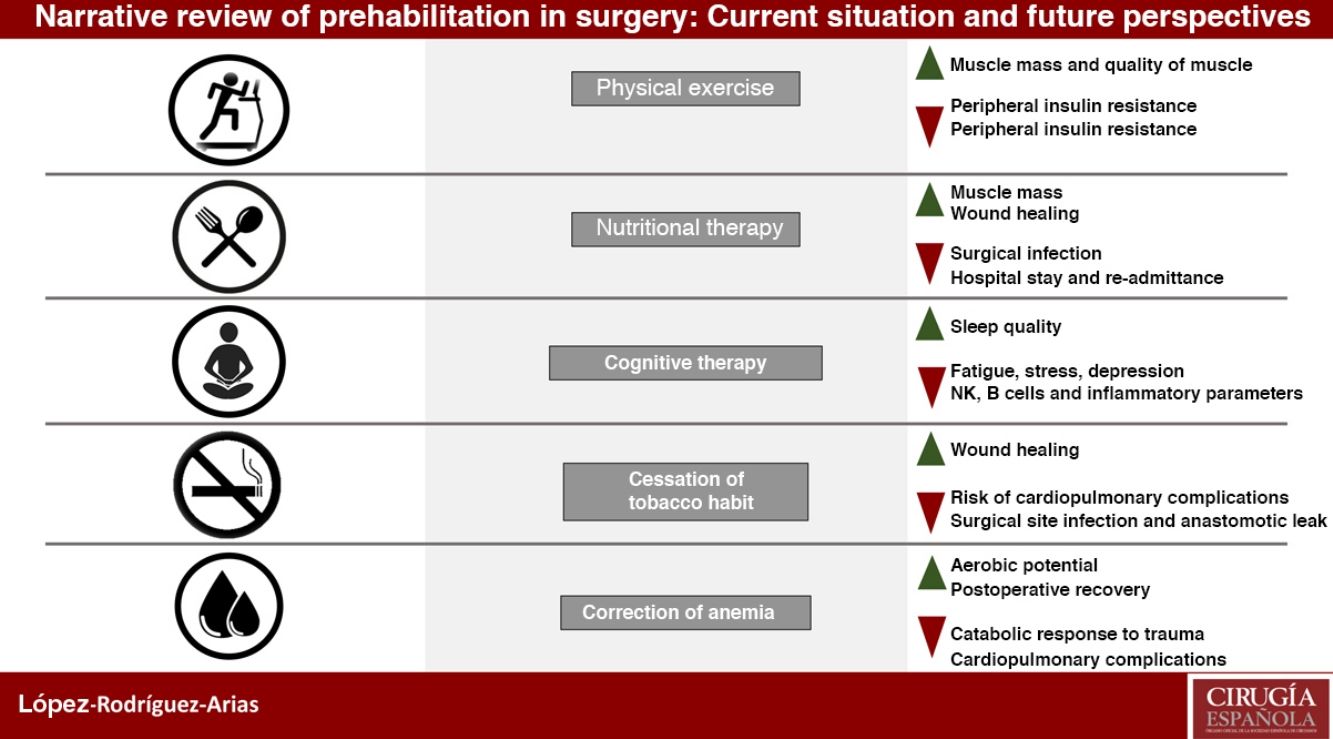
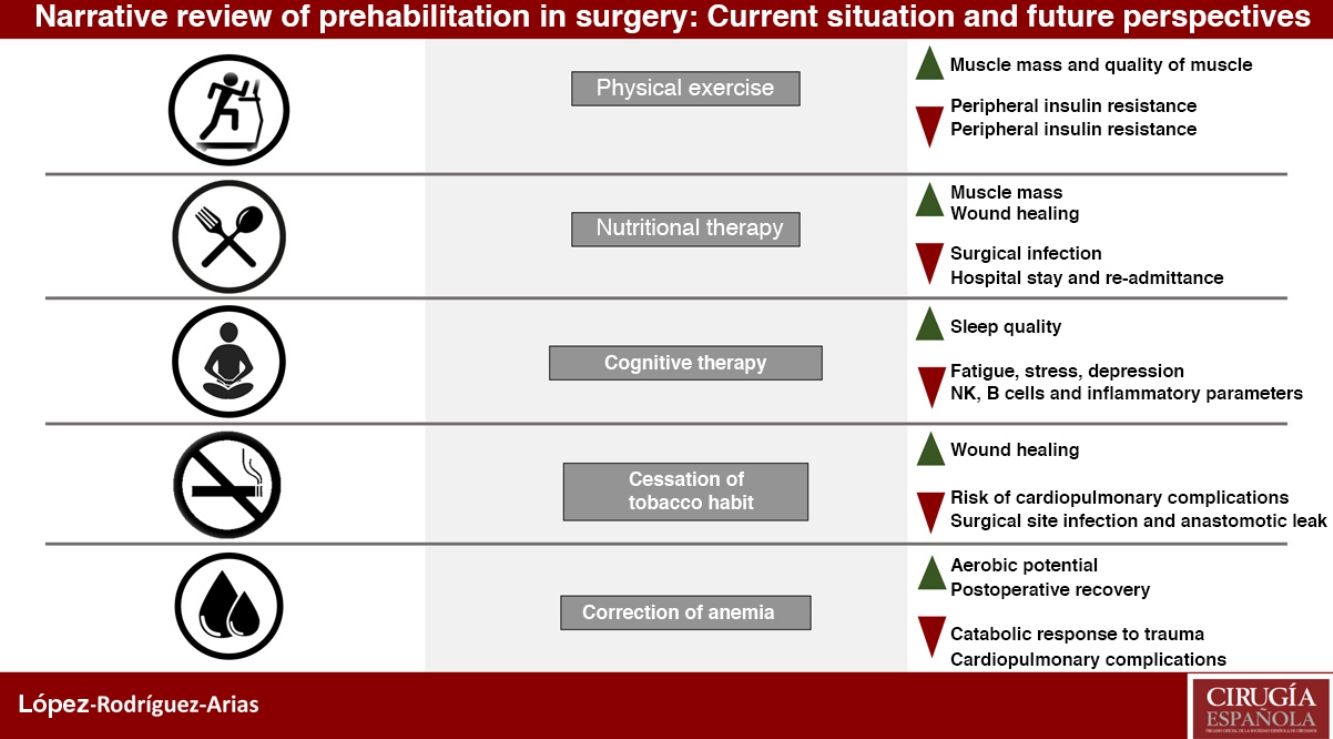
Prehabilitation has a multimodal conception based on three fundamental pillars: improvement of the patient's physical condition, nutritional optimization and cognitive intervention to reduce stress and anxiety, as well as other measures such as smoking cessation and correction of anemia.
The aim of prehabilitation programs is to optimize the patient from the moment of diagnosis until the surgical intervention in order to reduce postoperative complications.
As in the case of multimodal rehabilitation protocols, the actions of prehabilitation programs have synergistic effects, that is, small changes that, by themselves, do not have clinical significance but when added up, they produce a significant improvement in the postoperative evolution of patients.
Although more studies are required to evaluate the impact of these programs on patients groups with different pathologies, interventions and risk factors, their progressive implementation is necessary in the daily clinical practice of our patients. The objective of this narrative review is to evaluate the available evidence about prehabilitation in surgery, focusing on current established strategies, knowledge gaps and future research.
