The present work is an observational study of a series of variables regarding overall survival and disease-free survival in patients diagnosed with primary liposarcoma.
MethodsThe study is prospective with retrolective data collection that includes all patients with primary liposarcoma referred to Hospital Son Espases University Hospital, Palma de Mallorca, Spain from January 1990 to December 2019.
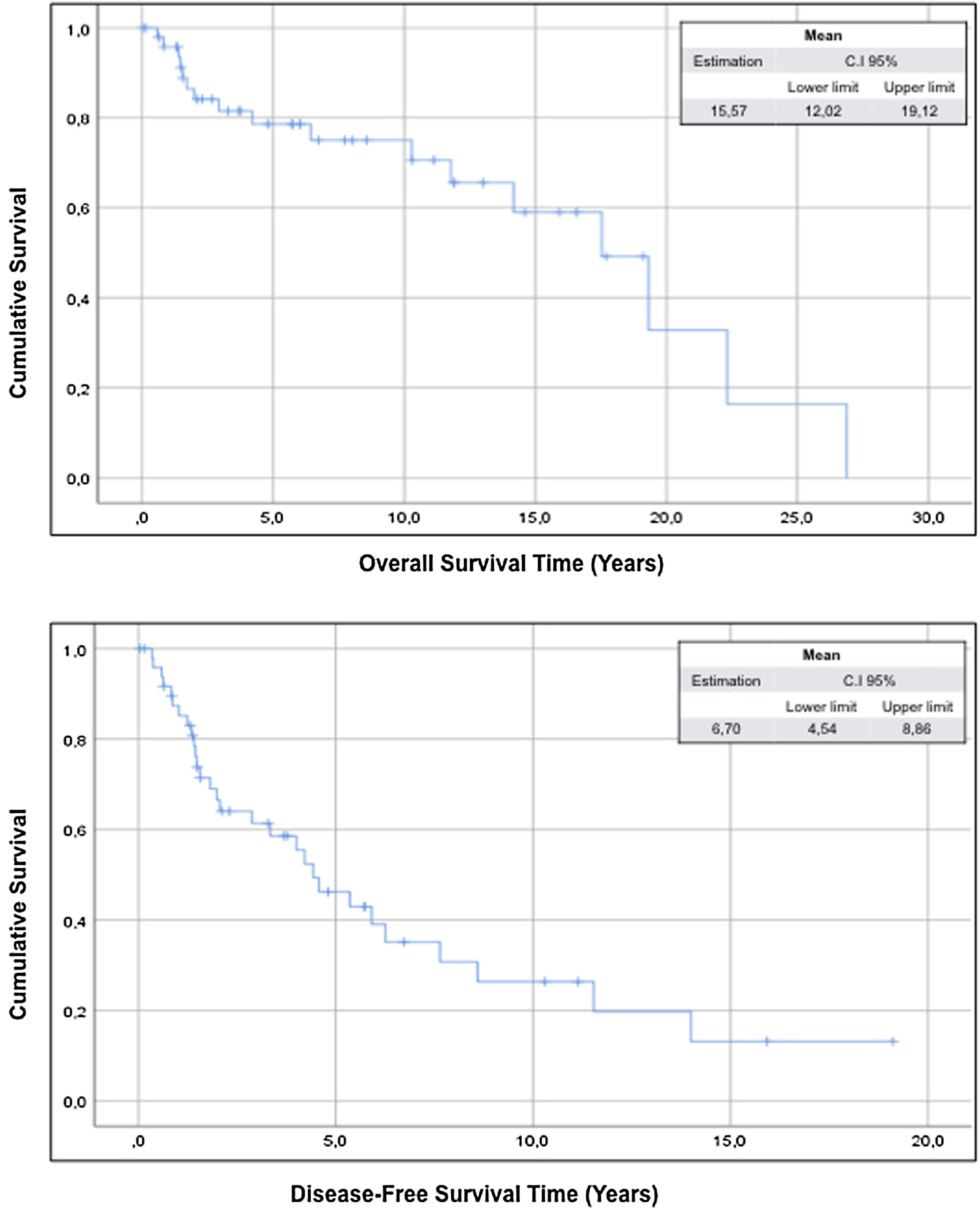
ResultsThe study includes 50 patients and the compartment surgery was performed in 18 patients (36%) of cases. The mean overall survival of the sample was 15.57 years (95% CI: 12.02–19.12) and the mean disease-free survival was 6.70 years (95% CI: 4.50–8.86).
ConclusionCompartment surgery has not shown benefits in terms of overall survival and disease-free survival. The ASA classification (≥3) predicts a poor prognosis in both overall survival and disease-free survival. Resection with free margins, described on the pathological results and defined in this work as R0, show better disease-free survival.
