Differentiated thyroid carcinoma includes 2 different tumour types, papillary (PC) and follicular carcinoma (FC), and although similar, their prognosis is different. FC is uncommon, and this has led to it often being analysed together with PC, and therefore the true reality of this tumour is difficult to know. As a result, the diagnostic and therapeutic management and the prognostic factors in differentiated carcinoma are more predictive of PC than FC. In this review we analyse the current state of many of the therapeutic aspects of this pathology. The best surgical technique and the usefulness of associated lymphadenectomy is also analysed. Regarding post-surgical ablation with 131I, the indications, doses and usefulness are discussed. For the remaining therapies we analyse the few indications for radiotherapy and chemotherapy, and of new drugs such as tyrosine kinase inhibitors.
El carcinoma diferenciado de tiroides incluye 2 tipos tumorales diferentes: el carcinoma papilar (CP) y el folicular (CF) y, aunque similares, su pronóstico es diferente. La infrecuencia del CF ha hecho que habitualmente se analice conjuntamente con el CP, lo cual dificulta conocer su verdadera realidad. En esta revisión se analiza la situación de los diferentes aspectos terapéuticos de esta dolencia. Se revisa cuál es la mejor técnica quirúrgica y la utilidad de realizar vaciamiento ganglionar asociado. Respecto a la ablación posquirúrgica con 131I se evalúan las indicaciones, las dosis y su utilidad. En el resto de terapias se analizan las pocas indicaciones que tiene la radioterapia y la quimioterapia, y la aparición de nuevos fármacos como los inhibidores de la tirosin-cinasa.
Differentiated carcinoma is the most common thyroid tumour, and in the majority of cases it is associated with a favourable prognosis.1 The denomination of differentiated carcinoma covers two tumour types with very different pathogenesis, biology and clinical behaviour. The current tendency is to consider them as different entities. These two tumours are papillary carcinoma (PC) and follicular carcinoma (FC), which although similar have different prognoses. Thus FC is more vascularised and usually presents a higher rate of vascular invasion and clinical aggressiveness.1
The incidence of FC is strongly related with iodine deficit, and it is decreasing due to iodine supplementation.1,2 Although there are major variations between populations, its incidence is estimated to stand at 1–2 cases for every 100000 women/year, and around 0.4–0.5 cases for every 100000 men/year. This rareness has led to its usually being analysed together with PC, hindering knowledge of the true nature of the former.1 Thus the majority of studies analysing the usefulness of certain diagnostic tests, the therapeutic efficacy of different treatments and which prognostic factors are the most significant for differentiated carcinoma, as the series include more PC than FC, their results are more predictive and useful for PC than they are for FC.3 Few studies contain a sufficient number of FC cases to allow it to be studied independently, and the results of these studies are not consistent.3–5
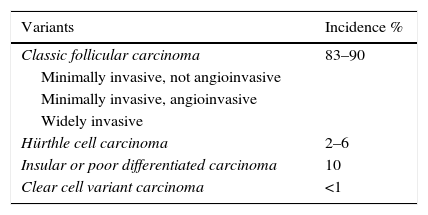
The treatment of FC currently depends on tumour extension. Thus patients with greater extension or a higher risk of recurrence are treated more aggressively and monitored more closely. However, in localised low risk tumours a more conservative treatment is equally effective.1 Additionally, the different subtypes of FC have to be taken into account, as they directly influence patient prognosis (Table 1).
Histological Types of Thyroid Follicular Carcinoma.
| Variants | Incidence % |
|---|---|
| Classic follicular carcinoma | 83–90 |
| Minimally invasive, not angioinvasive | |
| Minimally invasive, angioinvasive | |
| Widely invasive | |
| Hürthle cell carcinoma | 2–6 |
| Insular or poor differentiated carcinoma | 10 |
| Clear cell variant carcinoma | <1 |
Many controversies surround the diagnosis, treatment and evolution of FC. This revision aims to analyse the current state of the different therapeutic options for FC and determine which is the best medical and surgical treatment for this group of patients, based on the scientific evidence.
To this end two bibliographical searches were performed. On the one hand scientific publications indexed in the different databases (Pubmed, Embase and Conchrane Library) and, on the other, the guides and consensus documents of different Spanish, European and American scientific societies on the treatment of FC.