La prehabilitación tiene una concepción multimodal con tres pilares fundamentales: mejora en la condición física del paciente, optimización nutricional e intervención cognitiva para reducir el estrés y la ansiedad, además de otras medidas como la deshabituación tabáquica o la corrección de la anemia.
El objetivo principal es la optimización del paciente durante el periodo de tiempo preoperatorio (diagnóstico-intervención) con la finalidad de mejorar la capacidad funcional y disminuir las complicaciones derivadas de la cirugía.
Al igual que ocurre con los protocolos de rehabilitación multimodal, las acciones de los programas de prehabilitación tienen efectos sinérgicos, es decir, pequeños cambios que por sí solos no tienen transcendencia clínica pero que al sumarse producen una mejoría significativa en la evolución postoperatoria de los pacientes.
Aunque se requieren más estudios que evalúen el impacto concreto de estos programas en poblaciones de pacientes con diversas patologías, intervenciones y distintos factores de riesgo, se hace necesaria su implementación progresiva en la práctica clínica habitual de nuestros pacientes. El objetivo de esta revisión narrativa es evaluar la literatura disponible sobre la prehabilitación en cirugía, haciendo especial hincapié en las estrategias actualmente establecidas, así como en las lagunas de conocimiento actuales y futuros focos de investigación.
