
During the peripheral nervous system evaluation (PNSE), the bowel movement diary was completed (21 days) selecting those patients who were not candidates for permanent implants. At the end of the study bowel function, FI severity and QOL were assessed.
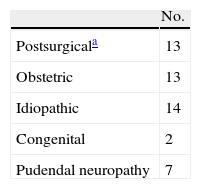
FI's aetiology is shown in Table 1. All the patients were medically managed and biofeedback was performed in 31 cases (63%), without improvement. The patients were informed and their consent was obtained for the surgical technique and the different examinations. This study was approved by the Ethical Committee of Clinical Research in our hospital.
Aetiology of Faecal Incontinence.
| No. | |
| Postsurgicala | 13 |
| Obstetric | 13 |
| Idiopathic | 14 |
| Congenital | 2 |
| Pudendal neuropathy | 7 |
Haemorrhoidectomy (2), haemorrhoidectomy and internal sphincterotomy (2), haemorrhoidectomy and fistulotomy (1), internal sphincterotomy (1), stapled anopexy (1), anterior rectum resection (4), right colectomy (1) and transanal resection of rectum adenoma (1). Anal atresia (1), spina bifida and myelomeningocele (1).
SNS is a 2-stage treatment administered in the outpatient department under local anaesthetic: PNSE and permanent stimulation.
For the first stage treatment, the sacral vertebrae S2, S3 or S4 which presented the best sensitive or motor response with the lowest stimulation threshold was selected. An electrode was then inserted, which was connected to an external generator (stimulation parameters: pulse width 210μs, frequency 25Hz and variable amplitude between 1 and 10V). In this stage, for the first 2 years we used a percutaneous monopolar electrode (Medtronic Interstim® 3065, Medtronic Inc, Minneapolis, U.S.A.) and from 2003 onwards, this was replaced by a self-anchoring tetrapolar electrode (Medtronic Interstim® 3889, Medtronic Inc, Minneapolis, U.S.A.). The patient returned to his or her daily activities and filled in the bowel habit diary. The final score was calculated by the sum of the number of episodes of FI or urge incontinence, interchangeably. If bowel function improved, defined as at least a 50% reduction in the daily score in the diary over the baseline, permanent stimulation was proposed.3
In the definitive implant the impulse generator was housed in a subcutaneous pouch in the homolateral gluteus and activated. During follow-up the stimulation parameters could be varied by telemetry. Clinical improvement criteria during follow-up were the same as those defined previously for PNSE.
Statistical MethodThe statistical programme SPSS® 13.0 was used for the analysis (SPSS Inc. Chicago, Illinois, U.S.A.).
Results are expressed as mean, standard deviation (SD), and range (minimum and maximum) or median and interquartile range (IQR). The Kolmogorov–Smirnov test was used to determine adequacy of fit of the samples to a normal distribution. Statistical analysis was performed using Student's t-test to compare 2 means for paired groups or the Wilcoxon T test for nonparametric samples, with a 0.05 level of significance.
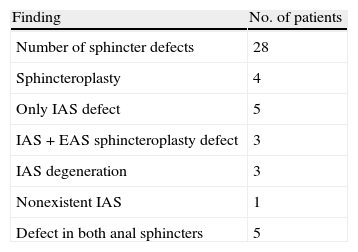
ResultsThe mean duration of symptoms was 90 months (r: 3–492 months). In 7 cases the cause of FI was pudendal neuropathy and another 13 patients (26%) with different aetiologies had associated FI. Thirteen patients presented urinary incontinence. Preoperative EAS findings are presented in Table 2.
Preoperative EAUS Findings.
| Finding | No. of patients |
| Number of sphincter defects | 28 |
| Sphincteroplasty | 4 |
| Only IAS defect | 5 |
| IAS+EAS sphincteroplasty defect | 3 |
| IAS degeneration | 3 |
| Nonexistent IAS | 1 |
| Defect in both anal sphincters | 5 |
EAS: external anal sphincter; IAS: internal anal sphincter; EAUS: endoanal ultrasound.