El presente trabajo es un estudio observacional de una serie de variables relacionadas con la supervivencia global y la supervivencia libre de enfermedad en pacientes diagnosticados de liposarcoma primario.
MétodosEste es un estudio prospectivo con recolección de datos retrolectiva que incluye a todos los pacientes con liposarcoma primario remitidos al Hospital Son Espases en Palma de Mallorca, desde enero de 1990 hasta diciembre de 2019.
ResultadosEl estudio incluye 50 pacientes y la cirugía compartimental se realizó en 18 (36%) de ellos. La supervivencia global media de la muestra fue de 15,57 años (IC 95% 12,02-19,12) y la supervivencia libre de enfermedad media fue de 6,70 años (IC 95% 4,50-8,86).
ConclusionesLa cirugía compartimental no ha mostrado beneficios en términos de supervivencia general y supervivencia libre de enfermedad. La clasificación ASA (≥3) predice un mal pronóstico tanto en la supervivencia global como en la supervivencia libre de enfermedad. La resección con márgenes libres, descrita en los resultados patológicos y definida en este trabajo como R0, muestra una mejor supervivencia libre de enfermedad.
Soft tissue sarcomas, a heterogeneous group of solid neoplasms of mesenchymal cellular origin, arising in the retroperitoneal space are defined as retroperitoneal soft tissue sarcomas (RPS).1 They are malignant tumors that represent 15% of all sarcomas and have an overall incidence of 0.3–0.4% per 100,000 of the population2 with a peak incidence that occurs during the fifth decade of life.3 Liposarcoma (LPS) is the most common histologic type assuming about 50% of RPS.4 Its manner of presentation is typically non-specific and its preoperative diagnosis usually requires preoperative needle biopsy, since the diagnosis by conventional radiological techniques does not allow to distinguish between benign conditions, non-sarcomatous neoplasms and other solid retroperitoneal masses.5
Surgery is the basis of the treatment of RPS and its quality is critical for any potential cure.6 The common characteristic of RPS is to involve or lean on multiple organs and compartment surgery, which includes the resection of healthy organs adjacent to the tumor, has been shown to reduce recurrence rates compared to simple mass excision, although this does not always translate into better survival.7 The margins of tumor resection influence both the local recurrence rate and mortality.8 However after radical R0 surgery, local and/or peritoneal recurrence occurs in more than 50% of cases.9 The recurrence-free time interval marks the evolution of the disease and is longer the more radical the primary surgery.9 The patient's prognosis is determined by the biological aggressiveness of the RPS (low/high grade) and more specifically, survival rates depend on the heterogeneity in histology and its differentiation. The loco-regional recurrence is the main cause of mortality.8,10 This study presents the results obtained from a regional reference center for this disease with the intention to identify factors that predict worse survival.
MethodsThe present work is an observational study of a series of variables regarding overall survival and disease-free survival in patients diagnosed with primary liposarcoma. The study is prospective with retrolective data collection that includes all patients with primary liposarcoma referred to Hospital Son Espases University Hospital, Palma de Mallorca, Spain from January 1990 to December 2019. During the study period, the multidisciplinary group of preferential dedication for retroperitoneal sarcomas was formed. Starting in 2013, a multidisciplinary committee led by oncologists, radiologists and surgeons trained in the treatment of retroperitoneal sarcomas assumed the treatment of this pathology in our center, basing the surgical treatment on the compartment surgery for the treatment of retroperitoneal liposarcomas described by Trans-Atlantic RPS Working Group.6 Before that, sarcomas had been operated by urologists, general surgeons and even vascular surgeons, not guaranteeing the same criteria for surgical resection and treatment. The study received appropriate institutional approval from a Hospital Ethics Committee. The most recent patients were informed of the use of the data by signing the preoperative informed consent while due to the characteristics of the study, it was not possible to achieve it in older patients.
Primary liposarcomas were defined as those untreated lesions before definitive surgical treatment and were classified according to the WHO classification system for soft tissue tumors11 and grade of dedifferentiation (high grade: grade 2 or 3 of the Fédération Nationale Des Centros De Lutte Contre Le Cáncer (FNCLCC); low grade: grade 1 of the FNCLCC).12 Tumors were also classified according to their location, identifying three categories: right hemi abdomen, left hemi abdomen and pelvis. Other pathological characteristics were the size of the tumor and considering the maximum diameter they were classified as<10cm, 10–20cm and >20cm.
Demographic data of the patients, such as sex, age, body mass index (BMI), the American Society of Anaesthesiologists (ASA) classification13 and the Eastern Cooperative Oncology Group status (ECOG)14 were analysed. The first diagnostic test was collected and whether the patient received a tru-cut needle biopsy to complete the diagnosis.
