Currently, variability in surgical practice is a problem to be solved. The aim of this study is to describe the variability in the surgical treatment of breast cancer and to analyze the factors associated with it.
MethodsThe study population included 1057 women diagnosed with breast cancer and surgically treated. Our data were from the CaMISS retrospective cohort.
ResultsThe mean age at diagnosis was 59.3±5 years. A total of 732 patients were diagnosed through screening mammograms and 325 patients as interval cancers. The mastectomy surgery was more frequent in the tumors detected between intervals (OR=2.5 [95%CI: 1.8–3.4]), although this effect disappeared when we adjusted for the rest of the variables.
The most important factor associated with performing a mastectomy was TNM: tumors in stage III–IV had an OR of 7.4 [95%CI: 3.9–13.8], increasing in adjusted OR to 21.7 [95%CI: 11.4–41.8].
Histologically, infiltrating lobular carcinoma maintains significance in adjusted OR (OR=2.5 [95%CI: 1.4–4.7]).
According to the screening program, there were significant differences in surgical treatment. Program 3 presented an OR of non-conservative surgery of 4.0 [95%CI: 1.8–8.9]. This program coincided with the highest percentage of reconstruction (58.3%).
ConclusionsThis study shows that, despite taking into account patient and tumor characteristics, there is great variability in the type of surgery depending on the place of diagnosis.
Actualmente la variabilidad en la práctica quirúrgica constituye un problema a resolver. El objetivo de nuestro estudio es describir la variabilidad en la realización del tratamiento quirúrgico del cáncer de mama y analizar los factores asociados a la misma.
MétodosLa población de estudio comprende 1.057 mujeres diagnosticadas de cáncer de mama y tratadas quirúrgicamente procedentes de la cohorte retrospectiva CaMISS.
ResultadosLa edad media en el momento del diagnóstico fue de 59,3±5 años. Se diagnosticaron 732 pacientes mediante mamografías de cribado y 325 pacientes como cánceres de intervalo. La realización de mastectomía fue más frecuente en los tumores detectados entre intervalos (OR=2,5; [IC 95%: 1,8-3,4]), aunque este efecto desaparece al ajustar por el resto de variables.
El factor más determinante asociado a la realización de una mastectomía fue el TNM: los tumores con estadio III-IV presentaron una OR de 7,4 (IC 95%: 3,9-13,8), aumentando en la OR ajustada hasta 21,7 (IC 95%: 11,4-41,8).
Histológicamente el carcinoma lobulillar infiltrante mantiene la significación en la OR ajustada (ORa=2,5; [IC 95%: 1,4-4,7]).
Según el programa de cribado existen diferencias significativas en el tratamiento quirúrgico. El programa 3 presenta una ORa de cirugía tipo mastectomía de 4 [IC 95%: 1,8-8,9]. Este programa coincide con el de mayor porcentaje de reconstrucción (58,3%).
ConclusionesEste estudio muestra cómo a pesar de tener en cuenta las características de las pacientes y del tumor, existe una elevada variabilidad en el tipo de cirugía en función del lugar de diagnóstico.
In Spain, breast cancer is very prevalent, with 30000 neoplasms diagnosed per year and an annual mortality rate of 6200 patients/year.1 There are population screening programs for the early detection of breast cancer that mail invitations to all women aged 50–69 years of age to actively participate every 2 years, following the “European Guidelines for Quality Assurance in Mammographic Screening” and meeting the required standards for quality.2 Advances in treatment along with early detection have led to a reduction in the breast cancer mortality rate over the last 25 years.3,4 Despite the current controversy regarding the risk-benefit balance of population-based breast cancer screening programs, there is consensus that attributes 20% of the reduction in mortality to screening.5 Women who participate in these programs have a greater probability of early detection with a smaller tumor size at diagnosis and therefore a greater probability of receiving less aggressive treatments and breast-conserving surgery.6–8
When the evidence about the effectiveness and safety of a given treatment is high, little variability in medical practice is expected.6 However, variability in surgical practice is a problem yet to be resolved9; in the surgical treatment of breast cancer, this variability is moderate to high.6,10–12
The aim of our study is to describe the variability in the surgical treatment of women participating in early detection population screening programs diagnosed with breast cancer and to analyze the associated factors.
