Classically, intermittent claudication, an intermediate stage in peripheral arterial disease, has been considered as a benign condition when considering only the muscular pain on walking.
In this paper our aim is to attract attention about the effects linked to ischaemic pain and the oxidative injury resulting from episodes of ischaemia/reperfusion. Throughout this process alterations in calcium homeostasis as well as uncontrolled generation of reactive oxygen species, in association with the mitochondrial dysfunction and inflammatory phenomena, could lead to accelerate atherosclerosis, with an increased cardiovascular risk stated by means of a reduced ankle-brachial index.
Taking this idea into account we propose a possible new classification for the management of the peripheral arterial disease, combining the Fontaine and Rutherford classifications and thinking about the described systemic effects in order to change the traditional management of peripheral arterial disease.
La claudicación intermitente, estación evolutiva intermedia de la enfermedad arterial periférica, se ha considerado tradicionalmente como un estadio benigno, atendiendo al hecho exclusivo de la clínica de dolor que se produce durante la marcha.
En este artículo pretendemos llamar la atención sobre las posibles consecuencias asociadas al dolor isquémico y el consiguiente estrés oxidativo desencadenado por su «sombra», el fenómeno isquemia/reperfusión. Durante el mismo se produce deficiente manejo del calcio y aparición incontrolada de radicales libres de oxígeno, con daño mitocondrial y aumento del fenómeno inflamatorio que podría estar asociado a una progresión acelerada de la arteriosclerosis sistémica con aumento del riesgo cardiovascular, directamente proporcional a la disminución del índice tobillo/brazo.
Ante estos acontecimientos, proponemos una nueva clasificación integradora de las actuales de Fontaine y Rutherford, que considera las posibles consecuencias sistémicas expuestas y sirva para modificar nuestro manejo tradicional de la enfermedad arterial periférica.
The grading systems for peripheral artery disease (PAD) by R. Fontaine (4 grades) and R.B. Rutherford (6 grades)1,2 are well known. Intermittent claudication is stage or grade ii (a and b) in the former and grades 2, 3 and 4 in the latter, and it is classified as mild, moderate or severe depending on the distance walked before claudication. As medical professionals, we continue to base the staging of the process on these grades.