Controversies in the Surgical Treatment of Follicular CarcinomaThe Utility of Molecular Cytological Markers in Preoperative Diagnosis and Surgical PlanningFine needle aspiration (FNA) is currently the gold standard for differential diagnosis between a benign nodule and thyroid cancer, and its sensitivity largely depends on the cytologist's experience.1,6 Nevertheless, the main problem with this technique is its lack of sensitivity in the evaluation of follicular neoplasia, as it is unable to distinguish between benign lesions (follicular adenoma) and malignant entities (thyroid follicular carcinoma and the follicular variant of papillary carcinoma),1,7 given that it is no longer possible to diagnose vascular or capsular invasion.1,8 To improve the diagnostic sensitivity of FNA in follicular neoplasia, immunohistochemical and molecular diagnosis techniques are being analysed. Several molecules have therefore been said to be involved in the carcinogenic process, and they have been proposed as thyroid malignity markers to increase the diagnostic precision of FNA. They include telomerase, thyroperoxidase, keratan-sulphate, the group of high mobility proteins I (Y) (HMGI[Y]), the cell surface mesothelial antigen HBME-1, thyroperoxides, cytokeratin 19 and galectin-3 (GAL3).9,10 Several gene expressions are also being analysed, and the expression of more than 100 genes has been detected.11 It should be pointed out that the fusion of oncogene PAX8/peroxidase proliferator–gamma receptor (PPARγ) has been identified in approximately 25%–50% of FC, with a translocation between regions 3p25 and 2q13.
Although advances are occurring very quickly, the results on the utility of the different proposed malignancy markers often disagree.12–15 Some studies have shown that, in comparison with using markers in isolation, a sequential combination of two markers is more useful. The combination of GAL3 and HBME-1, or GAL3 and cytokeratin 19 in the case of oncocytic lesions therefore improve the diagnostic sensitivity of FNA.12,14 Cytological markers are not currently in widespread use.
Lastly, although analysis of the BRAF (V600E) mutation has been shown to be of use in selecting nodules with indeterminate cytology (AUS/FLUS), it is highly specific for PC and not FC, so that it is not useful for the diagnosis of CF.16–19 However, the RAS mutation may be important in the identification of the follicular variant of PC and even FC, although more studies are required to confirm these preliminary results.20,21
Which is the Best Initial Surgery for Follicular Carcinoma?The main problem which tends to arise when considering surgery for FC is that the operation is usually indicated by the diagnosis of follicular neoplasia, but without knowing that it is a carcinoma. This means that the definitive diagnosis of FC usually takes place after the patient has been operated on. Due to this, we have a patient diagnosed with FC who has usually already been subjected to a hemithyroidectomy. There is now consensus and it is accepted that hemithyroidectomy is the correct surgery in only 2 cases: (1) microcarcinoma (tumour<1cm) that is unifocal with no vascular invasion or prior exposure to radiation, and (2) minimally invasive FC smaller than 3–4cm without vascular invasion.2,22
In the other cases, the majority of studies indicate that lobectomy is an independent factor which affects tumour recurrence, so that it is recommended that the thyroidectomy be completed if it was not complete in the first operation.23 However, depending on the size of the remaining thyroid, when the risk of disease persistence is low, and an effective option to completing the thyroidectomy is radioiodine ablation.24 In cases where an entire lobe has been left, 131I is not recommended, due to its low efficacy in these cases. Moreover, completing the thyroidectomy has low complication rates when it is performed by experienced surgeons, facilitating post-surgical ablation using 131I and permitting a more suitable follow-up.25 Some authors accept almost total thyroidectomy or Dunhill's technique, although this gives rise to the problem that the thyroid remnant will hinder the evaluation of possible relapses at a cervical level and ablation with 131I.1,2
How much time should pass between the first surgery and complete thyroidectomy is a controversial question. Although there is no consensus, Glockzin et al.,26 in a series of 128 thyroid carcinomas re-operated to complete the thyroidectomy, state that there is less morbidity when at least 3 months have elapsed since the first surgery.