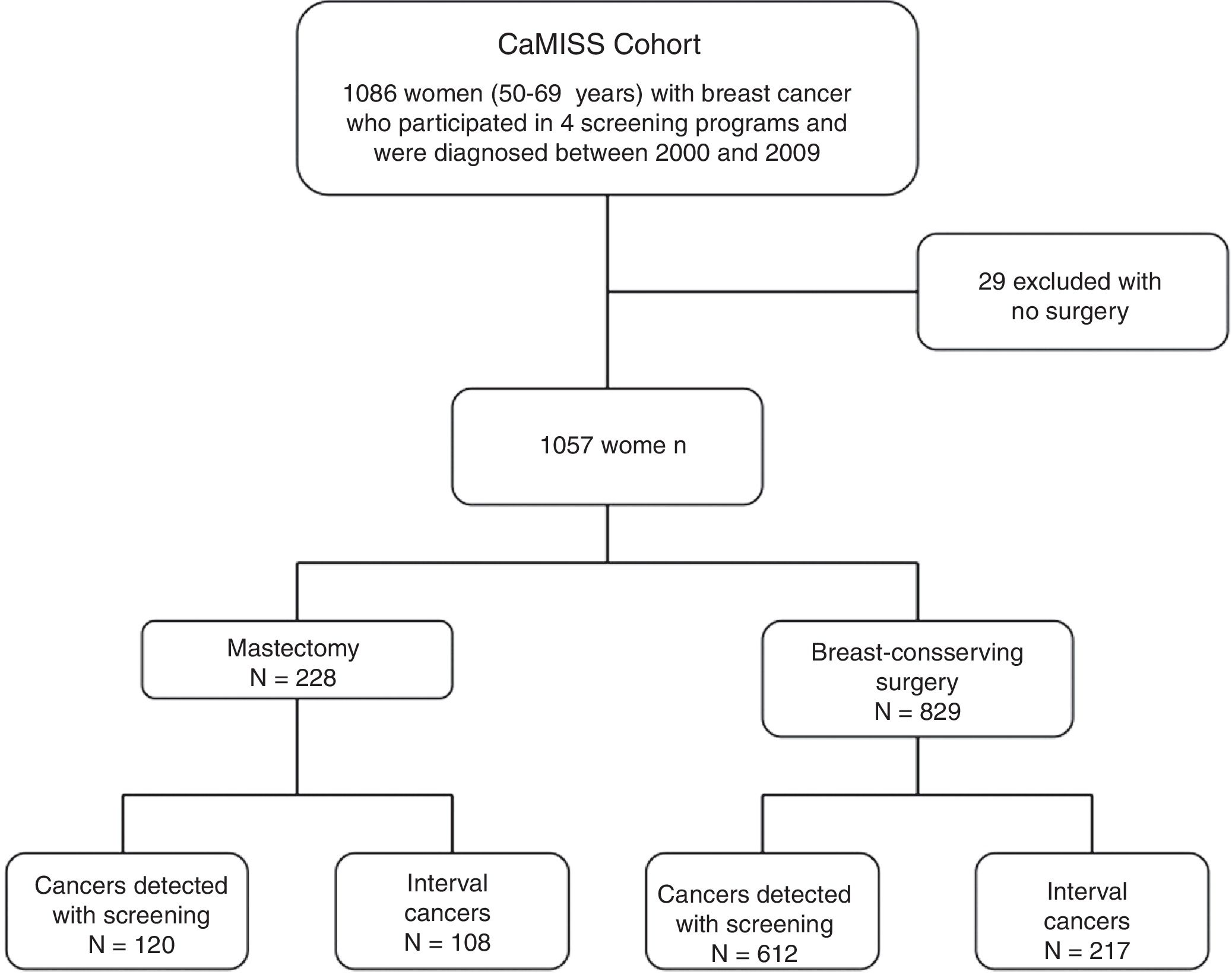
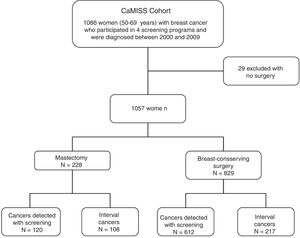
MethodsCaMISS cohort and exclusion criteria. The study population included 1086 women from the retrospective CaMISS cohort (Health Care Research in Breast Cancer). This cohort includes data regarding the diagnostic procedure and treatment of patients with breast cancer between 2000 and 2009 within the framework of the screening program for the early detection of breast cancer in 2 autonomous regions of Spain (Catalonia and the Canary Islands). In addition, data were collected for the follow-up, complications and mortality until June 2014.