But this view of the disease, which only focuses on the pain-free distance walked in patients with claudication, is based on the old segmental and evolutionary perception of arteriosclerosis in general and PAD in particular. This viewpoint may be becoming obsolete, however, as it only contemplates the symptom of pain, without considering the cause or the ischaemia/reperfusion (I/R) phenomenon that is always associated.
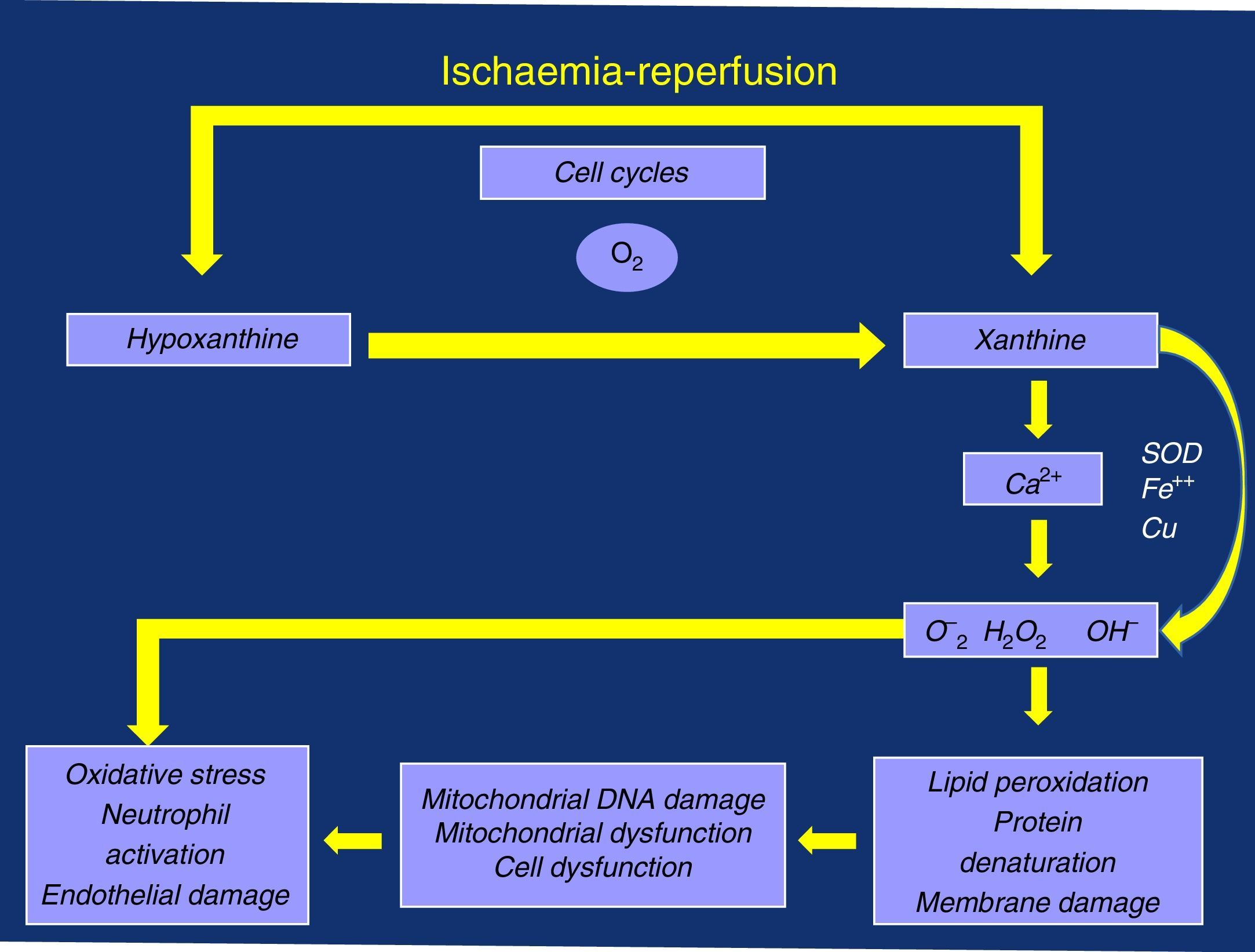
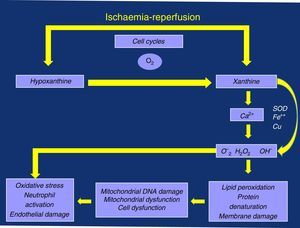
Perhaps we are paying attention to something that is not very transcendental (albeit very limiting for the active lives of these patients), such as ischaemic pain and the distance walked before associated claudication. We may not be giving enough importance to the possible consequences associated with the oxidative stress triggered by its “shadow”, I/R episodes, due to the deficient management of the calcium ion (Ca2+) and the production of oxygen free radicals (OFR), with damage to the mitochondrial and cell membranes, as well as the resulting local and systemic inflammatory response that is linked to neutrophil activation and endothelial dysfunction (damage)3–5 (Fig. 1).
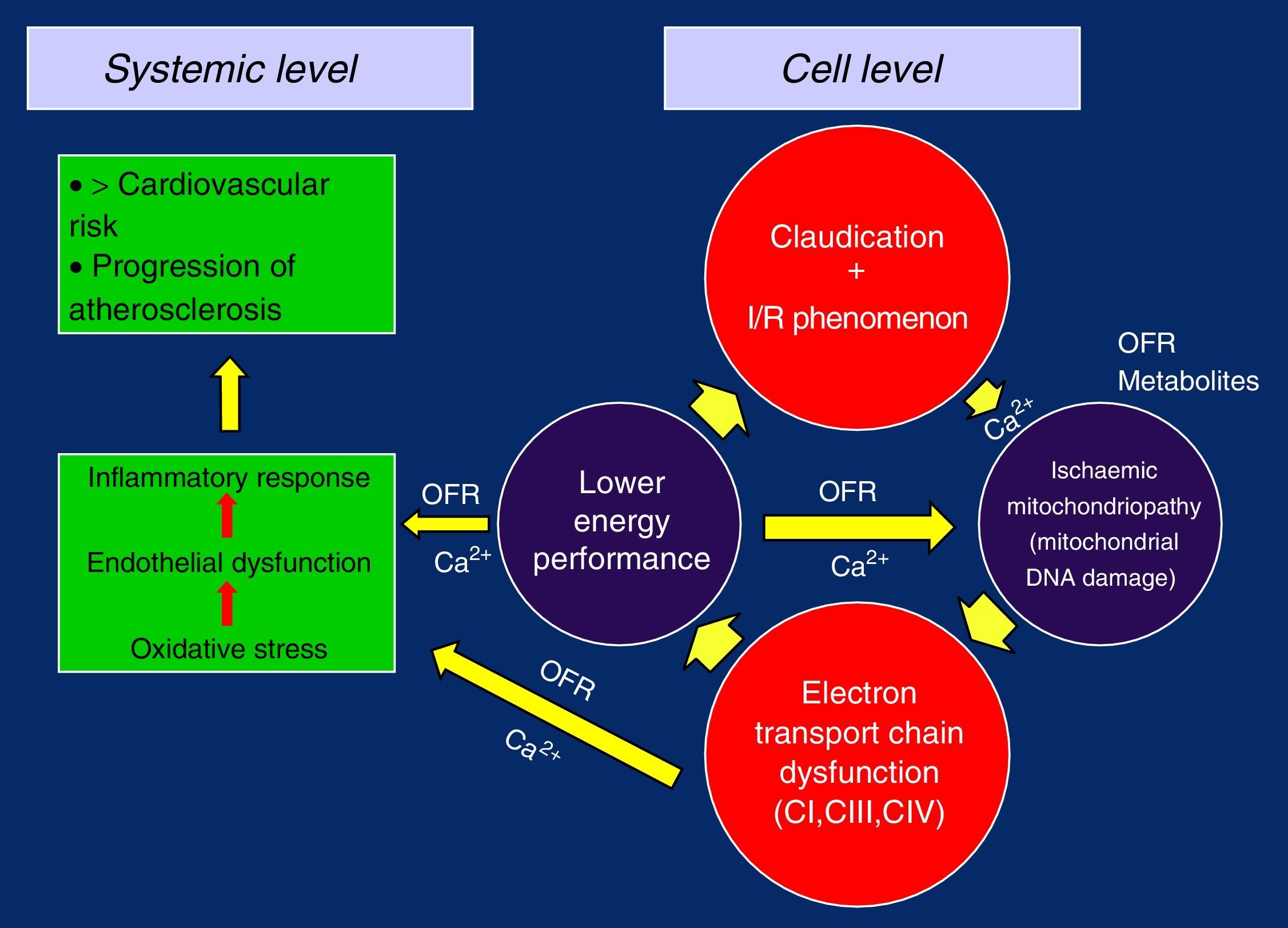
Claudication, Ischaemia/Reperfusion Syndrome, Haemodynamic ChangesEach time these patients need to slow down and/or stop walking, I/R has developed. This amounts to many times throughout the day and thousands over the course of a year. It is not reasonable to think that the cardiovascular risk (which is doubtlessly elevated in patients with claudication) without associated arteriosclerosis, coronary or cerebrovascular disease, is due to muscle pain in the affected calf, for example, or is merely coincidental. It is at least as plausible to think that there may be other phenomena associated with the physiopathology of the pain that might explain it.
The I/R that occurs in muscle masses that become temporarily ischaemic during claudication could trigger the process of oxidative stress. This is similar to what was originally described by Jennings et al.6 in an experimental study of the myocardium.
The existence of massive cell damage caused by I/R in the myocardium was described as the calcium paradox by Zimmerman et al.,7 who observed membrane damage with massive necrosis in rat cardiomyocytes after the administration of cardioplegic solution without Ca2+, followed by reperfusion using saline solution with calcium in physiological concentration.
Understanding the complex underlying physiopathological mechanisms of arteriosclerosis and its manifestation as intermittent claudication in PAD is essential for an awareness of the entire process as well as to propose new prevention and treatment strategies.8