The surgical technique was defined as simple dissection and block exeresis when the plane between the tumor and the adjacent viscera was dissected. The compartment surgery described by Trans-Atlantic RPS Working Group,6 consisted of aggressive surgery with removal of adjacent organs with the aim of obtaining a wide margin of healthy tissue surrounding the tumor area. An R0 resection was considered when in the pathology report the resection margins were free of disease. An R1 resection was considered when the pathological anatomy result showed the involvement of the margins of the surgical piece by tumor tissue. Postoperative morbidity and mortality were considered when deaths and/or complications occurred within 30 days after surgery and were classified according to the Clavien-Dindo classification.15
Post-surgical clinical follow-up was performed by the Surgery and Oncology Department. Patients were evaluated every 3 months by ambulatory clinical evaluation, blood tests and ultrasound, and every 6 months by computed tomography. Primary outcomes were disease-free survival and overall survival. Recurrence was defined as either pathologic or radiographic evidence of recurrence following resection. Overall survival was defined as the length of time between surgery and death from any cause with time censored at last follow-up.
A descriptive analysis of all the variables was carried out to define the characteristics of the study group with frequencies and percentages for the qualitative variables and with measures of central position and dispersion for the quantitative variables. Once the normal distribution of the numerical variables was confirmed, using normality tests and graphs, these have been expressed with the mean and standard deviation, except for the variable number of organs removed, which follows a non-normal distribution and is expressed with the median and the interquartile range. In the survival analysis, the variables were classified into three groups: sample-related variables (age, sex, BMI, ASA classification, ECOG status, initial diagnostic test and diagnostic biopsy), tumor-related variables (position, histological subtype, tumor grade, tumor dimension and tumor recurrence) and finally surgery-related variables (type of resection, organ resection, R0 resection and postoperative complications). In order to evaluate whether age effected survival, we first calculated the mean age of the sample and subsequently compared the two groups, one group of patients with ages under the mean age and the other group over. The same was made with the BMI, and patients were divided into those with a BMI<30kg/m2 compared with those with a BMI≥30kg/m2. Respects to the tumor characteristics, well-differentiated liposarcomas were contrasted with the other histological subtypes. On assessing survival, simple dissection and block exeresis were compared together versus compartment surgery. The Kaplan–Meier method was used to analyze the overall survival and survival times free of reoperation. The evaluation of risk factors on survival times was carried out using a univariate analysis according to the proportional hazards model of the Cox regression. In accordance with the main risks obtained, the survival curves were compared using the log-rank test. A value of p<0.05 was considered as an indicator of a significant difference. The statistical software used to analyze the data has been SPSS v.26.
ResultsFifty patients were included in the study period, 30 (60%) males and 20 (40%) females with an overall mean age of 57.3±14.0 years and mean BMI of 27.0±4.7kg/m2. Regarding the ASA classification, 31 (62%) patients were classified as grade 2 and 13 (26%) patients as grade 3, while the distribution in the ECOG classification was more heterogeneous, with 18 (36%) patients with ECOG 0, 19 (38%) patients with ECOG 1 and 10 (20%) patients with ECOG 2 respectively. The first radiological test most used to obtain the diagnosis of the disease was computed tomography in 30 (60.0%) patients. In one patient (2.0%) the magnetic resonance (MRI) was used and in 19 (38.0%) patients the first diagnosis was made by ultrasound. All the patients diagnosed by ultrasound, completed the study before surgery by performing a computed tomography. A core needle biopsy was performed in 19 (38.0%) patients (Table 1).
Patient characteristics and their influence on survival.