We excluded 29 women who had not undergone surgery. The final cohort included 1057 women diagnosed with breast cancer who had been treated surgically (Fig. 1).
Population programs. Four population screening programs were part of this study: 3 located in Catalonia (Barcelona, Sabadell and Girona) and the program that encompasses the autonomous community of the Canary Islands. All the women diagnosed with cancer in these programs were treated at their referral hospitals (university hospitals with more than 400 beds).
Diagnosis. Breast cancer (invasive or in situ) was detected by screening mammograms or as interval cancers. The definition of interval cancer proposed in the European guideline is “primary breast cancer diagnosed after a negative screening episode, with or without additional evaluation, and before the next screening invitation, or within 24 months for women who exceed the age limit”.13 Interval cancers were identified by cross-referencing data from population programs and regional cancer registries, the minimum basic data set and/or with hospital tumor registries. The diagnosis of breast cancer was obtained by biopsy of the radiologically detected lesion and subsequent anatomic pathology study in all cases.
Variables and data collection. We collected data for patient age, detection method (screening or interval) and the screening program itself from the databases of these programs (randomly identified as numbers 1–4 for blind analysis). Information on tumor characteristics (TNM, histology, phenotype), surgical treatment, reconstructive treatment and comorbidities at the time of diagnosis (Charlson index) was obtained from the medical records.
Surgical treatment was classified into 2 groups: breast-conserving surgery (resection of the tumor with a concentric margin of healthy tissue, preserving part of the breast tissue) and mastectomy (excision of the entire breast).
Statistical AnalysisA bivariate descriptive analysis using the chi-squared test was conducted to compare the characteristics of patients who underwent breast-conserving surgery and those with mastectomy. In order to explain which variables influence the probability of performing a mastectomy, crude odds ratios (OR) and adjusted odds ratios (ORa) were estimated along with their confidence intervals (95%CI). Statistical tests were bilateral, and all P values <.05 were considered statistically significant. The analyses were done with SSPS version 23.0 (SPSS Inc, Chicago, Illinois, USA) and version R 3.3.2 (Development Core Team 2014) software.
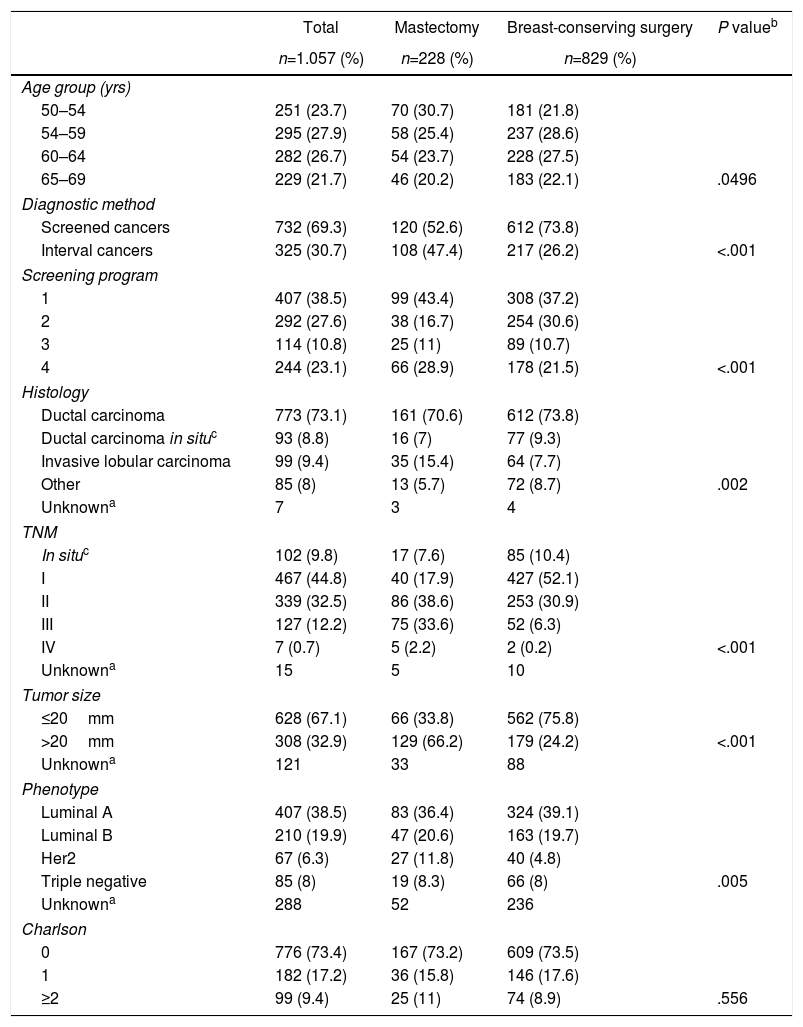