The arterial flow, dependent on systemic pressure and vascular resistance, is able to adapt to the needs of muscles during exercise due to decreased peripheral resistances, in the absence of stenosis. When there is stenosis in the circuit, Poiseuille's law reminds us that, among other factors, the radius (to the fourth power), length and blood viscosity are contributors. The radius is the primordial factor to determine the reduction in pressure, since a transversal stenosis of 50% multiplies the resistance by 16. The length of the lesion has little significance, although 2 non-critical stenoses one after the other would have an effect on flow.9,10
Critical arterial stenosis is considered to have diminished distal flow, but this concept relates stenosis with the flow velocity and the drop in distal flow. This means that an arterial stenosis may not be critical at rest but then become critical during exercise. At rest, the blood flow velocity in the femoral artery is between 10 and 20cm/seg.11 When walking, however, the flow velocity easily increases to 150cm/seg.
Claudication and Flow Variations in Peripheral Artery DiseaseIn the intermittent claudication stage, there are no signs of ischaemia at rest because the tissue perfusion distal to the lesion is sufficient to meet metabolic needs. But with exercise, due to the need for increased flow because of the drop in peripheral resistances, the increase is linear until there is a maintained flow plateau, during which oxygen consumption is delayed in spite of the possible metabolic compensations at work, such as increase in the muscular extraction of oxygen (increased oxidative enzymes, increased short-chain acylcarnitines, non-oxidative production [anaerobic] of adenosine triphosphate). Meanwhile, upon interrupting exercise, the hyperaemic phase is longer in these patients than in healthy subjects.8,12–15 Although the haemodynamic situation is determinant in the circulatory status of patients with claudication, there is no linear correlation between the decrease in the ankle-brachial index (ABI) and the claudication-free distance walked, or at least the correlation is inconstant,16 so other factors are probably involved. Other authors assess the ABI at rest and after exercise, carefully recording the pain-free initial distance and the claudication distance until the patient is forced to stop, observing that the data do not always correlate with the ABI. These authors therefore describe a regression model including other parameters.17
Furthermore, another randomised study18 observed that, in spite of tripling the distance before claudication in the training group over the control group, the muscle flow measured with xenon 133 showed no improvement in either of the groups, which demonstrated that the flow is not the only determinant for muscle function in this type of patients.