The Utility of Intraoperative BiopsyGiven that the majority of FC patients are operated with the diagnosis of follicular neoplasia,2,27 intraoperative biopsy (IOB) has the purpose of deciding the definitive surgical procedure during the first operation, avoiding the need for a second operation as well as unnecessarily aggressive surgery.4 However, although some authors have described its utility,28 the majority show that it is not a cost-efficient technique for the diagnosis of CF.29,30 Hamburger and Hamburger31 therefore state that only 3 of 359 IOB (0.8%) contributed to the decision on surgical treatment, while Shaha et al.32 show similar limitations. It also has to be said that there are problems with the reproducibility of results due to differences in interpretation between observers and the same observer in histopathological diagnosis.33 Although it is not accepted by the majority of pathologists, IOB has also been said to have the drawback of the deterioration in the surgical specimen resected due to freezing, which may affect its subsequent study in paraffin.
As there are no randomised prospective studies (the only one that exists indicates a low level of utility29), the tendency is to cease using IOB routinely for follicular neoplasias (Bethesda III and IV).34 Some authors state that IOB should be reserved for cases in which surgical exploration gives rise to the suspicion of a carcinoma.29,30,34 Lastly, it should be pointed out that although some groups found no false positives, they did detect a high percentage of false negatives, so that they continue to recommend IOB as it prevents a repeat operation in a group of patients.
The lack of sensitivity of IOB in thyroid follicular neoplasias could theoretically be improved by using molecular techniques (see Introduction), although studies would be required to confirm this.
The Type of Node Dissection to be UsedUnlike PC, FC usually has less of a tendency to spread into the lymphatic system. Thus removal of the ganglia either before or during the operation is indicated if lymphatic involvement is suspected.35 The benefits of prophylactic central dissection are arguable, as there is no evidence that this improves the relapse or mortality rates, and it increases postoperative morbidity.1,2 Nevertheless, it does permit greater precision in disease staging.
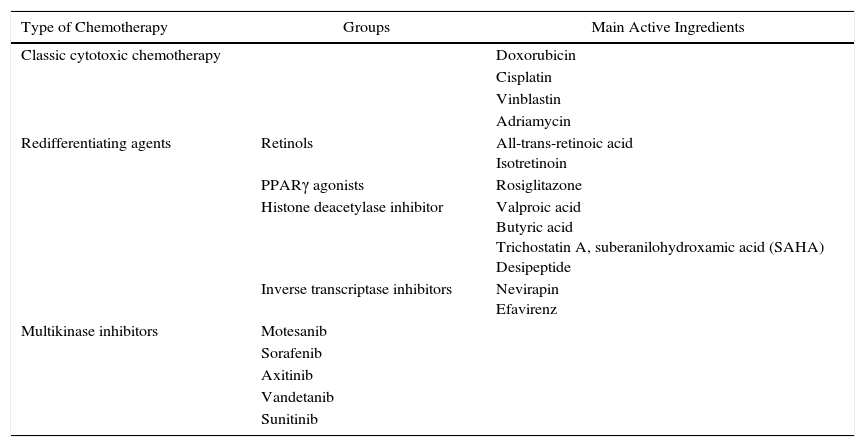
The Most Appropriate Surgery for Minimally Invasive CarcinomasAlthough total thyroidectomy is recommended in FC, minimally invasive carcinoma is the least aggressive FC and it may be treated by hemithyroidectomy.5,36 Although there is no consensus on size, in cases when it attains a size larger than 3–4cm it is advised that thyroidectomy be completed on the contralateral side (Table 2).
Classification of the Drugs Which Are Potentially Useful in Follicular Carcinoma.
| Type of Chemotherapy | Groups | Main Active Ingredients |
|---|---|---|
| Classic cytotoxic chemotherapy | Doxorubicin | |
| Cisplatin | ||
| Vinblastin | ||
| Adriamycin | ||
| Redifferentiating agents | Retinols | All-trans-retinoic acid Isotretinoin |
| PPARγ agonists | Rosiglitazone | |
| Histone deacetylase inhibitor | Valproic acid Butyric acid Trichostatin A, suberanilohydroxamic acid (SAHA) Desipeptide | |
| Inverse transcriptase inhibitors | Nevirapin Efavirenz | |
| Multikinase inhibitors | Motesanib | |
| Sorafenib | ||
| Axitinib | ||
| Vandetanib | ||
| Sunitinib |
The latest classifications take into account not only whether FC are minimally or widely invasive, but also consider angioinvasion. Thus minimally invasive FC is classified into types with and without angioinvasion (Table 1). This is important because angioinvasive types have a poorer prognosis and require more aggressive treatment.37
More Aggressive Variants of Follicular CarcinomaHürthle cell or oncocytic carcinoma has a higher rate of multicentricity and metastatic dissemination, above all in the cervical lymph nodes (25% of cases), although it also spreads to other organs. Given this greater presence of lymph node involvement and the fact that these tumours take up little iodine (so that radioiodine ablation is less effective), lymphatic surgery is the classical recommendation as performed in PC, advising ipsilateral central dissection.