ResultsDescriptive analysis. Mean patient age at the time of diagnosis was 59.3±5 years. In 732 patients (69.3%), the lesions were detected by screening mammograms, while in 325 patients (30.7%) they were diagnosed as interval cancers (Table 1). Program 1 yielded the highest number of cases (n=407; 38.5%). Regarding the tumor characteristics, the most frequent stages in the invasive neoplasms were I and II with 467 patients (44.8%) and 339 patients (32.5%), respectively; in 67.1% of the lesions had a size equal to or less than 20mm.
Clinical/pathological Characteristics of the Population According to Surgery Type.
| Total | Mastectomy | Breast-conserving surgery | P valueb | |
|---|---|---|---|---|
| n=1.057 (%) | n=228 (%) | n=829 (%) | ||
| Age group (yrs) | ||||
| 50–54 | 251 (23.7) | 70 (30.7) | 181 (21.8) | |
| 54–59 | 295 (27.9) | 58 (25.4) | 237 (28.6) | |
| 60–64 | 282 (26.7) | 54 (23.7) | 228 (27.5) | |
| 65–69 | 229 (21.7) | 46 (20.2) | 183 (22.1) | .0496 |
| Diagnostic method | ||||
| Screened cancers | 732 (69.3) | 120 (52.6) | 612 (73.8) | |
| Interval cancers | 325 (30.7) | 108 (47.4) | 217 (26.2) | <.001 |
| Screening program | ||||
| 1 | 407 (38.5) | 99 (43.4) | 308 (37.2) | |
| 2 | 292 (27.6) | 38 (16.7) | 254 (30.6) | |
| 3 | 114 (10.8) | 25 (11) | 89 (10.7) | |
| 4 | 244 (23.1) | 66 (28.9) | 178 (21.5) | <.001 |
| Histology | ||||
| Ductal carcinoma | 773 (73.1) | 161 (70.6) | 612 (73.8) | |
| Ductal carcinoma in situc | 93 (8.8) | 16 (7) | 77 (9.3) | |
| Invasive lobular carcinoma | 99 (9.4) | 35 (15.4) | 64 (7.7) | |
| Other | 85 (8) | 13 (5.7) | 72 (8.7) | .002 |
| Unknowna | 7 | 3 | 4 | |
| TNM | ||||
| In situc | 102 (9.8) | 17 (7.6) | 85 (10.4) | |
| I | 467 (44.8) | 40 (17.9) | 427 (52.1) | |
| II | 339 (32.5) | 86 (38.6) | 253 (30.9) | |
| III | 127 (12.2) | 75 (33.6) | 52 (6.3) | |
| IV | 7 (0.7) | 5 (2.2) | 2 (0.2) | <.001 |
| Unknowna | 15 | 5 | 10 | |
| Tumor size | ||||
| ≤20mm | 628 (67.1) | 66 (33.8) | 562 (75.8) | |
| >20mm | 308 (32.9) | 129 (66.2) | 179 (24.2) | <.001 |
| Unknowna | 121 | 33 | 88 | |
| Phenotype | ||||
| Luminal A | 407 (38.5) | 83 (36.4) | 324 (39.1) | |
| Luminal B | 210 (19.9) | 47 (20.6) | 163 (19.7) | |
| Her2 | 67 (6.3) | 27 (11.8) | 40 (4.8) | |
| Triple negative | 85 (8) | 19 (8.3) | 66 (8) | .005 |
| Unknowna | 288 | 52 | 236 | |
| Charlson | ||||
| 0 | 776 (73.4) | 167 (73.2) | 609 (73.5) | |
| 1 | 182 (17.2) | 36 (15.8) | 146 (17.6) | |
| ≥2 | 99 (9.4) | 25 (11) | 74 (8.9) | .556 |
Histologically, invasive ductal carcinoma was most frequent (73.1%), well above invasive lobular carcinoma (9.4%) and ductal carcinoma in situ (8.8%).