The aim of prehabilitation programs (PP) is to optimize patients during the period between the time of diagnosis and surgery in order to reduce complications arising from surgery.1
Until recently, prehabilitation has been based on a trimodal concept of three fundamental pillars: improvement of physical condition, optimization of nutritional situation and cognitive intervention to reduce stress and anxiety. However, recent studies show benefits when applying other measures, such as smoking cessation, preoperative improvement of anemia or medication reconciliation, so it would be more appropriate to call pre-rehabilitation a multimodalstrategy.2–4
By themselves, each of these elements included in the PP measures package may not be clinically significant. But together, they have been shown to significantly improve the postoperative evolution of patients, which is why they are considered to have a synergistic effect.
Following in the wake of multimodal rehabilitation with Enhanced Recovery After Surgery (ERAS) protocols, PP represent a revolution in the perception of patient preparation for surgical treatment. The objective of this review is to analyze the current evidence for PP and their influence on postoperative evolution after abdominal surgery.
MethodologyTwo authors (FLR-A and LS-G) conducted a narrative literature review using the keywords “surgery”, “prehabilitation”, “exercise”, “preoperative care” and “mindfulness” on MEDLINE, Cochrane Library, SCOPUS, ISI web of Science and Ovid databases. The authors identified 311 articles published between September 2002 and March 2019, 40 of which were included in this review. Inclusion criteria were: clinical practice guidelines, controlled clinical trials, cohort studies, meta-analyses and systematic reviews.
Prehabilitation Within ERAS ProgramsThe aggression caused by surgery triggers a double inflammatory response: activation of the immune system, mediated by the release of neuroendocrine hormones; and, stimulation of the sympathetic hypothalamic system, with the consequent release of catecholamines and cortisol. This triggers an initial phase of peripheral insulin resistance and an increase in protein catabolism.5
The magnitude of the inflammatory response is proportional to the degree of surgical aggression; therefore, the greater the surgical wound, organ manipulation and tissue dissection, the greater the triggered metabolic response.
The purpose of ERAS protocols is to optimize the patient perioperatively, thereby minimizing the catabolic effect caused by surgery, avoiding peripheral insulin resistance and favoring an early anabolic phase. There is evidence that a 70%–80% adherence to the protocol produces a significant improvement in postoperative results.6–9 Furthermore, high adherence to the ERAS protocol seems to be associated with better 5-year survival in patients treated surgically for colorectal cancer.10,11
While ERAS protocols play their role in the immediate perioperative period, pre-rehabilitation anticipates this period by initiating patient optimization for several weeks before the operation, starting at the moment of the indication for surgery. The implementation of preparatory measures and a longer patient preparation period before surgery allow PP to achieve greater benefits when they are carried out within the framework of an intensified recovery program.
Physical Assessment: Recommendations for Physical ActivityPhysical exercise improves cardiorespiratory capacity, favors a decrease in blood pressure levels, increases the muscle mass index and helps to decrease stress and anxiety levels. At the metabolic level, it causes a decrease in peripheral insulin resistance and favors a lower response to inflammation due to trauma.12
The stress of surgery leads to a substantial energy expenditure in patients, both intraoperatively and during the postoperative stage in which fasting, the inflammatory ‘storm’ triggered by surgery, and the healing phase test the physical capacity of patients and influence prognosis. Patients in a poor physical condition will need to carry out this process above their anaerobic threshold, therefore using a less effective metabolic pathway with the accumulation of lactic acid.
The main tests that allow us to assess the functional capacity or physical condition of patients are:
- •
6-minute walk test (6MWT): This test is used to assess the prognosis of patients with cardiopulmonary pathology, but, given its simplicity, it is frequently used to determine the global preoperative physical condition of patients.