The Ischaemia/Reperfusion Phenomenon, Metabolic Failure: Ca2+ and Oxygen Free RadicalsThe muscle ischaemia that occurs while walking and the later reperfusion that appears during the pain-related rest period (I/R) should likely cause in the affected sarcolemma consequences similar to what occurs in the myocardium.6
Inadequate perfusion reduces aerobic metabolism, leading to an initial depletion of energy reserves (phosphocreatine) with an eventual decrease in the production of adenosine triphosphate. There is an attempt to compensate for these losses through anaerobic metabolism, with production of lactic acid and OFR, resulting in oxidative stress and cell damage. This damage continues in the reperfusion phase, at rest and when the supply of oxygen and nutrients is restored.19
As anaerobic performance is lower, the decrease in adenine-nucleotides leads to a chain of metabolic failures: depletion and lack of substrate for the production of adenosine triphosphate; collapse of the mitochondrial membrane potential, with its damage and aperture of the mitochondrial permeability transition pore (mPTP), as well as difficult management of Ca2+; accumulation of intermediate products of anaerobic metabolism, including lactic acid and OFR, which entails the inhibition of several metabolic pathways, accumulation and overload of this ion with all its damaging consequences.19
The role of mPTP is key because it can allow for a fatal reperfusion injury. The internal membrane of the mitochondria is relatively impermeable under normal conditions as the pore remains closed during the ischaemia phase, as long as the membrane potential can be maintained. Nonetheless, it may open during reperfusion due to metabolic inability to maintain said potential, causing Ca2+ intoxication and explosion of OFR and leading to mitochondrial and cell damage.19,20
The endothelial regulation mainly mediated by nitric oxide is one of these factors, which can be seriously altered by the appearance and undesirable effects of the superoxide anion and other free radicals that appear during the I/R of patients with claudication, with the resulting oxidative stress.21 As a consequence of the altered function it favours the appearance of the systemic inflammatory response through the inflammatory mediators, activation of platelets and the migration of lymphocytes and monocytes.
Plasma concentrations of malondialdehyde, which is a marker for the formation of OFR, are high at rest in PAD and increase even more with exercise.22 In an attempt to better study the effects of this oxidative stress, we have analysed the effects of walk tests in a group of patients with claudication, studying the oxidation of antipyrine by OFR through the appearance of metabolites. Both while walking as well as during later reperfusion, the 2 products derived from antipyrine, consequence of the oxidation by the OFR, increased significantly in plasma. These metabolites are more precise for measuring the oxidative effect than when measured by the malondialdehyde test, which, due to its biochemical instability, can give rise to false positives for the appearance of lipid peroxidation.23
When we analysed local oxygenation of the ischaemic muscle tissue by means of infrared spectroscopy14 (placing a catheter over the calf muscle in healthy and ischaemic patients on a treadmill at maximum effort), we observed that desaturation was faster in healthy subjects, who also recovered more quickly. Meanwhile, in patients with claudication this process was slower, especially during the recovery time, which was significantly longer. These metabolic changes can be explained because the mitochondrial function in claudication would be diminished, producing a type of myopathy that would alter the response to exercise.
