In 1980, Sackett and Guyatt developed the concept of evidence-based medicine (EBM), defined as: “The conscious, explicit and judicious use of current best evidence in making decisions about treating individual patients”1. EBM focuses on: 1) the patient as an individual, with their specific characteristics; 2) the search for the most up-to-date information in terms of efficacy, results or cost; and 3) the judgment and experience of the surgeon who will apply this information according to the characteristics of the patient. The fundamental objective of EBM is to support any medical action with the best scientific basis possible. EBM has been extrapolated to surgery, coining the term ‘evidence-based surgery’ (EBS)2,3.
Methodology for obtaining evidence-based informationWhen we intend to apply the EBM concept, it is necessary to obtain the information in the most structured and objective manner possible. To this end, the most important instruments are outlined in the PICO concept:
- -
P: Patient or population: reason for the study.
- -
I: Intervention: procedure or exposure being studied.
- -
C: Comparison intervention: procedure with which it being compared.
- -
O: Outcomes: result being evaluated.
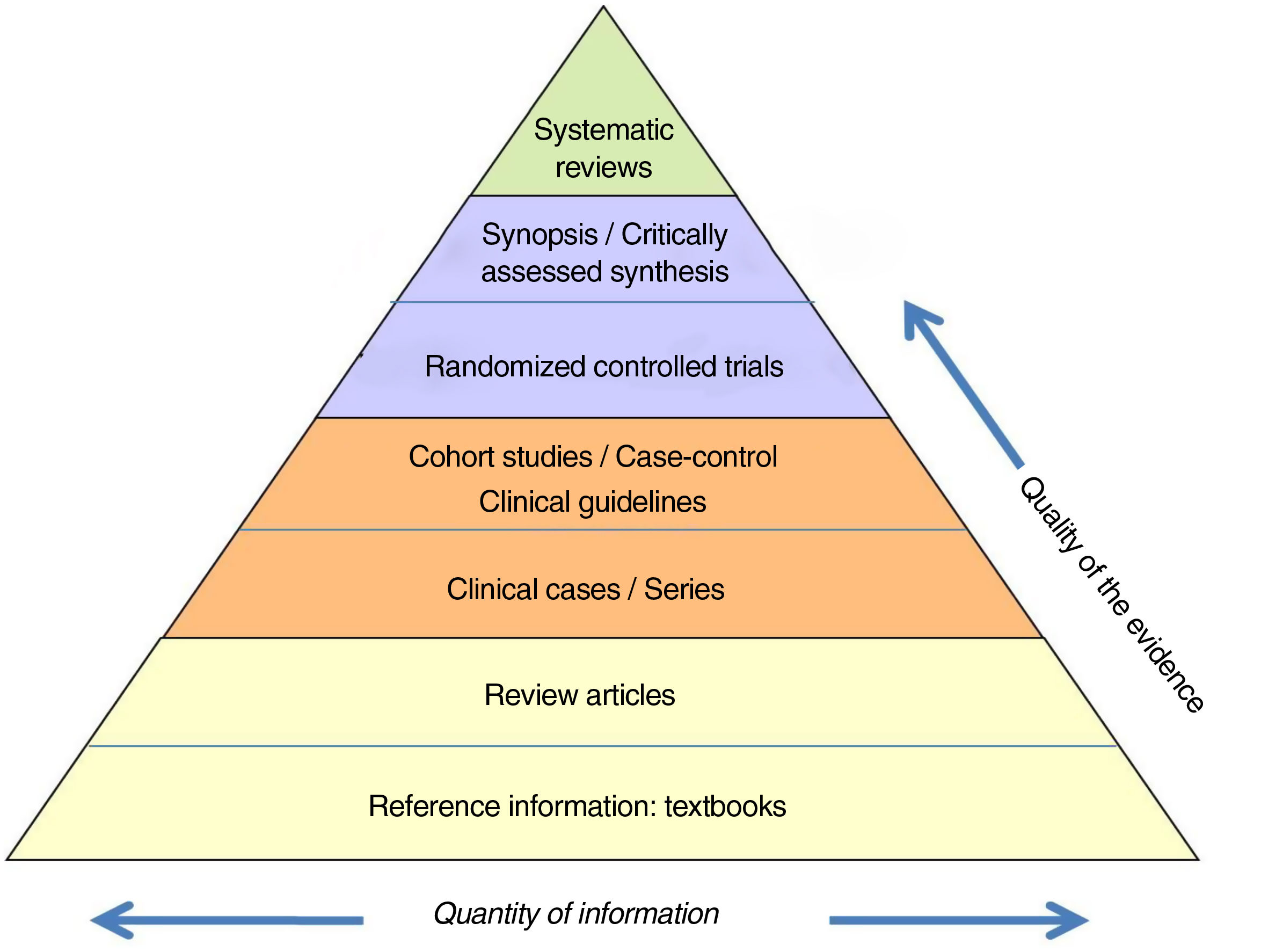
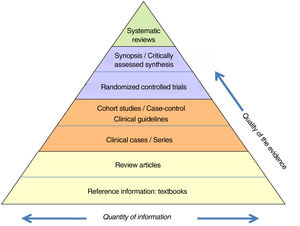
The information is also classified according to a ‘hierarchy’ based on its scientific quality (Fig. 1). This ranges from the concept of the lowest credibility, such as the opinion of an expert, to the meta-analysis of methodologically correct randomized controlled trials (RCT).