- •
A 6MWT distance of less than 250m is related with a significant increase in morbidity, mortality and hospital stay, although some authors have raised this figure to 350m.13 An increase of more than 100m has been related with a significant increase in survival in liver transplant candidates.14 Furthermore, it is a validated test to assess recovery after gastrointestinal surgery.15
- •
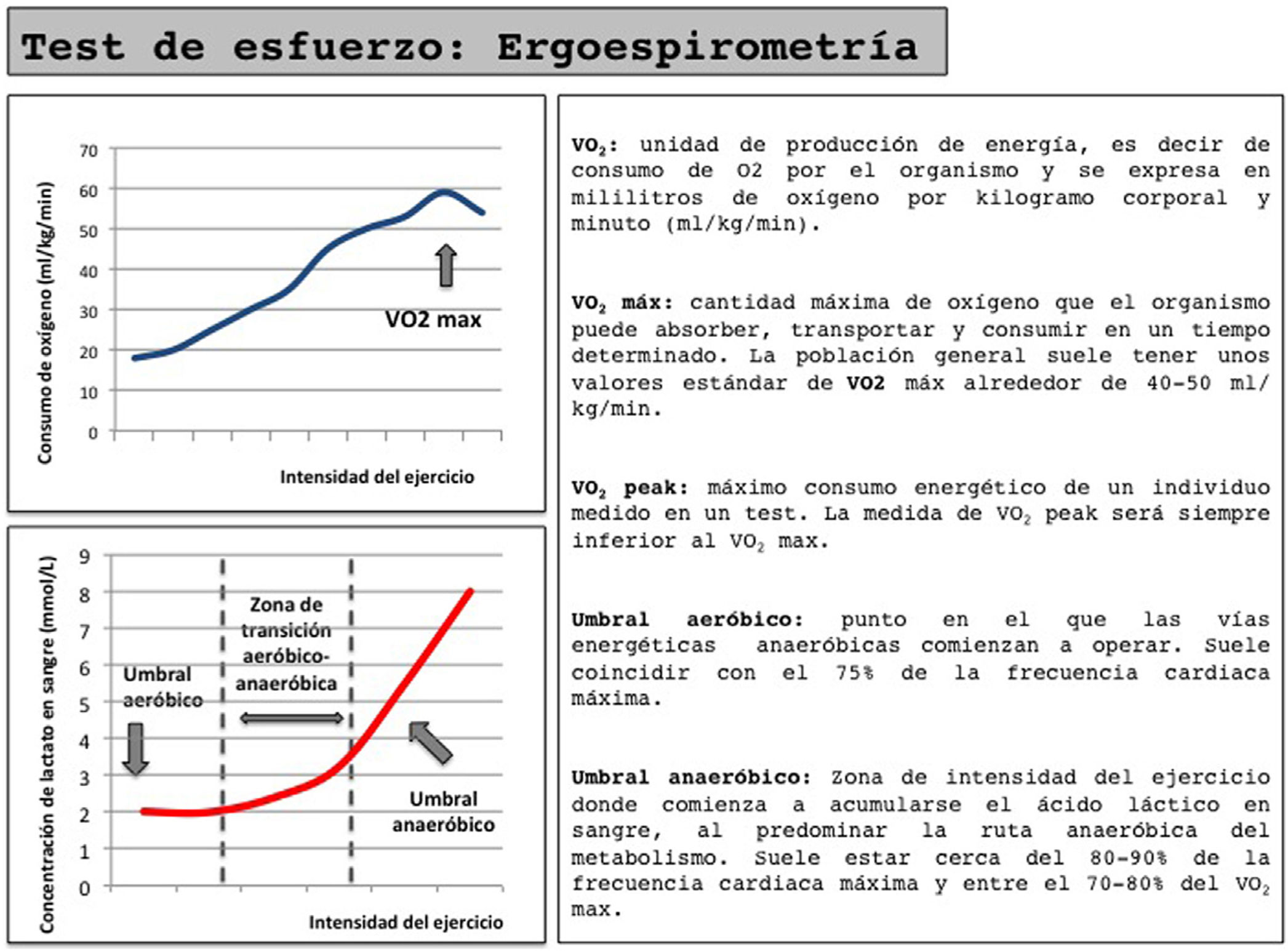
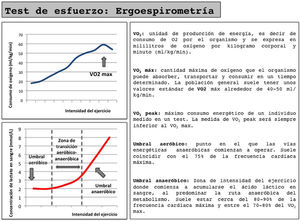
Cardiorespiratory stress test (ergospirometry) (Fig. 1): This test provides a more in-depth assessment of the patient's cardiopulmonary function and gas exchange, but requires specialized equipment and personnel. VO2max levels above 15mL/kg min have shown better 90-day survival rates in patients treated with cardiovascular surgery.13 However, the inability for most patients to reach VO2max makes it necessary to use the evaluation of submaximal parameters, such as anaerobic threshold. A low anaerobic threshold (<10mL/kgmin) determines a significant increase in postoperative morbidity, mortality and hospital stay, and therefore entails a significant increase in healthcare costs.16
- •
In addition to its diagnostic accuracy, ergospirometry enable us to determine the reference heart rate at different effort thresholds, which can be used as a reference in a training program and accurately assess the improvement obtained in physical condition after applying a PP.