The most frequent phenotype was luminal A (407 cases; 38.5%) and the least was triple negative (8%). Most of the women (73.4%) had no comorbidity at the time of diagnosis.
Regarding the surgical treatment received, 829 women (78.4%) underwent breast-conserving surgery and 228 (21.6%) mastectomy.
Bivariate descriptive analysis. The characteristics associated with conservative surgery vs mastectomy are also shown in Table 1. Statistically significant differences were observed by age groups, with the youngest group (50–54) having the highest mastectomy rate (30.7%). There was also a higher percentage of mastectomies among the interval cancers compared to the conservative ones (47.4% vs 26.2%; P<.001).
Regarding tumor characteristics, statistically significant differences were observed according to the TNM (in the mastectomy group, 33% of patients were stage III versus only 6.3% in breast-conserving surgery), histology (lobular tumors were observed in 15.4% of mastectomies vs 7.7% of breast-conserving procedures) and tumor phenotype (HER2 amplified in 11.8% of mastectomies vs 4.8% of breast-conserving surgeries) (P=.005). No differences were observed regarding morbidity.
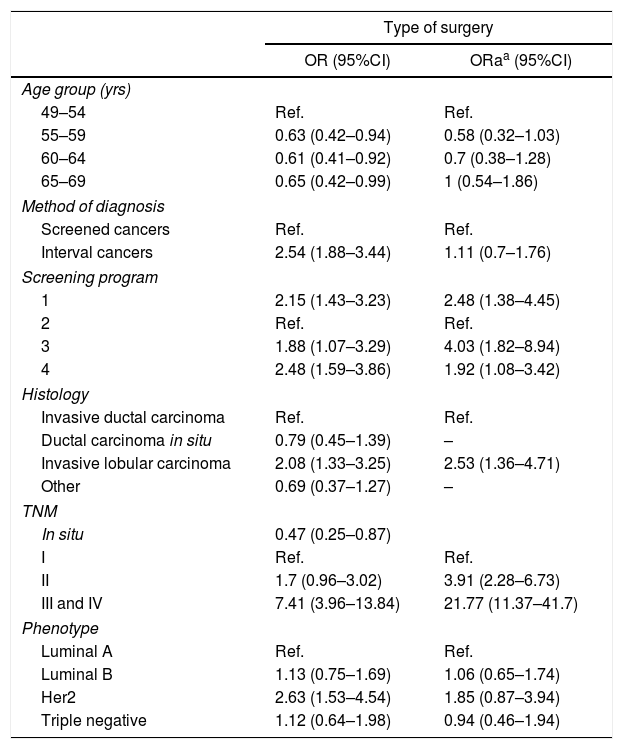
Risk estimation analysis. In the risk estimation models for performing mastectomy (Table 2), in patients >54 years of age there is a lower probability of performing this surgery, but the statistical significance disappears in the adjusted model.