However, there is no consensus and, in spite of the description of a higher risk, some institutions such as the College of American Pathologists state that the biological behaviour of Hürthle's carcinoma depends more on the size and extension of the tumour than it does on its histology. They therefore consider it to be a variant of FC with a similar prognosis, and believe that it should be treated in the same way as the equivalent stage of non-Hürthle cell FC.38,39
The insular or poorly differentiated type is usually located in the sinus of a multinodular goitre. It is characterised by intermediate morphology and behaviour between differentiated carcinomas and anaplasic carcinoma. The clinical profile corresponds to women over the age of 50 years old who present rapid growth of a pre-existing thyroid lesion. It is characterised by high metastatic power and a high relapse rate, while metastasis to lymph nodes, the lungs and bones is often found at diagnosis. The majority of patients die within the first 3 years after diagnosis. The prognosis depends on the initial TNM classification, whether surgery was complete and if it responded to treatment with radioactive iodine.40
Lastly, the clear cell variant is more aggressive, although it is also rare. Clear cell follicular carcinoma with metaplastic changes and a mitochondrial formation of intracytoplasmatic vesicles, glycogen and fat accumulation and thyroglobulin deposits. The majority of primitive clear cell tumours present histologically as microfollicular or trabecular.
In all of these more aggressive variants surgical treatment must also be more aggressive.
The Most Appropriate Surgery for Locally Advanced CarcinomaCervical structure invasion is rare but increases the possibility of complications that compromise the patient's life and limit the utility of non-surgical therapies. Due to this, in the absence of uncontrolled disseminated disease, the possibility of surgical resection has to be evaluated.41 The indication for surgery depends not only on local resectability but also on the individual condition of the patient. Depending on the extension of the invasion, the technique used may vary from simple tracheal up to complex laryngotracheal or oesophageal resection.4
Although there is no consensus on the optimum surgery for carcinoma with laryngotracheal invasion, aggressive resection is generally recommended to reduce morbidity and maintain the integrity of the airway.41 Residual macroscopic disease has an unfavourable affect on evolution, above all in young patients.
Which Surgical Option is the Best for Distant MetastasisThe best therapeutic option for distant metastasis of FC is resection, whenever it is accessible.42,43 This treatment must be completed, or sometimes treated only with 131I, whenever they are iodine-absorbing. In the other cases and in cases that are refractory to 131I treatment, the best therapeutic option is local radiotherapy.42,43 As a general rule these patients need a multidisciplinary therapeutic approach, as they usually require surgical resection, ablation with 131I, radiotherapy and sometimes chemotherapy to control or relieve the symptoms.42,44
Controversies in the Ablation of Residual Post-Surgical Thyroid Tissue Using Radioactive IodineWhen Should Ablation Using Radioactive Iodine Be Indicated?Ablation using 131I makes it possible to destroy thyroid remnants that may remain in the thyroid bed, reducing relapses and facilitating the monitoring of thyroglobulin.24,45,46
The systematic use of 131I is accepted in those FC cases with persistent disease, vascular invasion and factors leading to a poor prognosis, as it reduces the relapse rate. Nevertheless, in unifocal microcarcinomas and in non-angioinvasive minimally invasive carcinomas it has not been proven to have any benefit, so that ablation is not recommended in these cases. In other cases which do not have a poor prognosis but are neither unifocal microcarcinomas nor minimally invasive, there is no consensus on its utility, as its benefits are controversial.