| Global survival since first surgery | Disease-free survival since first surgery | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Items | Category | Descriptive | HR (risk) | CI 95.0% | p | HR (risk) | CI 95.0% | p | ||
| Sex [n (%)] | ||||||||||
| Female | 20 (40.0) | |||||||||
| Male | 30 (60.0) | 1.455 | 0.533 | 3.970 | 0.464 | 0.913 | 0.437 | 1.906 | 0.808 | |
| Age [years; mean±SD] | 57.3±14.0 | 1.035 | 0.998 | 1.074 | 0.061 | 1.001 | 0.979 | 1.022 | 0.953 | |
| <57 | 22 (44.0) | |||||||||
| ≥57 | 28 (56.0) | 2.760 | 0.850 | 8.961 | 0.091 | 1.093 | 0.520 | 2.296 | 0.815 | |
| BMI [kg/m2; mean±SD] | 27.0±4.7 | 1.017 | 0.906 | 1.143 | 0.770 | 0.986 | 0.908 | 1.071 | 0.734 | |
| <30 | 38 (76.0) | |||||||||
| ≥30 | 12 (24.0) | 1.206 | 0.326 | 4.457 | 0.779 | 0.746 | 0.303 | 1.837 | 0.524 | |
| ASA [n (%)] | ||||||||||
| 1 | 4 (8.0) | |||||||||
| 2 | 31 (62.0) | |||||||||
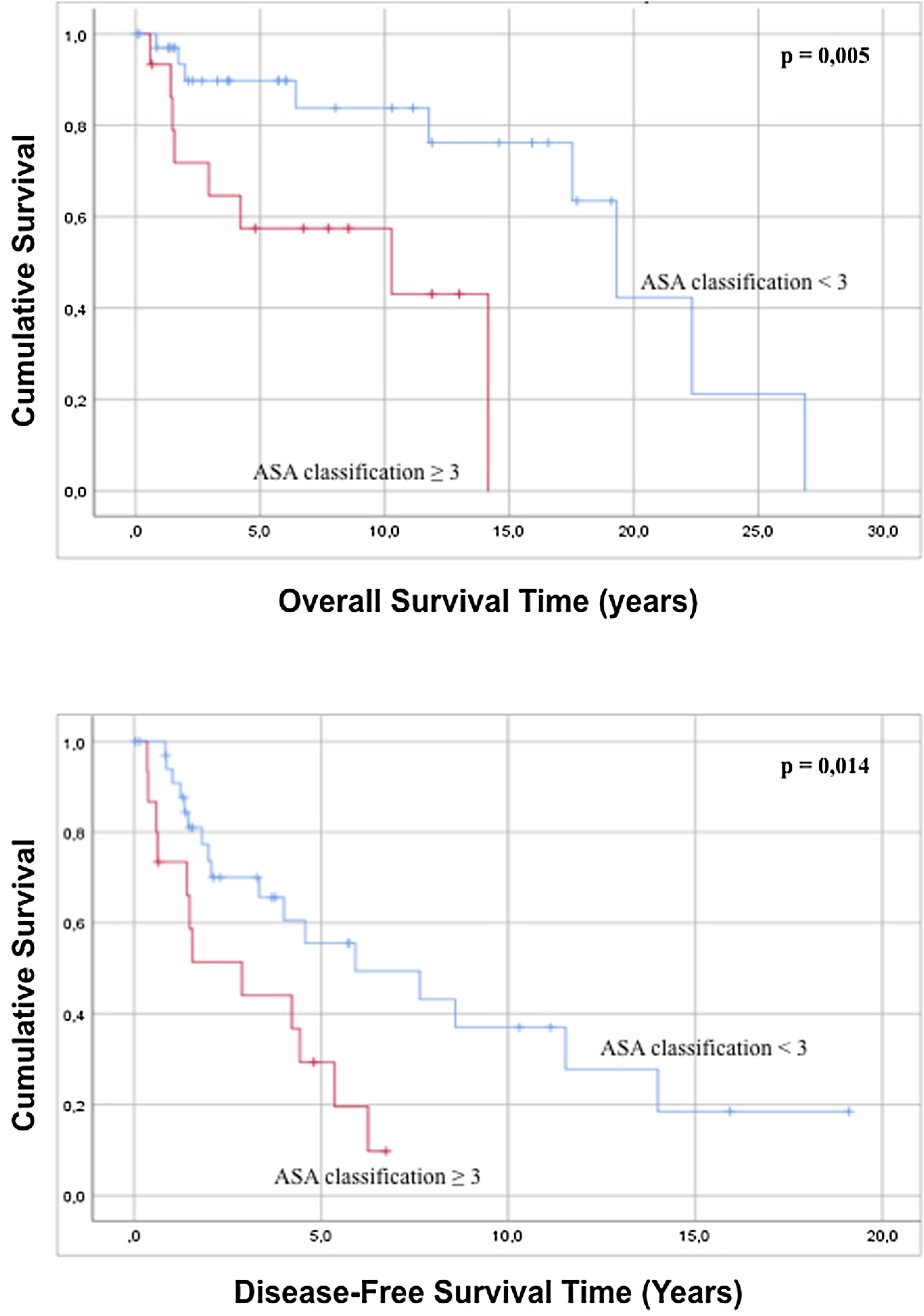
| 3 | 13 (26.0) | 4.466 | 1.433 | 13.922 | 0.010 | 2.592 | 1.179 | 5.699 | 0.018 | |
| 4 | 2 (4.0) | |||||||||
| ECOG [n (%)] | ||||||||||
| 0 | 18 (36.0) | 0.425 | 0.135 | 1.336 | 0.143 | 0.476 | 0.210 | 1.078 | 0.075 | |
| 1 | 19 (38.0) | |||||||||
| 2 | 10 (20.0) | |||||||||
| 3 | 3 (6.0) | |||||||||
| Inicial diagnostic test [n (%)] | ||||||||||
| CT | 30 (60.0) | 0.553 | 0.193 | 1.586 | 0.271 | 1.077 | 0.498 | 2.327 | 0.850 | |
| MRI | 1 (2.0) | |||||||||
| US | 19 (38.0) | |||||||||
| Biopsy prior to surgery [n (%)] | ||||||||||
| No | 31 (62.0) | |||||||||
| Yes | 18 (38.0) | 1.314 | 0.432 | 3.997 | 0.631 | 1.132 | 0.518 | 2.476 | 0.756 | |
| Tumor position [n (%)] | ||||||||||
| Pelvis | 5 (10.0) | 1.269 | 0.161 | 10.000 | 0.821 | 0.406 | 0.055 | 3.001 | 0.377 | |
| Right hemiabdomen | 24 (48.0) | 1.061 | 0.392 | 2.874 | 0.907 | 1.503 | 0.711 | 3.176 | 0.286 | |