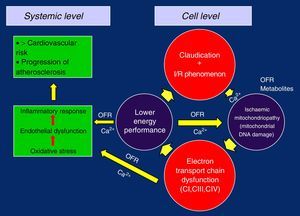
Oxidative Injury, Dysfunction and Mitochondrial DamageWhen mitochondrial respiration is measured at baseline and after the stimulation of the Krebs cycle with substrates and ADP, we can observe in patients with PAD (when compared to healthy subjects) the decrease in oxygen consumption in the myofibrils of the skeletal muscles, indicating that a defect in the electron transport chain (ETC) is the origin of the mitochondrial dysfunction.24
By separately analysing the ETC complexes in these patients in order to study their function and thus be able to evaluate their possible defects (both through in vitro respirometry as well as their enzymatic activity by spectrometry), we can observe that the CI, CIII and CIV complexes are where most OFR are generated, measuring a diminished function of mitochondrial respiration of 17%, 30% and 17% as well as a lower enzymatic activity of 22%, 15% and 32%, respectively. These findings are particularly important to clearly quantify the mitochondrial dysfunction caused by ischaemia25 and show the logical connection between the ETC defects, compromised mitochondrial respiration, decreased production of oxidative energy in the skeletal muscles and the low exercise performance in patients with PAD.26
But the role of OFR is a permanent topic of discussion because the antioxidant substances that should neutralise them have not been able to validate their properties in different clinical trials conducted to avoid their clinical effects regarding protection over oxidative stress and the progression of systemic arteriosclerosis. Until now, no consistent trials have been published demonstrating any benefits with the different antioxidant therapies.27
Nonetheless, if we contemplate the loss of the mitochondrial membrane potential, appearance of the pore (mPTP), inability to maintain proper intracellular Ca2+ levels, which is the result of the coordinated action of a series of different transport systems located in the plasma membranes and membranes of different cell organelles, as well as the link with diverse cytosolic proteins,28 together with the release of OFR in the CI, CIII and CIV complexes of the ETC, it is logical to think that the first place where alterations may occur is in the transport chain itself, progressively worsening its function, as well as in the content of the mitocondria.28,29 Curiously, the CII is not affected in these separated studies, possibly because this complex is entirely codified through the DNA of the cell nucleus, while the ADNmt partially controls the rest of the complexes by means of the codification of 13 of its proteins.30
This damage in the ADNmt can be the mechanism responsible for the specific defects found in those complexes, as this DNA is particularly vulnerable to oxidative damage, with a mutation average 10 times greater than that caused in the genome of muscle cells.31 This hypothesis has gained support since other studies have demonstrated a damage 17 times greater in ADNmt mediated by the specific substrate of the 4977-bp deletion compared to control groups.32
In this way, with the defective replication of the ETC complexes, the vicious circle of ischaemia/reperfusion injury is closed, which perpetuates its failure and gives rise to the ischaemic mitochondrial myopathy in claudication. The loss of the membrane potential and the problems in the management of Ca2+ due to the lower energetic performance would be the trigger, causing oxidative stress, endothelial dysfunction and overall inflammatory response, with the progression of generalised systemic arteriosclerosis and increased cardiovascular risk5,19,26,28,32 (Figure 2).
Alterations in Muscle Structure and Function in Peripheral Artery DiseasePatients with PAD present histological anomalies in their skeletal muscles.
In muscle biopsies, deep changes are observed both in morphology and in the recruitment number of the different slow-contracting myofibrils (type i), as well as in 2 subtypes a and b of type ii, which are for faster and more energetic activity. These latter, which under normal circumstances contain fewer mitochondria and become fatigued sooner, would be diminished, with associated muscle weakness; electrophysiology also demonstrated the presence of distal axonopathy, which affects the nerve fibres of all sizes.33,34
As a consequence of the limitation of their activity when walking, patients with claudication pain tend to be more sedentary. Therefore, the number of mitochondria and their activity should be reduced but, to the contrary, several studies show an increase in the number of mitochondria.26,35 This increase in mitochondrial expression can be a direct and proportional consequence of the degree of ischaemia in the extremity, which could reflect to a point the severity and intensity of the ischaemia.
Indeed, an increase in the mitochondrial mass can improve the extraction of oxygen to meet their metabolic functions in the presence of ischaemia and become established as a compensatory mechanism. Interestingly, however, the increased mitochondrial expression is also associated with degenerative ETC diseases, and this allows us to establish a mechanistic and functional link with the previously described ischaemic mitochondrial myopathy.24,35
Systemic AlterationsIntermittent claudication is associated with haemorrheologic alterations of the blood with greater viscosity, due to an increase in the plasma concentration of fibrinogen, fibrin replacement, von Willebrand factor and plasminogen activator inhibitor. Meanwhile, the deformability of the erythrocytes is lower, so their passage through the microcirculation becomes difficult.36
It has also been demonstrated that exercise during claudication is associated with excess thrombin production both in smoker and non-smoker groups when compared to control groups.37
The hidden effects that are produced in the organism as a result of the oxidative stress while walking in patients with claudication are even more numerous, with the possible presence of microalbuminuria, an indicator of increased permeability of the renal glomeruli, a phenomenon involved in the context of a generalised increase in vascular permeability.38 In any event, microalbuminuria in other occasions does not appear when walking, but the NAG enzyme appears at statistically significant proportions, with a more severe marker profile, at more advanced stages of critical ischaemia, and with a serious risk for causing renal dysfunction.39
Another distant effect that merits attention is the increased intestinal permeability of patients while walking (200m on a treadmill with a slope of 12%), a fact that is demonstrated by the lactulose-mannitol test. This increase in intestinal permeability was known as a consequence of I/R after aortic clamping, both in animal models as well as in human clinical practice, but after this study it has been demonstrated that, as a consequence of I/R, intestinal permeability significantly increases while walking and diminishes to the point of near disappearance after successful revascularisation of these patients.40
Intermittent, Ankle-Brachial Index and Cardiovascular RiskThe apparent benign nature of claudication is well known, given its evolution within the current classifications for PAD. In the lower extremities, only 25% tend to worsen at a rate of 7%–9% annually and later 2%–3% annually.