Hierarchy of the evidence.
Adapted from Targarona et al.8.
In recent years, several initiatives have been developed to protocolize information collection and publication methodology. The www.equator-network.org website summarizes the standard protocols and checklists to be used for different types of scientific studies. Its objective is to favor the publication of results in a transparent and exact manner, while increasing the credibility of biomedical publications4.
The best assimilated methodological system is CONSORT (Consolidated Standards of Reporting Trials), which is recommended for reporting RCT with a checklist that includes all the items that must be included in the publication of these trials. There are other protocols for other types of studies: Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) or Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT). The current opinion is that RCT should be public knowledge, and for this there is a specific database (Clinical Trial.gov [U.S. National Library of Medicine]).
An especially useful concept in EBM is the systematic review or meta-analysis. Meta-analyses can contain many biases; therefore, a very demanding system has been described (Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA]). Its specific registry is the International Prospective Register of Systematic Reviews (PROSPER).
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) is the checklist for reporting observational studies, and the methodology for their meta-analysis is summarized in the Meta-analysis of Observational Studies in Epidemiology (MOOSE).
In the event that there is not enough information available, the opinion of experts following a DELPHI-type consensus may be useful.
The ultimate goal of a systematic literature search may be the development of clinical protocols or guidelines. Appraisal of Guidelines for REsearch & Evaluation (AGREE) is an instrument designed to normalize the qualitative variability of clinical guidelines
Resources to obtain evidence-based informationAdvances made in information systems have facilitated the storage of biomedical information. This topic has been extensively addressed in another Methodological Letter by Fernandez-Ananin et al.
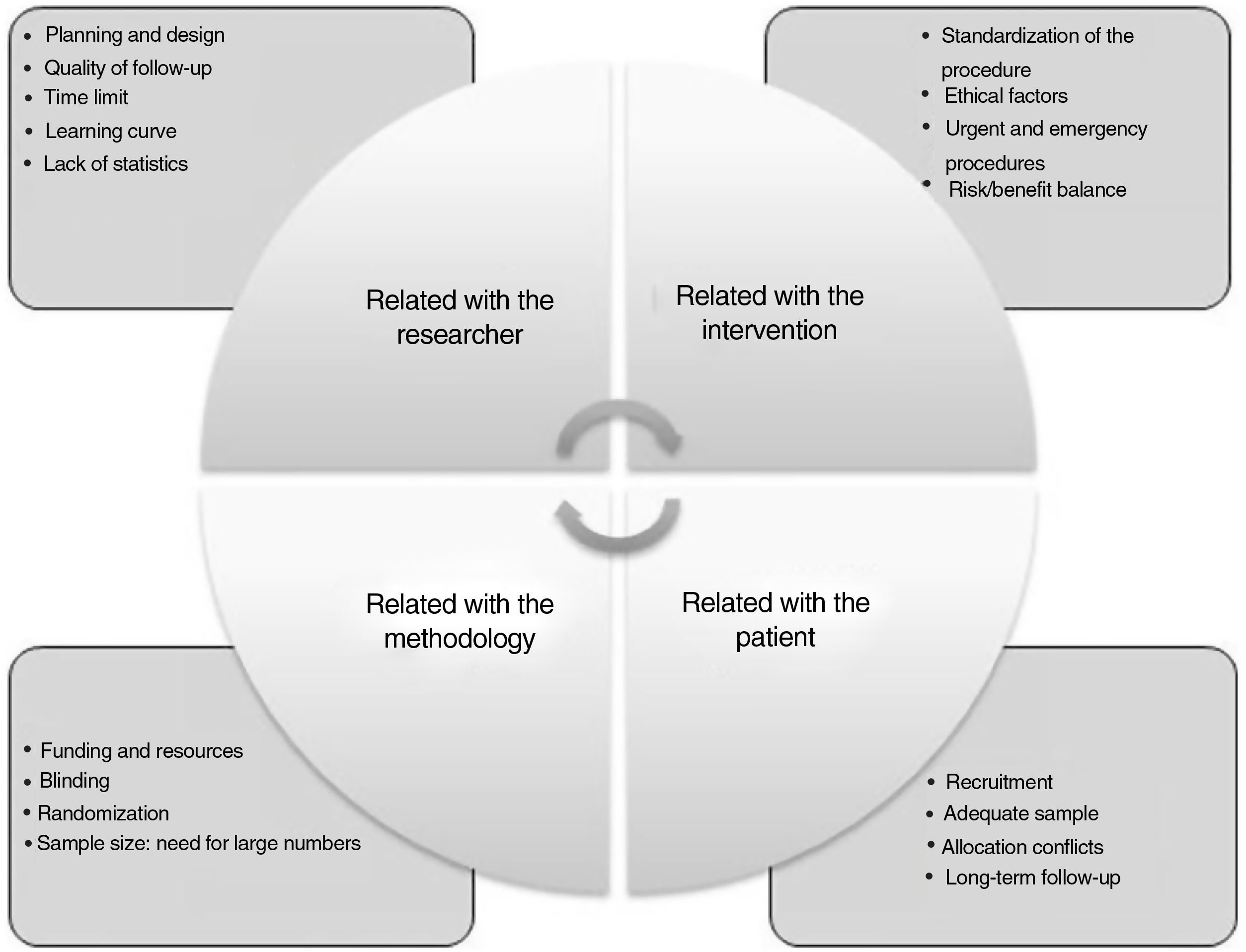
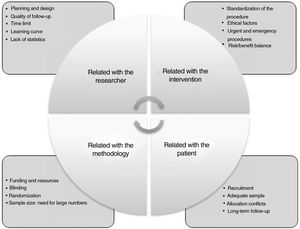
Difficulties to obtain and apply evidence-based surgeryRCT in surgery poses essential differences with medical-type studies, and there are numerous difficulties that arise when conducting this type of study (Fig. 2): a) mastery of the technique: surgeons who develop new techniques do not want their patients to be treated in a traditional way; another difficulty is randomization because, once both options are explained, the patient chooses; b) difficulty to obtain absolutely objective results, since blind randomization is more difficult; c) another factor is the duration of the study, since, depending on the type of intervention, the study may not be feasible, or the procedure may have already been implemented; and d) an additional factor is the lack of research culture in the surgical community.
Difficulties in conducting randomized studies in surgery.
Adapted from Targarona et al.8.
In the last 30 years, we have witnessed how difficult it is to validate and share surgical techniques or instruments in a controlled manner. In the development and evaluation of medicines, research is perfectly regulated by the Medicines Agency. In contrast, the development of new surgical techniques does not have its own validation method. In 2007, a group of epidemiologists led by Barkun and McCulloch developed a series of recommendations to safely apply innovations in surgery5. IDEAL is the acronym for I: Idea, D: Development, E: Exploration, A: Assessment and L: Long-term study, which represent the natural history of a surgical innovation.