The proposed training schemes vary greatly. There is evidence that a 6-week program of supervised training in the hospital (consisting of three weekly sessions of aerobic exercise with short intervals of moderate and severe intensity using as a reference the heart rate measured at VO2peak and at the anaerobic threshold) achieves improvements of 2.6mL/kgmin at VO2peak and 2.12mL/kgmin in the anaerobic threshold.17
However, Dunne et al.18 achieved similar improvements with only 4 weeks of supervised interval training at the hospital and using VO2peak as a reference.
Although supervised training has been shown to be highly effective, the need for facilities and trained personnel makes it difficult to implement PP, which is why some groups are assessing home training programs. These programs vary in their effectiveness depending on patient adherence to physical exercise, which seems to be closely related to its complexity. A simple exercise program, such as walking 30min a day and a series of breathing exercises, has been shown to have high adherence, with a significant improvement later found in the 6MWT.19 Adding respiratory exercises to the program manages to strengthen the respiratory muscles in a short period of time and is highly recommended in patients with concomitant pulmonary pathology or a very poor initial physical condition.20
There is no defined physical training program, and each group interprets this freely, making it difficult to extrapolate the results obtained. However, a recommended training program would have a series of highly effective supervised sessions with intense intervals, together with simple home sessions to complement aerobic activity. In addition, muscle resistance exercises and breathing exercises should not be forgotten.
Nutritional Assessment and Body Composition; Nutritional Recommendations; ImmunonutritionMalnutrition is closely related to disease and aging, leading to a significant increase in postoperative complications, hospital stay and readmission rates. Therefore, age and a digestive neoplasm are two risk factors for a patient to present a poor nutritional status. Some 50% of patients diagnosed with colorectal cancer are over the age of 65.21 Furthermore, Burden et al.22 show that two out of every three patients with colorectal cancer experience preoperative weight loss, and in one out of five it is more than 10%. In esophagogastric neoplasms, the incidence of malnutrition is even more striking and can reach 19% in patients with gastric cancer. In these patients, the incidence of surgical site infection is more than double in malnourished patients.23
The nutritional assessment of a patient can be carried out through nutritional screening, anthropometric tests, lab work and/or tests that allow us to assess patient body composition. The main objective of nutritional screening is to identify patients at risk of malnutrition or malnourished patients who require treatment in a reliable, reproducible and practical way. The two most widely used tests are:
- •
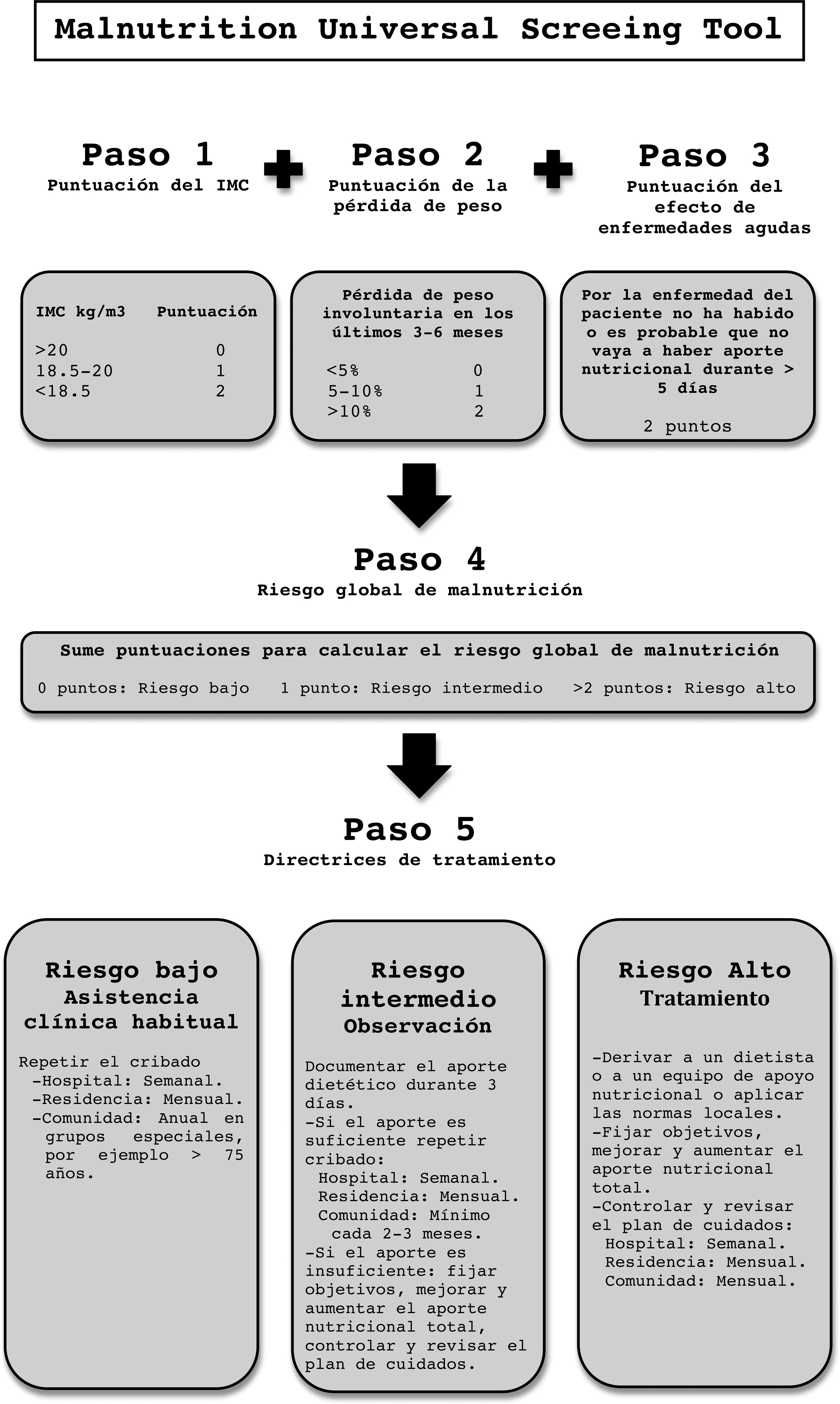
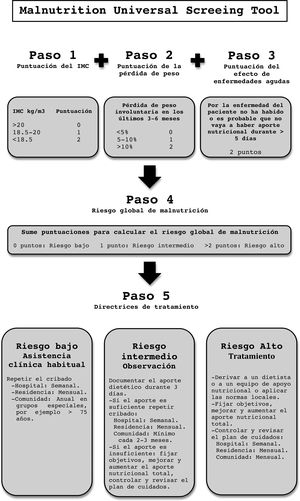
Malnutrition Universal Screening Tool (MUST): This is a quick and simple test. It analyzes BMI, weight loss in the last 3–6 months and the effect of acute illness on intake in the last 5 days.24 It adapts to the new criteria for malnutrition of the European Society for Clinical Nutrition and Metabolism (ESPEN), so its use is on the rise (Annex A: MUST).
- •
NRS 2002: This requires a certain amount of training for its implementation. It has proven to be a useful tool in hospitalized patients with malignancies.25
The role of the degenerative loss of muscle mass, or sarcopenia, as a predictor of postoperative morbidity has led to a boom in the use of tests evaluating body composition, such as bioelectrical impedance or computed tomography assessment. The latest ESPEN guidelines extend the recommendations for nutritional intervention to patients with low lean mass index (<15 in women and <17kg/m2 in men),24,25 and several studies have correlated sarcopenia and area in cm2 of skeletal muscle at the level of the third lumbar vertebra as predictors of complications and postoperative mortality.26–28
ESPEN recommends nutritional supplementation prior to surgery in patients with severe nutritional risk for a period of 7 to 14 days, guaranteeing a minimum protein intake of 1.2 to 1.5g/kg of weight per day.29,30
However, it has been shown that protein supplementation for 4 weeks produces an improvement of more than 20m in the 6MWT compared to non-supplementation,31 so future guidelines are likely to extend the recommended supplementation time to 3–4 weeks, including PP.
Immunonutrition, consisting of supplementing the patient with specific nutritional elements such as omega-3 fatty acid, arginine, glutamine and/or ribonucleic acid, has shown a significant decrease in surgical site infection rates in malnourished patients after oncological surgery, with the consequent decrease in hospital stay and costs.32 However, this does not seem to present a significant difference compared to standard nutritional supplementation in well-nourished patients,33,34 so its standardization within the PP still requires further evidence.
Cognitive Assessment and RecommendationsThe indication of the need for surgical intervention, especially when accompanied by a serious disease such as a tumor pathology, creates a situation of uncertainty for patients, derived from the procedure itself as well as future events related to their own life stage and their family environment. This triggers a situation of sustained stress in patients, which activates the hypothalamic-pituitary-adrenal system and the sympathetic system with elevation of cortisol and catecholamines. In turn, with the release of cytokines, this causes immune alterations.