The risk for loss of a limb is estimated in the Framingham study at 2%, but more recent studies over a 5-year period report percentages of 1%–3%. In this context, it is therefore logical to consider it a benign disease.
Nevertheless, this same claudication can be considered a disease with a very poor prognosis given its systemic complications. The survival rate of claudication patients is much lower than the general population, with 5, 10 and 15-year mortality rates of 30%, 50% and 70%, respectively. Their risk for mortality is 2.5 times greater compared with control groups, which is even greater in the more advanced subgroup of critical ischaemia (3–4 times higher), with mortality rates of 20% for the first year, 50% after 5 years and 90% within 10 years.41 We can establish a clear stratification between the stages of PAD and ABI: stage i (asymptomatic) correlates with indices ≥0.9; stage ii (intermittent claudication) has an ABI of 0.9–0.5. The distance to claudication is shorter as the index drops, although there is no linear correlation between the distance before claudication measured on treadmill and ABI.16
Moreover, the ABI has been revealed as the most powerful marker for cardiovascular risk. In older women, an ABI<0.9 increases between 3 and 8 times the risk for cardiovascular mortality and between 2 and 5 times the risk for all-cause death when compared with an ABI>0.9.42 Patients with more extensive coronary death and with a greater prevalence of multivascular disease are those with a more pathological ABI.43
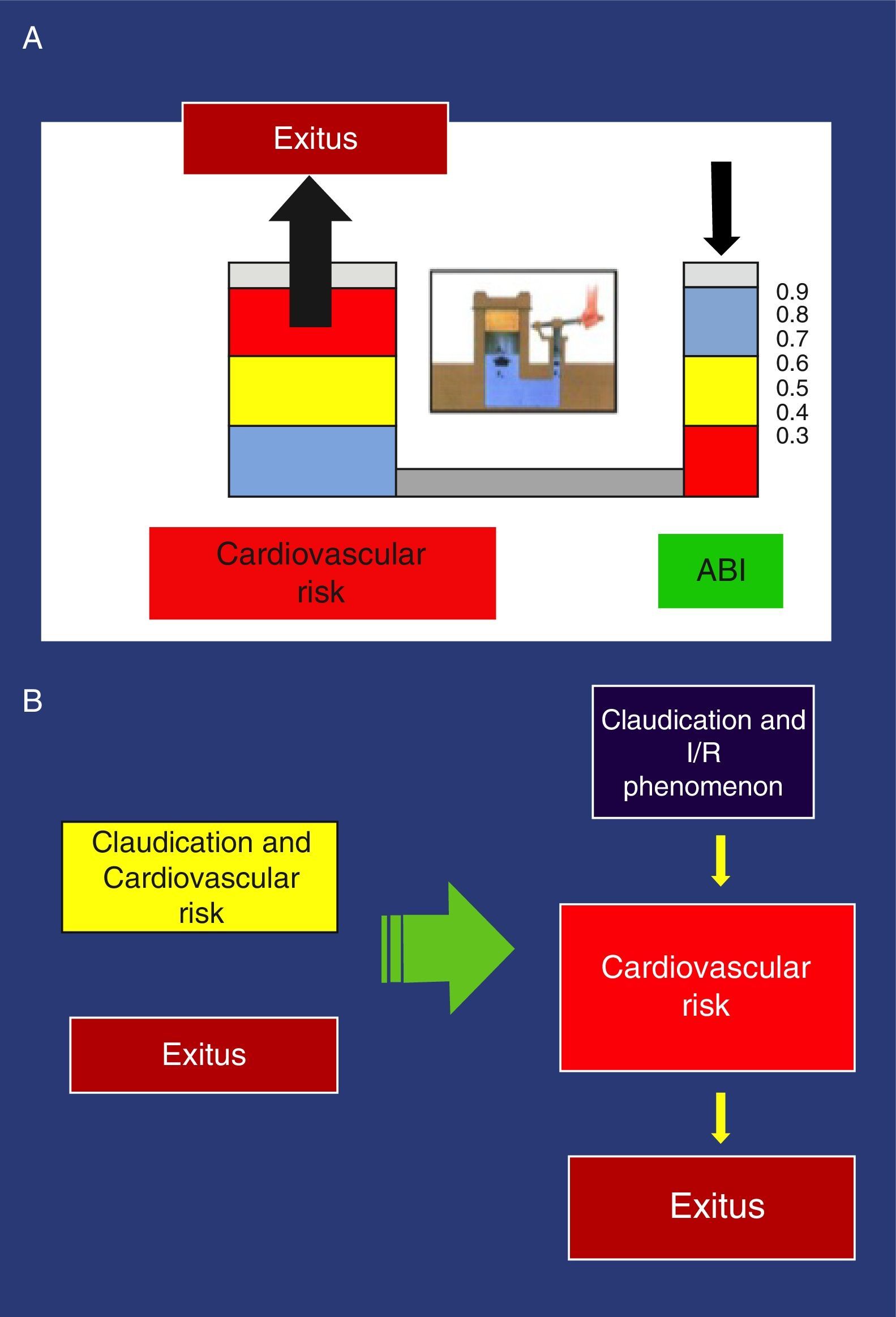
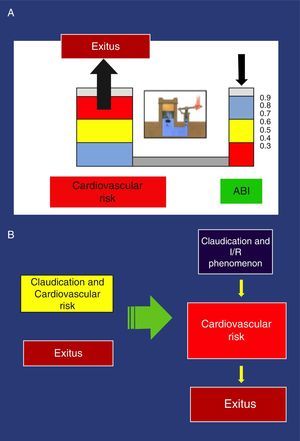
These findings have been confirmed in several studies. Thus, an ABI<0.9 is associated with approximately twice the total 10-year mortality due to cardiovascular causes, and the vital prognosis is much worse due to cardiovascular risk as the ABI diminishes.44 Pascal's principle applied to the hydraulic clamp is an example that illustrates this association (Fig. 3).