What Is the Most Effective Dose of 131I?The dose of 131I to be used may vary depending on several factors. To use low doses of 131I, from 30 to 50mCi, it is fundamental that any remnant be small or non-existent, evaluated by total body gammagraphy and using 2–3mCi 131I.47 The dose of 30mCi became quite popular as it was a way of avoiding hospitalisation. Although this is not the case in all countries, currently it is possible to carry out ablation with 131I on an out-patient basis, with a dose of up to 60mCi, given that those who are close to the patient are exposed to a minimum amount of radiation.
It is important to select the correct dose, as several authors state that the success rates of ablation increase with the dose of 131I, although some of them find the differences between doses to be minimal.24,47 Thus in a randomised study Johansen K et al.48 show that the ablation index with 30mCi stands at 81%, as opposed to 84% with 100mCi. Nevertheless, another randomised study49 shows that with 30mCi the complete ablation index is 63%, with 50mCi it is 78%, with 90mCi it is 74% and with 155mCi it is 77%. Recently, Schlumberger et al.50 in a phase 3 randomised study, showed that in low risk patients treatment with low dose 131I (30mCi) is effective. Likewise, the clinical trial by Mallick et al.51 shows that the dose of 30mCi may be as effective as a 100mCi dose (85% versus 88.9% of complete ablation, respectively).
What Is the Therapeutic Impact of Ablation With 131I?There is much discrepancy about the results of ablation using 131I, although the majority of authors mention lower rates of relapse and mortality afterwards. Another factor that may justify these differences is the extension of the thyroidectomy performed, as it is not homogeneous in all cases.1,2
There is sufficient scientific evidence to indicate that in high-risk tumours carcinoma recurrence, distant recurrence and deaths due to cancer are significantly less after ablation with radioiodine 131I than is the case with L-T4 or only medical treatment.1,2
The Utility of Other TreatmentsIs Treatment With Thyroid Hormone Useful to Obtain TSH Suppression?The theoretical aim of TSH suppressive therapy with l-T4 is to inhibit the growth, which requires TSH, of the thyroid tissue that may remain following the initial treatment. Some authors state that rates of recurrence fall with treatment using l-thyroxin,52 although the optimum level of TSH required is still unknown. Initially the dose of l-T4 should be sufficient to reduce levels of TSH to ≤0.1mU/l, as there is no evidence that suppression of TSH below this level improves results.
The retrospective study by Pujol et al.53 shows that disease-free survival increases when TSH is constantly suppressed (TSH <0.05U/ml), while the degree of TSH suppression is an independent predictor of recurrence. However, a prospective study with 617 patients in the National thyroid cancer treatment cooperative study found that disease stage, patient age and treatment with 131I were all independent predictive factors of disease progression, while this was not so for degree of TSH suppression.54
All of this is important, as TSH suppression to undetectable levels (subclinical thyrotoxicosis) is not innocuous, and over the long-term is may have side effects at a cardiac and bone level.55 Thus low concentrations of TSH in serum in individuals aged 60 years old or more is associated with an increase in mortality from all causes, and in particular from circulatory and cardiovascular diseases. Due to this, in these patients TSH suppression should be avoided. During subclinical thyrotoxicosis, one aspect that should be taken into account is that the majority of patients have a prothrombotic profile. TSH suppression is therefore unnecessary in patients considered to be in complete remission after a suitable follow-up period, so that therapy can be switched from suppression to replacement. TSH suppressive therapy should be indicated for patients with evidence of persistent disease. Additionally, in high-risk patients who have undergone remission, suppressive therapy is advised during 3–5 years.56
Radiotherapy in Follicular Carcinoma?External radiotherapy of the neck is rarely used, and it is usually indicated for tumours or recurrent tumours that cannot be resected, above all if they do not absorb 131I. External radiotherapy is also indicated for bone and brain metastasis.57 In cases of microscopically invasive FC a higher disease-free rate was described when radiotherapy was used in comparison with when it was not (53% versus 38%).58
The fact that radiotherapy requires careful planning must be taken into account, with precautions to prevent post-radiation myelopathy.4 For residual microscopic disease a total dose of 50–60Gy must be given for the neck and upper mediastinum, in 25–30 sessions with 5 sessions per week. An increase of 5–10Gy may be given if there is a large residual neoplastic centre.