Fifteen patients (31%) presented morbidity. Infection (trauma) occurred during the PNSE phase which resulted in removal and renewed stimulation. In the permanent stimulation stage morbidity consisted of a section of the electrode during the definitive implantation which meant a second test had to be performed, 3 haematomas, pain or paresthesias in 14 cases (28.5%) (anal area: 3, extremities: 4 and locating the generator: 7). In 10 cases this was resolved with analgesics or by reducing the extent of stimulation, in 3 cases an explant was required, and in one patient the generator was relocated with improvement; there was one case of constipation treated with enemas.
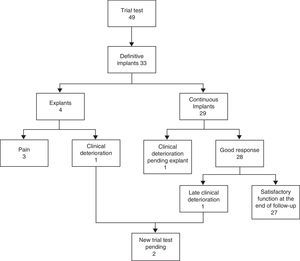
Functional ResultA total of 33 patients (67%) presented clinical improvements of 50% during the PNSE stage and received a definitive implant. Of these, 17 (51%) achieved complete continence (CC) during PNSE. A further PNSE was indicated for another 3 patients with insufficient improvement: in one a different vertebra was stimulated and in another, 2 further tests were carried out (different vertebra and bilateral, respectively), with no clinical improvement in any case. The third refused further surgery. The response of the last patient was mixed: urgency had improved but leakage had not. PNSE was continued for another 2 weeks with some improvement and a definitive device was implanted.
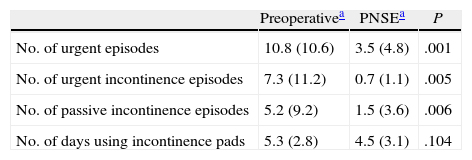
Following temporary implant, the number of weekly episodes of urgent need to evacuate the bowels dropped significantly, as did urge leakage and passive FI episodes compared to the baseline situation (Table 3).
Variation of Weekly Bowel Movement Diary.