(a) Example of the Pascal principle, applied to the association of the ankle-brachial index and increased cardiovascular risk. (b) New perception of claudication: Left, conventional perception, non-causal relationship of >cardiovascular risk. Right, new orientation with causal relationship.
With the traditional perception of claudication that obviates physiopathology, the possible consequences of the I/R phenomenon are completely ignored as it only deals with the distance walked, which is subjectively expressed by the pain-free distance before claudication. In addition to the distance walked, the new vision contemplates I/R and its possible relationship with increased cardiovascular risk in patients with claudication. The greater its intensity, the higher the risk becomes (Fig. 3).
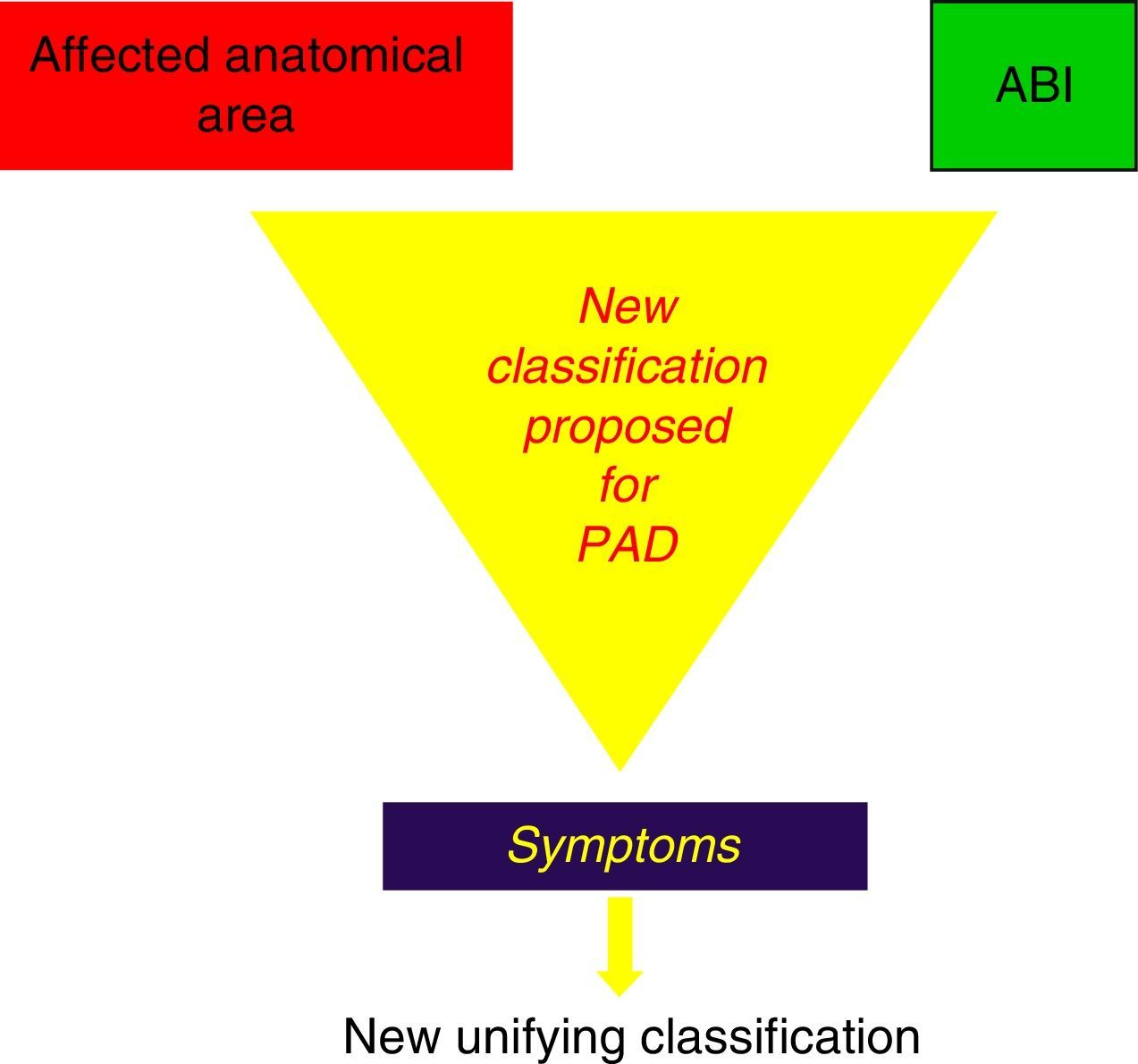
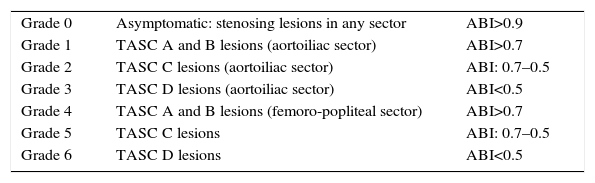
In light of these events, we suggest the need for a new PAD classification that, by integrating the current recommendations of Fontaine and Rutherford,1,2 would contemplate not only claudication symptoms (which are subjective and variable even on the same day) but also the possible metabolic consequences of I/R, expressed by the muscle masses that enter into ischaemia in the affected topographic sectors, by the stenosing or obstructive lesions included in the TASC II classification,41 as well as by its intensity expressed by the ABI44,45 (Fig. 4).
An obstructive lesion of the tibioperoneal trunk artery and Leriche syndrome should not have the same long-term metabolic effect because of the magnitude of the I/R that they generate. The 2 lesions can have clinical expression of moderate claudication at 150m and are classified as Fontaine G.IIb and Rutherford 2. However, we should concur that the possible repercussions linked to ischaemia/reperfusion injury (>oxidative stress, >endothelial dysfunction and >systemic inflammatory response) should not be comparable.