- -
Idea: stage I. What is the concept of the new treatment, and why is it necessary?
- -
Development: stage IIa. Is the new intervention sufficiently developed to be replicated by others?
- -
Exploration: stage IIb. Have the factors that may favor the development of an RCT been assessed?
- -
Assessment: stage III. How does the new procedure compare with the usual results in clinical practice?
- -
Long-term study: stage IV. Are there unforeseen long-term results?
In stage I, Idea. First in humans: a series of criteria should be met with maximum transparency. The patient selection criteria must be reported, as well as the number of individuals that have been accepted or rejected. The technique must be described in detail and the short-term results given in terms of safety. This phase includes multiple ethical requirements; therefore, authorization by the hospital research committee is required.
During stage IIa, Development, Toward stabilization of the technique: the procedure is refined by a few authors. Here, the study corresponds with a prospective cohort. The CUSUM6 analysis is of interest to establish the learning curve. The development of this phase, with safety results, provides for its reproduction in other hospitals.
In stage IIb, Exploration, Bridge to a pivotal trial: the need for an RCT arises. The ideal instrument in this phase is the multicenter series and the design of a possible RCT. Other types of study (case-controls) can be evaluated. Ethical requirements regarding transparency, training and monitoring are important.
Stage III, Assessment: key study/RCT: the definitive RCT should be performed at this stage, immediately after the technique is considered stable and before it can be widely -used. The objective is to make a comparison with the best possible established intervention and validate the efficiency of the new procedure.
In stage IV, Long-term study IV: the objective is to identify unforeseen long-term results. This is the phase to develop registries and databases. The conflicts of interest derived from clinical registries are determined by who depends on or exploits them and the definition of authorship.
Registries and big dataThe collection of objective information has also been based on the development of clinical registries. The evolution of digital platforms has facilitated the creation of large databases that include thousands of clinical records, allowing for them to be analyzed practically in real time. The best example is the NSQIP (The American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP®)7,8, which makes it possible to compare clinical results between different hospitals (benchmarking) and allows clinical information to be obtained7: a) evaluate treatment results; b) identify risk factors and develop risk prediction models; c) compare results between different interventions; and d) evaluate variations in the use of healthcare resources. The evolution of the digital world is heading towards the application of artificial intelligence (AI) to manage clinical information generated by big data, which will ultimately favor personalized medicine. Both forms of information management, evidence-based surgery and AI, are convergent and will complement each other in the future8–10.
Please cite this article as: Targarona Soler EM, Bollo García J, Fernández Ananin S. Qué significa la cirugía basada en la evidencia. Cir Esp. 2022;100:371–374.