| Preoperativea | PNSEa | P | |
| No. of urgent episodes | 10.8 (10.6) | 3.5 (4.8) | .001 |
| No. of urgent incontinence episodes | 7.3 (11.2) | 0.7 (1.1) | .005 |
| No. of passive incontinence episodes | 5.2 (9.2) | 1.5 (3.6) | .006 |
| No. of days using incontinence pads | 5.3 (2.8) | 4.5 (3.1) | .104 |
Definitive devices were implanted in S3 in 26 patients, S4 in 6 and S2 in one. Median (RIQ) follow-up after definitive implant was 37 (52) months (r: 6–118 months).
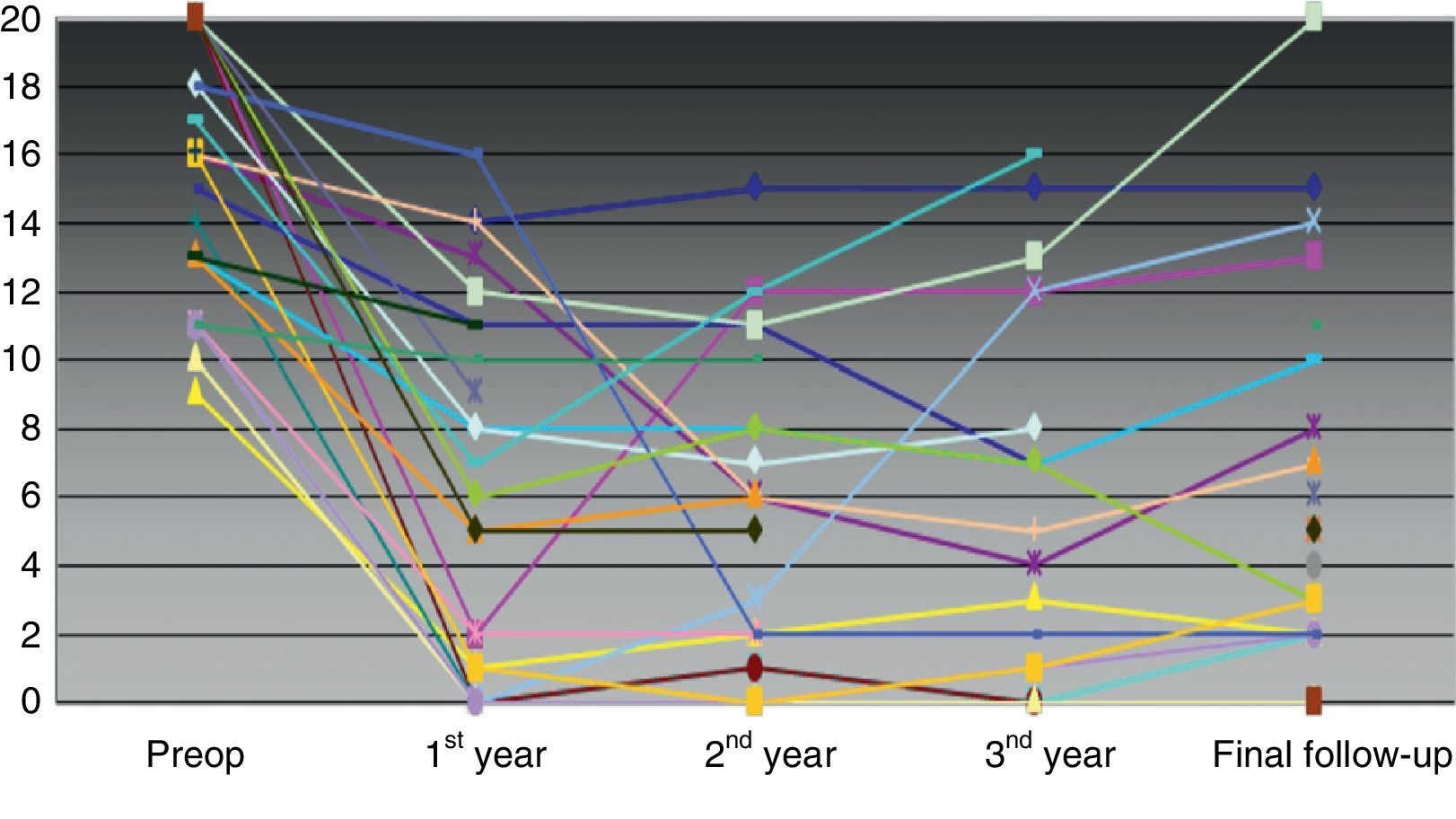
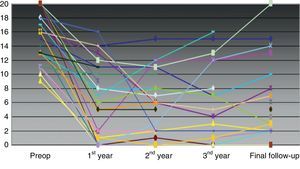
As a whole, the severity of FI had improved from a preoperative mean score (SD) of 16.04 (3.9)–5.6 (5.4) (P<.001) at the end of the follow-up. There was a significant improvement between the number of urgent bowel movement episodes, leakage due to urgent need and passive FI leakage preoperatively and at the end of the follow-up, but the number of days per week using an incontinence pad did not vary (Table 3). Individual variation on the severity scale throughout follow-up is shown in Fig. 1.
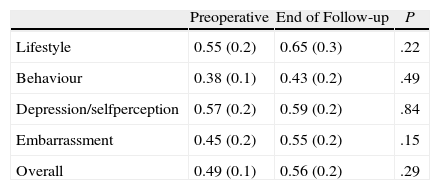
The QOL scale was completed at the end of the study by 13 of the 29 patients who continued with the definitive implant. Although an improvement in all parameters was present, statistical significance was not achieved compared with preoperative figures (Table 4).
Changes in Quality of Life.
| Preoperative | End of Follow-up | P | |
| Lifestyle | 0.55 (0.2) | 0.65 (0.3) | .22 |
| Behaviour | 0.38 (0.1) | 0.43 (0.2) | .49 |
| Depression/selfperception | 0.57 (0.2) | 0.59 (0.2) | .84 |
| Embarrassment | 0.45 (0.2) | 0.55 (0.2) | .15 |
| Overall | 0.49 (0.1) | 0.56 (0.2) | .29 |
Preoperative Student's t-test-end of follow-up (n=13).