| Left hemiabdomen | 21 (42.0) | |||||||||
| Histological subtype [n (%)] | ||||||||||
| Well differentiated | 25 (50.0%) | 0.353 | 0.122 | 1.437 | 0.165 | 1.211 | 0.530 | 2.453 | 0.575 | |
| Desdiferentiated | 19 (38.0) | |||||||||
| Mixoyd | 4 (8.0) | |||||||||
| Pleomorfic | 2 (4.0) | |||||||||
| Grade [n (%)] | ||||||||||
| Low grade | 60.0% (30) | |||||||||
| High grade | 40.0% (20) | 1.536 | 0.531 | 4.448 | 0.429 | 0.593 | 0.269 | 1.307 | 0.195 | |
| Tumor dimension [n (%)] | ||||||||||
| <10cm | 12 (24.0) | |||||||||
| 10–20cm | 15 (30.0) | |||||||||
| >20cm | 23 (46.0) | 1.948 | 0.627 | 6.048 | 0.249 | 1.252 | 0.584 | 2.687 | 0.563 | |
| Type of resection [n (%)] | ||||||||||
| Local exeresis | 10 (20.0%) | |||||||||
| Block exeresis | 22 (44.0) | |||||||||
| Compartment surgery | 18 (36.0) | 0.279 | 0.035 | 2.226 | 0.228 | 0.432 | 0.147 | 1.271 | 0.127 | |
| Number of organs resected [n (%)] | ||||||||||
| 1 o 2 | 8/24 (33.3) | |||||||||
| >2 | 16/24 (66.7) | 0.256 | 0.027 | 2.429 | 0.235 | 0.934 | 0.240 | 3.639 | 0.922 | |
| Organs included [n (%)] | ||||||||||
| Kidney | 20 (40.0) | |||||||||
| Adrenal gland | 20 (40.0) | |||||||||
| Psoas muscle | 14 (28.0) | |||||||||
| Colon | 13 (26.0) | |||||||||
| Small bowel | 4 (8.0) | |||||||||
| Spleen | 4 (8.0) | |||||||||
| Pancreas | 3 (6.0) | |||||||||
| Cava vein | 3 (6.0) | |||||||||
| Duodenum | 2(4.0) | |||||||||
| Ovary | 1 (2.0) | |||||||||
| Resection [n (%)] | ||||||||||
| R0 | 42 (84.0) | |||||||||
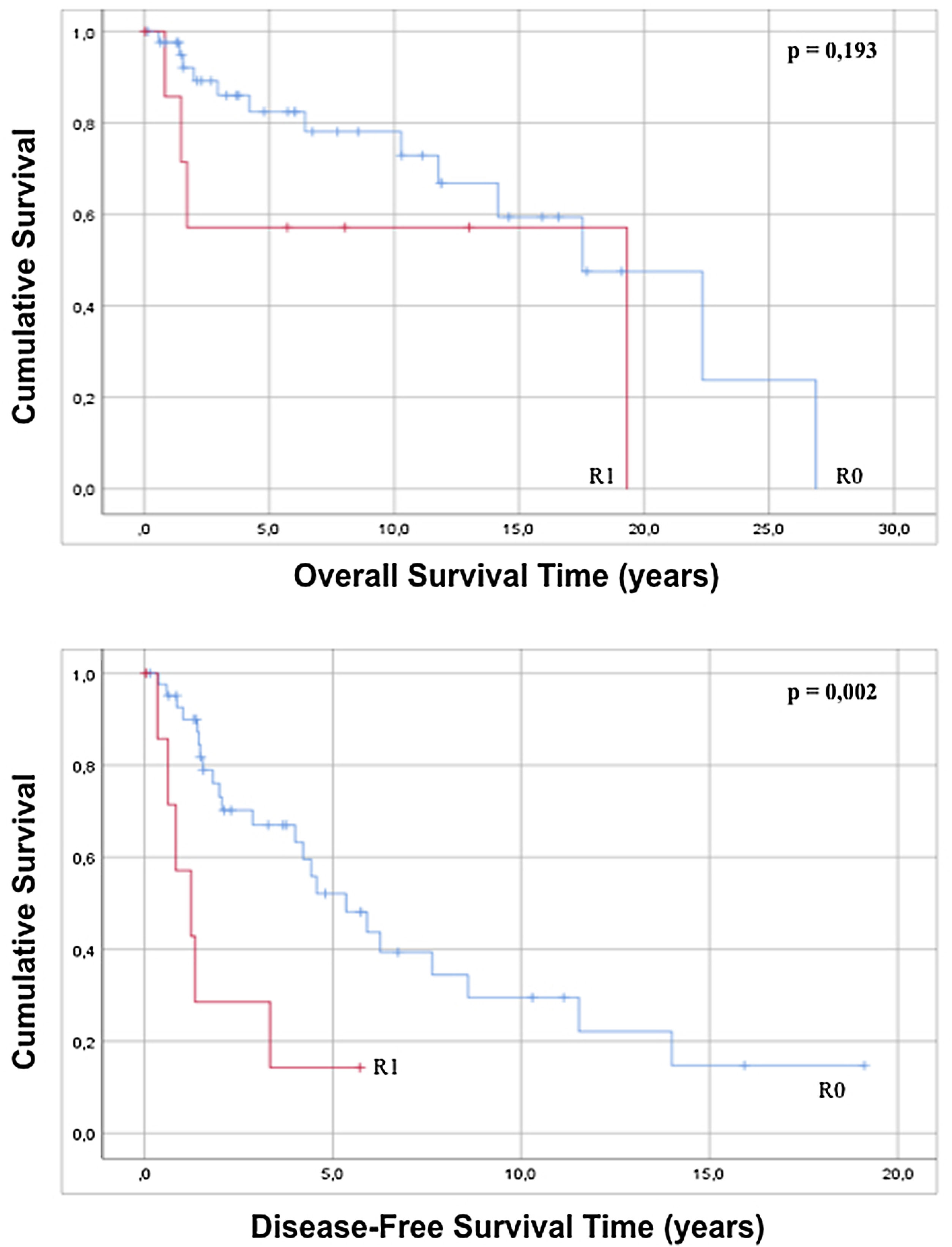
| R1 | 8 (16.0) | 2.125 | 0.666 | 6.775 | 0.203 | 3.862 | 1.506 | 9.904 | 0.005 | |
| Postoperative complications [n (%)] | ||||||||||
| No | 27 (54.0) | |||||||||
| Yes | 23 (46.0) | 0.862 | 0.289 | 2.570 | 0.790 | 1.216 | 0.573 | 2.579 | 0.611 | |
| Clavien-Dindo [n (%)] | ||||||||||