Odds Ratio for Performing Mastectomy in Breast Cancers, Unadjusted and Adjusted for Clinical-pathological Characteristics.
| Type of surgery | ||
|---|---|---|
| OR (95%CI) | ORaa (95%CI) | |
| Age group (yrs) | ||
| 49–54 | Ref. | Ref. |
| 55–59 | 0.63 (0.42–0.94) | 0.58 (0.32–1.03) |
| 60–64 | 0.61 (0.41–0.92) | 0.7 (0.38–1.28) |
| 65–69 | 0.65 (0.42–0.99) | 1 (0.54–1.86) |
| Method of diagnosis | ||
| Screened cancers | Ref. | Ref. |
| Interval cancers | 2.54 (1.88–3.44) | 1.11 (0.7–1.76) |
| Screening program | ||
| 1 | 2.15 (1.43–3.23) | 2.48 (1.38–4.45) |
| 2 | Ref. | Ref. |
| 3 | 1.88 (1.07–3.29) | 4.03 (1.82–8.94) |
| 4 | 2.48 (1.59–3.86) | 1.92 (1.08–3.42) |
| Histology | ||
| Invasive ductal carcinoma | Ref. | Ref. |
| Ductal carcinoma in situ | 0.79 (0.45–1.39) | – |
| Invasive lobular carcinoma | 2.08 (1.33–3.25) | 2.53 (1.36–4.71) |
| Other | 0.69 (0.37–1.27) | – |
| TNM | ||
| In situ | 0.47 (0.25–0.87) | |
| I | Ref. | Ref. |
| II | 1.7 (0.96–3.02) | 3.91 (2.28–6.73) |
| III and IV | 7.41 (3.96–13.84) | 21.77 (11.37–41.7) |
| Phenotype | ||
| Luminal A | Ref. | Ref. |
| Luminal B | 1.13 (0.75–1.69) | 1.06 (0.65–1.74) |
| Her2 | 2.63 (1.53–4.54) | 1.85 (0.87–3.94) |
| Triple negative | 1.12 (0.64–1.98) | 0.94 (0.46–1.94) |
As for the diagnostic method, mastectomy was more frequent in the tumors detected between intervals (OR=2.5 [95%CI: 1.8–3.4]) compared to the cancers detected by screening mammography, although this effect disappeared when adjusted for the remaining variables.
As for the relationship with tumor characteristics, the most determining factor associated with mastectomy was TNM stage: the tumors diagnosed in stages III and IV presented an OR of 7.4 (95%CI: 3.9–13.8) compared to stage I, an association that increased after adjusting for the remaining variables to an ORa of 21.7 (95%CI: 11.4–41.8).
Histologically, infiltrating lobular carcinoma was the only one to maintain significance in the adjusted OR, and the risk of performing a mastectomy in a patient with such a histology was twice that of invasive ductal carcinoma (ORa: 2.5 [95%CI: 1.4–4.7]).
The HER2 positive phenotype compared to luminal A tumors also presented a higher probability of non-conservative surgery with an OR of 2.6 (95%CI: 1.5–4.5), although in the ORa it lost statistical significance (ORa: 1.8 [95% CI: 0.9–3.9]).
Depending on the screening program in which the breast cancer was diagnosed, there were significant differences for the performance of mastectomy. Program 3 presented an adjusted OR for mastectomy of 4 (95%CI: 1.8–8.9) compared to the program with the lowest percentage of this type of treatment (program 2).
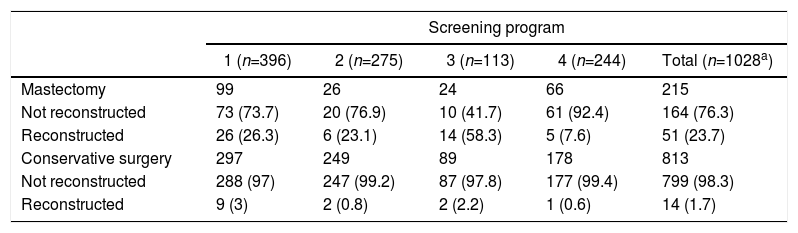
Out of all the patients treated, only 65 women underwent reconstructive surgery (6.3%), 51 of which were after non-conservative surgical treatment (Table 3). The program most likely to perform a mastectomy (program 3) coincided with the highest percentage of reconstruction (58.3%).