In the subgroup of patients who were followed up for more than 5 years (n=8; median [IQR] follow-up of 75 [2.5] months; r: 70–118 months), the severity of the FI improved from a preoperative mean score (SD) of 15.14 (3.8)–6.7 (5.7) (P=.025) until the end of the follow-up. There was no significant improvement in any of the parameters of the bowel movement diary or in the QOL between the preoperative period and the end of the study.
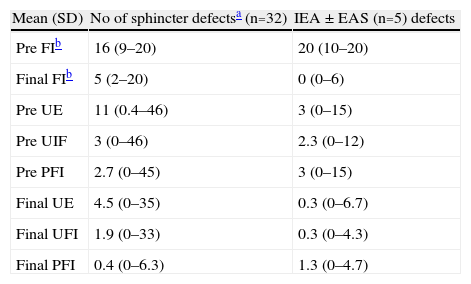
Data from the patient subgroup with sphincter defects (n=5) are detailed in Table 5. It can be seen that the clinical results are no worse than that of those of the patient group with sphincter integrity.
Description of Data in Patient Groups With/Without Sphincter Defects.
| Mean (SD) | No of sphincter defectsa (n=32) | IEA±EAS (n=5) defects |
| Pre FIb | 16 (9–20) | 20 (10–20) |
| Final FIb | 5 (2–20) | 0 (0–6) |
| Pre UE | 11 (0.4–46) | 3 (0–15) |
| Pre UIF | 3 (0–46) | 2.3 (0–12) |
| Pre PFI | 2.7 (0–45) | 3 (0–15) |
| Final UE | 4.5 (0–35) | 0.3 (0–6.7) |
| Final UFI | 1.9 (0–33) | 0.3 (0–4.3) |
| Final PFI | 0.4 (0–6.3) | 1.3 (0–4.7) |
EAS: external anal sphincter; IAS: internal anal sphincter; UE: weekly urgent episodes; FI: faecal incontinence; PFI: weekly passive FI episodes; UFI: weekly urgent FI episodes; Pre: preoperative.
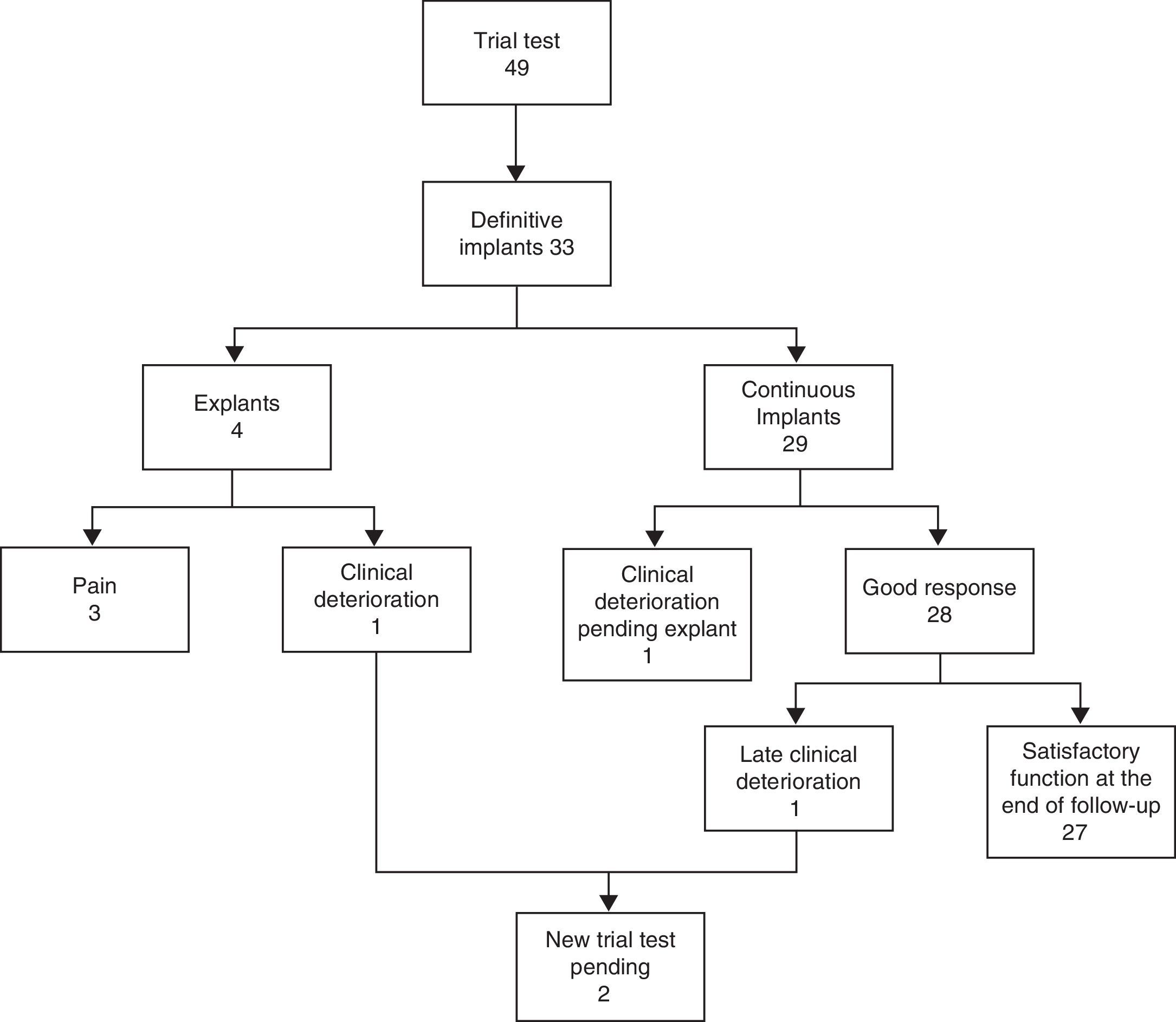
Of the 33 definitive implant cases, 4 (12%) required explantation (3 due to pain and 1 due to clinical deterioration). Another patient is pending an explant due to clinical deterioration and refuses further stimulation. Two cases with late clinical deterioration (the patient who has already been explanted and another pending explant) will undergo further stimulation (Fig. 2). Seven patients