Our proposal includes 6 grades (grade 0 asymptomatic), that integrating 3 sections that should be combined (Table 1):
- a.
the lesion extension of the affected anatomical area (TASC II classification)
- b.
haemodynamic expression (ABI)
- c.
patient symptoms (modified Fontaine and Rutherford), in 5 stages
New Unifying Classification.
| Grade 0 | Asymptomatic: stenosing lesions in any sector | ABI>0.9 |
| Grade 1 | TASC A and B lesions (aortoiliac sector) | ABI>0.7 |
| Grade 2 | TASC C lesions (aortoiliac sector) | ABI: 0.7–0.5 |
| Grade 3 | TASC D lesions (aortoiliac sector) | ABI<0.5 |
| Grade 4 | TASC A and B lesions (femoro-popliteal sector) | ABI>0.7 |
| Grade 5 | TASC C lesions | ABI: 0.7–0.5 |
| Grade 6 | TASC D lesions | ABI<0.5 |
Symptoms: (a) mild claudication>300m; (b) moderate claudication: 100–300m; (c) severe claudication<100m; (d) paraesthesia-pain at rest; (e) tissue damage.
Note: The new classification associates a numerical grade and a letter for symptoms: e.g. Grade 3d; Grade 5b.
In this manner, for instance, grade 5b is a patient with stenosing or obstructive lesions greater than 15cm of the femoro-popliteal sector of a limb (TASC C; ABI 0.7–0.5; and, a moderate claudication distance of 200m).
The consideration in the new classification of the possible consequences caused by I/R on ischaemic muscle mass (ischaemic mitochondrial myopathy), which triggers oxidative stress, endothelial dysfunction and systemic inflammatory response with possible generalised progression of arteriosclerosis and increased cardiovascular risk,5,19,26,28 would be key to provide integral treatment recommendations for these patients. In the mid-term, this could possibly reduce the elevated cardiovascular risk that these patients currently present.41–45
Conflict of InterestsThe author has no conflict of interests to declare.
Please cite this article as: Vaquero Morillo F. El impacto de la enfermedad arterial periférica: propuesta de una nueva clasificación. Cir Esp. 2016;94:266–273.