Reconstructive Surgery According to Surgical Technique and Screening Program.
| Screening program | |||||
|---|---|---|---|---|---|
| 1 (n=396) | 2 (n=275) | 3 (n=113) | 4 (n=244) | Total (n=1028a) | |
| Mastectomy | 99 | 26 | 24 | 66 | 215 |
| Not reconstructed | 73 (73.7) | 20 (76.9) | 10 (41.7) | 61 (92.4) | 164 (76.3) |
| Reconstructed | 26 (26.3) | 6 (23.1) | 14 (58.3) | 5 (7.6) | 51 (23.7) |
| Conservative surgery | 297 | 249 | 89 | 178 | 813 |
| Not reconstructed | 288 (97) | 247 (99.2) | 87 (97.8) | 177 (99.4) | 799 (98.3) |
| Reconstructed | 9 (3) | 2 (0.8) | 2 (2.2) | 1 (0.6) | 14 (1.7) |
The main objective of early detection programs for breast cancer is to reduce mortality from this disease. With the earlier diagnosis provided by screening mammograms, it has been estimated that mortality from breast cancer has been reduced by 15,14 20,15,16 20–3017 and even 35%.18 We should also mention that this point is controversial as some authors defend the hypothesis that breast cancer mortality has decreased in both groups of patients participating in screening programs and in non-participants due to therapeutic advances.19,20 Some authors advocate developing a predictive model for breast cancer risk (taking into account risk factors such as the age at menarche, age at first pregnancy, alcohol consumption, mammographic density and BMI) that provide the basis for a stratification of the population according to the different levels of risk, in order to offer different screening procedures and time between tests.21 Thus, an individual screening model is created to minimize damage and maximize benefits based on risk factors.22
In addition to decreasing mortality, population screening provides other benefits, such as the detection of cancer at earlier stages, whose treatment is more effective compared to cancers that present clinically and at generally more advances phases.8,14–16 In terms of surgical treatment, early detection identifies tumors that are smaller in size, which increases the frequency of breast-conserving surgery.6–8 The evolution of surgical treatment for breast cancer has led us to perform the least aggressive treatment possible, and a progressive increase has been observed in the number of patients treated with conservative surgery.7,23–25 In our study, early detection may have led to less aggressive treatments, as observed in the unadjusted logistic regression model. However, when adjusted for tumor characteristics, no differences were observed in the type of surgery according to the diagnostic method. On the other hand, when breast-conserving surgery was not possible, reconstructive surgery was offered more frequently. Therefore, a possible explanation for the observed surgical variability could be the accessibility of this type of surgery at these hospitals, since a program with the capability to offer immediate or deferred reconstruction could influence the decision toward more aggressive treatment in controversial cases.26 The availability of reconstructive surgery could influence the final decision, as our results indicate, where the program most likely to perform a mastectomy coincided with the highest percentage of reconstruction.
In Spain, the indication of breast-conserving surgery plus radiotherapy or non-conservative surgery is based on breast cancer clinical practice guidelines prepared by each autonomous community,27–29 which in turn reflects the criteria of international guidelines such as the Breast Cancer National Comprehensive Cancer Network Guidelines.26 The role of clinical practice guidelines is key to unify the criteria for surgical treatment.9 In the case of breast cancer, it is clear to perform mastectomy in multicentric tumors or with extensive microcalcifications that affect more than 30% of the breast tissue. The indication is also clear in patients with poor general condition that prevent performing complementary treatment and present a tumor with extensive skin involvement (sanitary purposes) or >3cm, as well as breast cancer in men.26–29 In our study, no differences were observed according to the Charlson index per se in terms of tumor size.
The therapeutic variability depends on the variability of the patients themselves. For example, whether a T2–T3 tumor is treated with breast-conserving surgery or mastectomy depends to a large extent on the ratio between the patient's breast volume and the tumor. The location of the tumor can also determine the type of surgery, resulting in non-conservative surgery if the location of the tumor is sub- or retroareolar, if we know that the histological type is lobular or with an extensive intraductal component, as well as if the mass presents a more aggressive phenotypic pattern (HER2 or triple negative).29–31 In our cohort, we also observed a greater risk of mastectomy in lobular tumors and HER2, although in the latter case it did not reach statistical significance. In addition to all these variables, the opinion of the patient must always be taken into account. The therapeutic approach should always be personalized and, therefore, unpredictable to a certain extent.