with satisfactory clinical results required a replacement generator (average lifetime: 56 months) due to battery depletion. Twenty of the 33 definitive devices (61%) needed the stimulation parameters to be reprogrammed (mean: 3.5 reprogramming sessions per patient). The most frequent adjustment was varying the extent of stimulation at a range between 0.8 and 6.4V to maintain continence.
DiscussionSNS is a simple and safe technique. In our study morbidity presented in a third of patients, the majority was mild and without repercussions for the implant. One patient presented with an infection (2%) which is in keeping with other series,6,7 and did not compromise the definitive implant. Like Michelsen et al.8 we highlighted that the alternatives available for severe FI until SNS was described were DG and AAS, both with high rates of infection and which could lead to permanent failure of the technique.9,10 In one definitive implant, the electrode was cut off. Care taken during the procedure was therefore increased, as although it is technically simple, high costs are involved.11
Permanent failure of the technique due to morbidity presented in 3 patients (9%), where pain or paresthesia led to explantation. Pain was controlled traditionally with analgesics in the other patients and we reduced the extent of stimulation to treat paresthesias. Similarly to Brouwer et al.,3 for a year we have been implanting the lateral generator higher and more laterally in the gluteus, to prevent patient discomfort when sitting using a more medial and lower location.
Response was satisfactory in 67% of patients during PNSE and they received the definitive implant, which is in keeping with literature data.12,13 During PNSE, 50% of the patients gained CC.