All these processes, in addition to causing multiple symptoms in patients, such as gastrointestinal alterations, tachycardia, palpitations or insomnia, favor a maintained catabolic phase after surgery, a lower capability for healing, and alterations at the immune level that could increase the risk of postoperative infections.
Given the difficulty in finding an analytical parameter that does not undergo changes with circadian rhythm and quantifies in a sensitive and specific manner the amount of stress to which a patient is subjected, qualitative questionnaires have demonstrated their validity not only to determine the degree of stress, but also for assessing the effect of cognitive interventions.
There is a wide variety of qualitative tests. The two most commonly used are:
- •
Hospital Anxiety and Depression Scale (HADS): With a widely demonstrated validity, HADS provides clinically significant results as a psychological screening tool in comparisons of clinical groups and in correlational studies with various aspects of disease and quality of life. It is sensitive to changes both during the course of illness and in response to psychotherapeutic and psychopharmacological intervention35 (Annex B: HADS).
- •
SF-36: This test provides a health status profile and is applicable to both patients and the general population. It has been useful in evaluating health-related quality of life in the general population and in specific subgroups, comparing the burden of very diverse diseases, detecting the health benefits of a wide range of different treatments, and assessing the health status of individual patients.36
Although theoretically cognitive recommendations aimed at reducing the level of anxiety prior to surgery are a central part of prehabilitation, in practice most multimodal PP underestimate their contribution, and there is no clearly defined course of action. Most interventions are based on audiovisual recommendations, and groups that conduct face-to-face cognitive intervention sessions are rare.
However, the role of mindfulness-based stress reduction (MBSR) as a cognitive intervention method against stress is thriving. In recent years, an exponential increase in studies about its possible benefits has been observed in pathologies such as inflammatory bowel disease and rheumatoid arthritis, which are suspected of playing an immunomodulatory role in the inflammatory response.37 Furthermore, in patients with breast cancer, the MBSR has statistically significant short-term effects in reducing fatigue, sleep, stress, anxiety and depression,38 so it is expected to have a more transcendental role within prehabilitation in coming years.39
On the other hand, cognitive training programs have been shown to be effective in preventing episodes of agitation and disorientation in patients with dementia. Although their usefulness has not yet been demonstrated within a PP, it is a tool to consider in patients with risk factors for delirium.40,41
Other RecommendationsSmoking CessationTobacco use is closely related to the development of tumors of the digestive tract: some 75%–80% of patients with squamous cell carcinoma of the esophagus have a history of smoking, which is considered the most important risk factor for the development of gastric cancer.42 In addition, smoking is an independent risk factor for pulmonary complications after gastroesophageal surgery, causing immune alterations that lead to a marked increase in surgical site infection rates and altering the wound healing process, as even increased anastomotic leakage is observed.43
Although it is not considered a fundamental pillar of prehabilitation, several studies support the effectiveness of smoking cessation during this period, since important benefits are achieved in the short term. Yoshida et al.3 demonstrated that after 4 weeks without smoking, the risk of developing pneumonia in the postoperative period decreased significantly in surgical patients with esophageal cancer; after 3 months, the patients had the same risk for postoperative complications as those who had never smoked. In the study by Jung et al.,4 the time limit without tobacco consumption that protected against the development of postoperative complications was established at 2 weeks for patients treated surgically for gastric cancer.
Interventions combining pharmacotherapy with nicotine replacement therapy, varenicline, and bupropion with behavioral support increased the success of smoking cessation.44
Correction of AnemiaPreoperative anemia, defined by the WHO as hemoglobin (Hb) <13g/dL in men and <12g/dL in women,45 is a risk factor for perioperative complications that has become more relevant in recent years. With an incidence of 30% in patients undergoing elective surgery, in up to 75% of cases this anemia is unknown to patients and their physicians.46
In anemia of chronic or inflammatory disorders, which are frequent in chronic or hospitalized patients, an altered immune response mediated by cytokines interrupts iron homeostasis, and a smaller number of circulating red blood cells causes impaired postoperative recovery secondary to a state of hypercatabolism due to decreased oxygen and aerobic capacity. Thus, several studies have established anemia as an independent risk factor in colorectal, hepatic, bariatric and oncological breast surgery, with an increase in postoperative complications, recurrence and mortality in the short and long term, associated with significant increases in healthcare costs.4
The analytical evaluation of perioperative anemia should include Hb, ferritin, mean corpuscular volume (MCV) and transferrin, knowing that anemia of chronic disorders presents with normal or increased ferritin levels.