| No | 27 (54.0) | 1.160 | 0.389 | 3.458 | 0.790 | 0.823 | 0.388 | 1.745 | 0.611 | |
| Clavien I | 13 (26.0) | |||||||||
| Clavien II | 4 (8.0) | |||||||||
| Clavien III | 3 (6.0) | |||||||||
| Clavien IV | 3 (6.0) | |||||||||
HR hazard ratio, CI confidence interval, p level of critical significance, *p<0.05, % percentage, BMI body mass index, ASA American Society of Anaesthesiologists, ECOG Eastern Cooperative Oncology Group status.
Tumours were located in the right hemi-abdomen in 24 (48%) patients, in the left hemi-abdomen in 21 (42%) patients and in the pelvis in 5 (10%) cases. All patients were primary retroperitoneal liposarcomas, 30 (60%) of them with a low tumor grade and the histological subtypes of liposarcoma are resumed in Table 1. Regarding the dimensions of the tumours operated on in this study, 23 (46%) patients had tumours with a maximum diameter greater than 20cm; in 15 (30%) cases the maximum diameter was between 10 and 20cm and only in 12 (24%) patients had a diameter of less than 10cm.
With respect to the applied surgical technique, simple dissection was performed in 10 (20%) patients, block exeresis was performed in 22 (44%) patients and finally a compartment surgery was performed in 18 (36%) patients. Regarding the results of the surgery, in 42 (84%) patients the resected tumour presented in the pathology report a R0 resection. Twenty-three patients (46%) suffered postoperative complications, of which the majority had Clavien-Dindo I in 13 patients (26%), with the rest of the patients as follows, 4 patients (8%) Clavien-Dindo II, 3 patients (6%) Clavien-Dindo III and in 3 patients (6%) Clavien-Dindo IV, respectively. Four patients (8%) had to be intervened due to immediate postoperative complications, while there was no perioperative or postoperative deaths (Table 1).