In 2011, Ridao-López et al. observed systematic variations in the use of conservative and non-conservative breast cancer surgery up to 4-fold in the 180 areas analyzed.6 They were explained by socioeconomic, technological and political differences, as well as the progressive implementation of conservative surgery among the different areas studied, coinciding with the previous study conducted in 2002 by Gilligan et al.11 Also in 2014, a study was carried out on the different surgical alternatives for breast cancer in which systematic variations were observed in the use of conservative surgery and mastectomy of up to 3-fold among the 199 health areas observed; these facts were not explained or analyzed as the objective of the study was economic costs.12
In our analysis, with individual information that has allowed us to adjust for tumor and patient characteristics, there is notable variability in the surgical treatment of breast cancer among the different population screening programs. Patients diagnosed by the same detection method, with the same age, comorbidity, identical TNM stage, histology and tumor phenotype present 4 times more risk of undergoing mastectomy surgery in screening program 3 compared to program 2. The study has been carried out in areas with similar socioeconomic, technological, political and clinical protocols. In the 4 screening programs studied, there was at least one university referral hospital, with its own radiotherapy unit or services at a nearby medical center, and each had magnetic resonance imaging testing. In all the hospitals participating in the study, there is a Breast Pathology Unit with technically trained personnel and preferential dedication (the majority worked exclusively in this regard) to breast cancer surgery. The 2 autonomous communities presented comparable clinical protocols.27–29 The characteristics of the early detection population programs were similar, with the same target population, same number of mammographic projections in the examinations, same reading method, as well as the same resources and the degree of development of the technical quality control system based on the European Protocol for the Quality Control of the Physical and Technical Aspects of Breast Cancer Screening proposed by the European Guidelines for Quality Assurance in Breast Cancer Screening.2,32
Despite being a retrospective cohort with patients who underwent surgery more than 10 years ago, this is the first study in Spain to analyze the variability in the surgical treatment of breast cancer in a cohort of women participating in screening programs using individual data for patient and tumor mass characteristics.
In conclusion, this study shows how, even when taking into account patient age and comorbidities together with the tumor characteristics, there is high variability in the type of surgery indicated depending on the place of diagnosis. It is necessary to determine the impact of this variability on recurrence and survival, as well as the impact on costs, in order to make the appropriate recommendations for the benefit of patients and the national healthcare system.
FundingThis study has received no funding in the form of grants.
Presentation at CongressesDescription of the treatment for breast cancer in women participating in population screening program: CAMISS 2000–2009 Cohort, at the 2nd Spanish Breast Congress, 22–24 October 2015 (Madrid)
Surgical variability of radical breast cancer treatment in 4 screening programs (2000–2009), at the 10th Catalonia Surgery Congress, 15–16 October 2015 (Barcelona)
Variability in performing radical surgery in women with breast cancer from four population screening programs (2000–2009), at the 19th SIS World Congress on breast health care, 5–8 May 2016 (Warsaw)
Variability in radical surgery for breast cancer, 7–10 November 2016
Contribution of the Authors to the ManuscriptLidia Blay: Study design, database adaptation, analysis and interpretation of the results, article composition, critical review and approval of the final version.
Javier Louro: Study design, analysis and interpretation of the results, critical review and approval of the final version.
Teresa Barata: Data collection.
Marisa Baré: Data collection.
Joana Ferrer: Data collection.
Josep Maria Abad: Critical review and approval of the final version.
Xavier Castells: Critical review and approval of the final version.
Maria Sala: Study design, analysis and interpretation of the results, critical review and approval of the final version.
Conflict of interestsThe authors have no conflict of interests to declare.
Dr. JF Julián, Dr. D Parés, Dr. J Valibrea, Dra. A Romero, Dr. J Camps, Grupo CaMISS and Arnau Sans.
Please cite this article as: Blay L, Louro J, Barata T, Baré M, Ferrer J, Abad JM, et al. Variabilidad en la práctica de la cirugía mamaria en mujeres participantes en el programa de cribado poblacional de cáncer de mama. Cir Esp. 2019;97:89–96.