Current recommendations of ERAS protocols suggest treating anemia with oral iron for 14 days prior to surgery.47,48 However, oral iron has uncertain or inhibited absorption in the presence of inflammation, so this period is insufficient to improve the value of Hb and/or replenish iron stores,47 resulting in the need for intravenous iron in many cases. Current guidelines for the treatment of preoperative anemia recommend an initial dose of 1000mg of iron with ferric carboxymaltose.
Therefore, its incorporation in PP seems essential to increase the efficacy of oral iron and to limit the use of intravenous iron and blood transfusions or erythropoiesis-stimulating agents to special situations, such as contraindication, resistance or refractoriness to oral iron or little time before surgery.49,50
Medication ReconciliationSurgery in elderly patients is related to an increase in mortality and a higher incidence of postoperative complications that lead to delayed hospital discharge, a higher rate of readmissions, as well as a significant deterioration in quality of life.51,52
Medication reconciliation has proven to be an effective method of detecting drug discrepancies and reducing the potential harm caused by them. However, current evidence is limited when evaluating its effect on hospital stay, readmission rate, or mortality.53 Nonetheless, we must assume that the adaptation and individualization of a prehabilitation protocol with adequate medication reconciliation will mean a greater benefit for geriatric patients, and its inclusion is a priority for correct evaluation.
Prehabilitation as a Multimodal Program: Future OutlookBeyond the initial trimodal concept of exercise, nutritional support and cognitive support of PP, current protocols integrate the individual benefits from each action previously described, and ultimately the favorable effects are combined synergistically. Several groups have begun to implement these protocols for comprehensive patient preparation prior to the immediate perioperative period, obtaining encouraging results. Li et al.,54 for instance, observed an improvement of about 40m in the 6MWT after 4 weeks of trimodal PP. This is a greater improvement than in studies of nutritional supplementation or isolated physical exercise, in which a non-significant improvement of only 10 to 20m is observed.19,31
Studies with PP in frail elderly patients have been shown to significantly decrease hospital stay and allow for an earlier return to the functional situation before surgery.55,56 In addition, in borderline situations with patients who present high comorbidity, the response to PP could be considered a screening method to help in therapeutic decision-making.
Furthermore, the benefits of PP should be evaluated not only in the immediate perioperative context. Their implementation has shown less loss of muscle mass in the long term and an earlier recovery of the functional situation prior to surgery, even when compared with rehabilitation therapy.57,58 This decrease in postoperative complications, added to the reduction in preoperative events related to lack of exercise, malnutrition, stress, tobacco, and anemia, suggest upcoming evidence about the importance of these programs.
Currently, only Van Rooijen et al.59 have evaluated the cost-effectiveness and economic feasibility of implementing PP within normal clinical practice. However, in addition to the direct costs of the program, cost-effectiveness studies will be necessary to evaluate, the savings in healthcare costs derived from the prevention of complications and an early return to work, just like those done for ERAS or major outpatient surgery programs.
ConclusionsIn summary, the current evidence allows us to recommend the need for prehabilitation in patients with low physical condition and high nutritional risk. With an increasingly fragile and aging population and a demand for rapid recovery of normal life while maintaining quality-of-life standards similar to before surgery, the implementation of prolonged preoperative optimization strategies for patients seems the way to go in surgery of the future.
AuthorshipFrancisco López Rodríguez-Arias, Luis Sánchez-Guillén and Antonio Arroyo Sebastián have participated in the design of the study, analysis and interpretation of the results, as well as the critical review and approval of the final version.
Laura Irene Armañanzas Ruiz and Carlos Díaz Lara have contributed to the acquisition and collection of data as well as the study design.
Francisco Javier Lacueva Gómez, Carmen Balagué Ponz and José Manuel Ramírez Rodríguez have participated in the analysis and interpretation of results as well as the critical review and final approval.
Conflict of InterestsThe authors participating in this study have no conflict of interests to declare.
Please cite this article as: López Rodríguez-Arias F, Sánchez-Guillén L, Armañanzas Ruiz LI, Díaz Lara C, Lacueva Gómez FJ, Balagué Pons C, et al. Revisión narrativa de la prehabilitación en cirugía: situación actual y perspectivas futuras. Cir Esp. 2020;98:178–186.