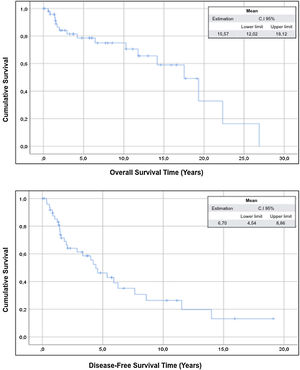
The mean overall survival of the sample was 15.57 years (95% CI: 12.02–19.12) and the mean disease-free survival was 6.70 years (95% CI: 4.50–8.86) (Fig. 1).
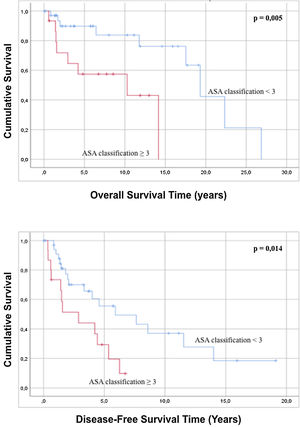
Regarding the characteristics of the sample, it was statistically observed that an ASA classification of less than 3 is related to a better overall survival and greater disease-free survival compared to patients with an ASA grade>3 (Fig. 2). Analysis of the age of the patients has not had a statistically significant influence on greater overall survival or disease-free survival (p=0.091 and p=0.815). In the same way, the evaluation of the ECOG scale does not predict any difference in both overall survival and disease-free survival (p=0.143 and p=0.075).
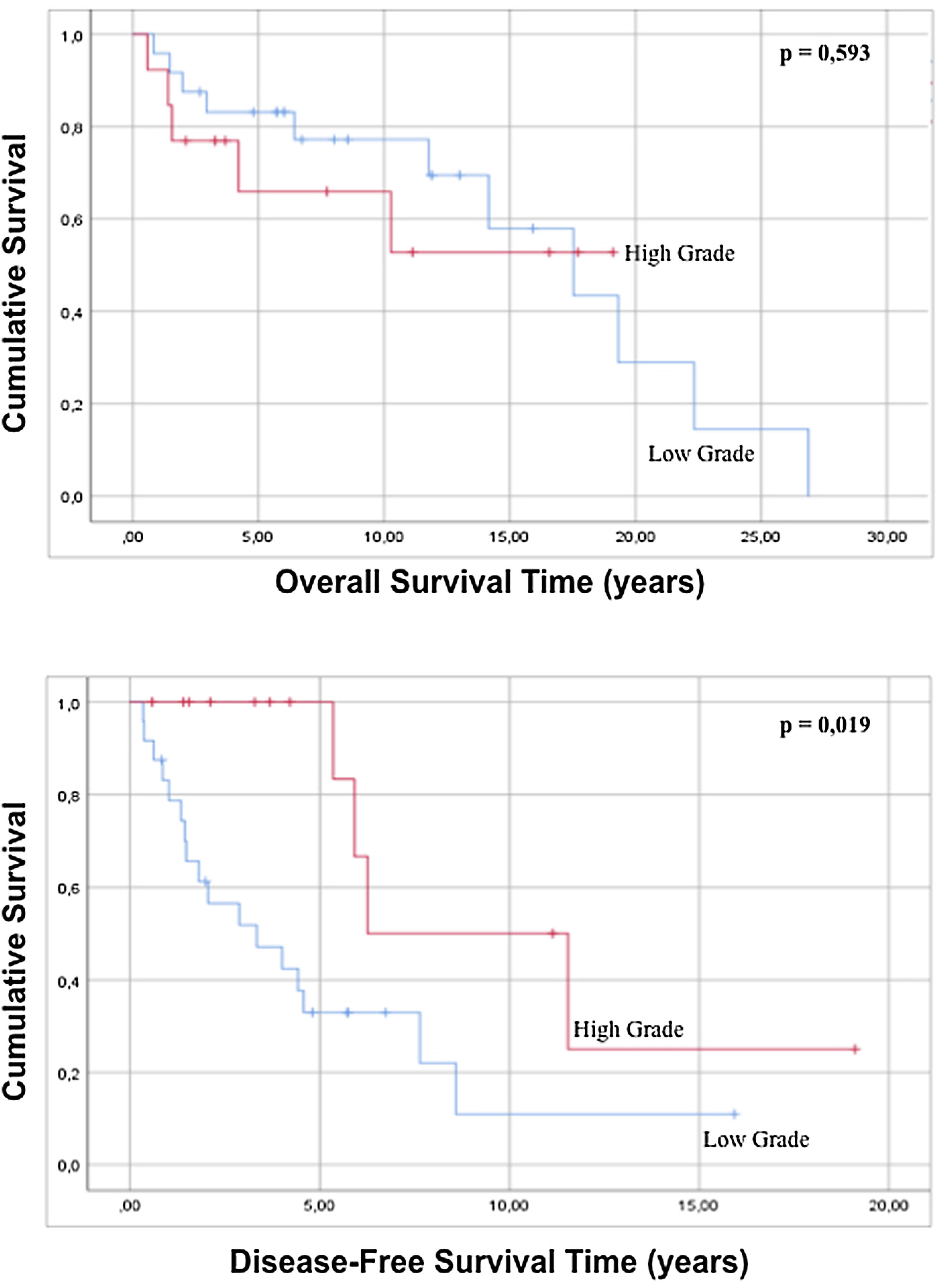
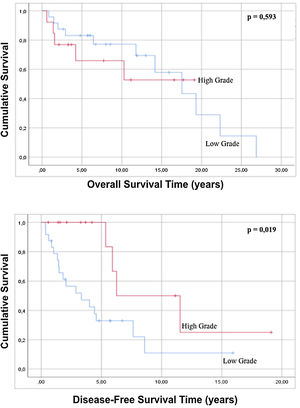
No statistically significant differences were found in either overall survival (p=0.165) or disease-free survival (p=0.575) when well-differentiated liposarcomas were contrasted with the other histological subtypes. Regarding the variable grade of the tumor, in the regression there is no statistically significant difference in both global survival and disease-free survival (p=0.429 and p=0.195 respectively), but comparing survival times by analyzing the curves survival rate, it is possible to observe that high-grade tumors show a better disease-free survival with statistical significance compared to low-grade tumors (p=0.019) (Fig. 3). When the tumor dimensions are analyzed, differences in both overall and disease-free survival between tumors with a dimension less than 20cm are observed with respect to tumors with a maximum diameter greater than 20cm, although without reaching a statistical significance (p=0.086 and p=0.879).
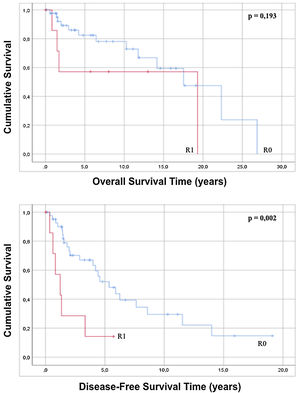
The approach to retroperitoneal liposarcomas using compartment surgery does not present a statistically significant difference in overall or disease-free survival when analyzed by regression (p=0.228 and p=0.127). The results of the regression show that an R0 resection, although it does not influence overall survival (p=0.203), increases disease-free survival with statistical significance (p=0.005) and when the Kaplan–Meier curve is analyzed, it is observed that patients with an R0 present an overall survival of 16.74 years (95% CI 12.71–20.78) with p=0.193 and a disease-free survival of 7, 33 years (95% CI 5.01–9.75) with p=0.002 with respect to patients with an anatomopathological report of R1 (Fig. 4).