Is Chemotherapy of Any Use Now?Classical Cytotoxic ChemotherapyCytotoxic chemotherapy is of no utility in the treatment of FC. Its use is restricted to patients with disease that progresses in spite of surgery, 131I or other forms of treatment.4,59 The response is poor, so much so that the best response is only 10%–20% with the use of doxorubicin or a combination of doxorubicin–cisplatin. However, recently vinblastin with or without adriamycin seems to improve results.59 In any case, responses are partial and transitory, without any clear lengthening of survival.
The response to chemotherapy seems to be better in poorly differentiated carcinomas, although this requires confirmation by more studies.60 In advanced cases, chemotherapy before surgery may sometimes be effective in reducing tumour size and aiding surgery.59
Redifferentiating AgentsThese agents have the purpose of redifferentiating those cases which have become dedifferentiated so that they absorb 131I, so that 131I can be used again as a therapy. Several substances have been used for this, with varying results. The retinoids are the group that has been studied the most, and existing clinical studies indicate that treatment using them is tolerated well, and that absorption of 131I increased by from 20% to 50%.61,62 In these cases thyroglobulin usually increases as a sign of tumour redifferentiation. Several studies are planned to evaluate which advanced thyroid cancer patient subgroups could benefit from its use. Another group of substances are the PPARγ agonists, although few studies show that they slightly increase the absorption of radioiodine and that they are well-tolerated. Histone deacetylase inhibitors inhibit cellular proliferation and permit dedifferentiation, although clinical studies to confirm their utility are lacking. Lastly, the use of reverse transcriptase inhibitors (nevirapin and efavirenz) was described recently in cancer, although these treatments are usually used for HIV. Nevertheless, given their toxicity it is not recommended that they be used until their efficacy has been proven.
Drugs That Inhibit the Intracellular Proliferation CascadeOther molecular therapies and antiangiogenic agents are being analysed and may represent an aid for some patients,63 although these studies are only preliminary. Several clinical trials are therefore evaluating tyrosin-kinase and angiogenesis inhibitors in the treatment of patients with metastatic disease or differentiated carcinomas that are refractory to other treatments. Although the results are hopeful, they are pending confirmation in large series of patients.63 Their main problem is their high percentage of side effects, which although not serious are very unpleasant (gastrointestinal symptoms, etc.).
Final Considerations- 1.
Molecular cytological markers are still not useful for clinical application in the preoperative diagnosis of FC.
- 2.
The best surgical technique for FC is total thyroidectomy, except in very carefully selected cases in which hemithyroidectomy may be sufficient.
- 3.
IOB is of limited use in ruling out FC, so that its use is not recommended.
- 4.
Node removal in FC is only recommended when lymphatic involvement is suspected. It should not be performed as prophylaxis.
- 5.
In minimally invasive FC it is important to differentiate between the angioinvasive and non-angioinvasive forms, as this has therapeutic and prognostic implications.
- 6.
The non-classical variants of FC (Hürthle, insular and clear cells) have a poorer prognosis and require more aggressive initial treatment.
- 7.
In locally advanced FC, more aggressive resection is generally recommended to reduce morbidity and maintain the integrity of the airway.
- 8.
Ablation of thyroid tissue in FC reduces relapses and facilitates monitoring of thyroglobulin, although some groups of FC do not require ablation to achieve a good prognosis.
- 9.
The latest clinical trials show the utility of low-dose radioiodine, facilitating the treatment of these patients on an out-patient basis. Due to this, some European groups, mostly Italian, are starting to use it in this way.
- 10.
TSH suppression therapy improves disease-free survival, although in elderly patients and those with high cardiovascular risk it increases mortality due to cardiovascular causes.
- 11.
External radiotherapy is indicated in relapses that cannot be resected that do not absorb 131I and in brain and bone metastatic lesions.
- 12.
Many therapeutic agents are being trialled for advanced thyroid cancer, and pharmacological gene therapy, chiefly agents that block the signalling route and redifferentiation agents, are now on the horizon as a therapeutic alternative.
The authors declare that they have no conflict of interests.
Please cite this article as: Ríos A, Rodríguez JM, Parrilla P. Tratamiento del carcinoma folicular de tiroides. Cir Esp. 2015;93:611–618.