At study completion after the definitive device had been implanted, the series as a whole (median follow-up: 37 months) presented significant improvement in the severity of FI and in the number of urgency and leakage episodes compared to that before the operation, and only 5 patients presented CC (17%), and another 7 (24%) only had occasional leakage. This means that 41% of the 29 patients continued with the implant at the end of the follow-up. At present, the patient subgroup with follow-up of more than 5 years maintained significant improvement in the severity of the FI but not in the diary parameters. We believe that the failure to find significant differences in this last group is due to a low sample size with a follow-up of over 5 years (n=8), although it may also be that the clinical outcome, even if still good, deteriorates over time. Another explanation would be that the desire to have the implant and obtain a good response causes the patients during PNSE and the first few years after definitive implantation to minimise symptoms of leakage and not reflect it in their diaries. Over time, they could become more demanding and consider leakage to be something that they had previously overlooked. This would also explain the differences in the CC figures achieved in the different stages of the study.
We have not confirmed the reduction in the use of incontinence pads which some series obtained.14,15 One explanation would be that their use responds to an acquired habit or fear of leakage and reflects a need for reassurance rather than a current situation. Moreover, several patients urinary incontinence and do not differentiate the reason for continuing to use them.
On study completion, there was no significant improvement in the 13 patients who responded to the QOL survey. This does not match the results of other series16,17 and may be due to low completion at the end of the follow-up. The survey was sent to be completed and returned by post. If it was not received a second survey was sent. Even so, only 13 patients with definitive implants (44%) responded to the request. Subsequent studies with a higher recruitment of patients will confirm whether our results with regard to QOL are real or not.
In our study, 61% of patients needed some form of reprogramming. We agree with Govaert et al.18 that these should be informed and follow-up arranged for the necessary adjustments. We currently have a Medtronic technician who regularly visits our patients to control the parameters and make the necessary adjustments.
Indications for SNS have extended over time for several reasons. It is a technique without perianal manipulation; therefore preoperative continence should not be compromised when it is performed. Furthermore, surgical alternatives (DG and AAS implant) are more aggressive with raised, and occasionally severe morbidity,19,20 which has resulted in the indications for SNS to be extended in order to obtain results which would avoid these other operations. Lastly, SNS has its own «patient selection process» (PNSE) and has been tested in groups with certain characteristics. When good results were shown, definitive implants were used. Gradually these new indications are becoming a reality.
Initially, the exclusion criteria for SNS included congenital anorectal malformations, severe pudendal neuropathy, anterior resection syndrome after proctectomy or sphincter defects,1–24 but at present none of these are exclusion criteria. In our series there are patients with all these characteristics and specifically with sphincter defects. Due to the small number of patients we have not been able to make comparisons, and we present the data descriptively but we underline that the result obtained in this group was not worse than that of patients with sphincter integrity.
Several studies have used SNS in patients with sphincter defects with a good clinical outcome3,25,26 with no differences compared to sphincteroplasty.27,28 Long-term deterioration in continence after sphincteroplasty has been published,29 but other authors30 observed no differences between follow-up periods of under or over 5 years. Our group has conducted a study which is pending publication in which, with a median follow-up of 11 months after sphincteroplasty, the severity of FI does not significantly worsen compared with that in studies with shorter follow-ups. Despite this, the majority of authors publish that the initial effectiveness of this technique deteriorates over time.31–33
At present we are starting a randomised study in which patients with sphincter defects are allocated for treatment with SNS vs sphincteroplasty. We believe that this study will help us to reach conclusions regarding these 2 techniques for FI due to sphincter defects.
ConclusionSNS is a safe technique with low morbidity. The majority of patients with satisfactory trial stimulation obtain good functional results after the implantation of a definitive device, although studies need to be made with a larger number of patients with long-term follow-up to confirm whether this is stable over time. We have found no significant differences in the QOL of our patients. The SNS could be considered as an alternative in FI due to sphincter defect if the preliminary results obtained here are confirmed in a series with greater number of patients. We believe that prospective randomised studies should be designed to compare SNS with sphincteroplasty to define the indications for SNS and its place in the management of the different types of FI.
FundingThe study did not receive any support in the form of a grant nor has it been previously presented at any conference.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Ruiz Carmona MD, Martín Arévalo J, Moro Valdezate D, Plá Martí V, Checa Ayet F. Neuromodulación sacra en el tratamiento de la incontinencia fecal grave: resultados tras 10 años de experiencia. Cir Esp. 2014;92:329–335.