Postoperative complications influence poorer overall (p=0.734) and disease-free survival (p=0.118), although no significant differences have been detected with respect to the cases that did not have any complications.
DiscussionIn the treatment of retroperitoneal liposarcomas, surgery plays a fundamental role. Complete resection remains to date the only effective treatment of choice8,16,17 and is the most important independent factor that predicts postoperative survival time.8,16,18 Surgical resections with disease-free resection margins may prolong postoperative survival time when compared to resections with a positive resection margin.8,19 Recently this concept has been further improved through a standardized approach based on histological behavior and site of origin with the aim of maximizing the possibility of achieving a complete resection.20 The importance of high-quality surgery was also observed in our results, where the pathological anatomical result of R0 resection was the factor related to surgery, which presents a statistically significant result in terms of disease-free survival. For this reason a complete resection with a clean microscopic margin must be pursued. To achieve this, most of the time it is necessary to remove the adjacent organs.21 Unlike other published articles,22 in our study the kidney and the correlated adrenal gland were the most commonly resected organ, followed by the colon. Resection of organs that are in contact with the RPS has been shown to reduce local recurrence rates, although it has no impact on prolonging survival time.17,23,24 The introduction of compartment surgery has had a positive effect on the survival of patients. Although there are no statistically differences, we believe that this is due to the small number of the sample and the differences in terms of follow-up time compared to patients who underwent another surgical approach. This aspect has been described by different authors9 and we trust that in the near future we could obtain the same results described in other publications. For this it would be essential to be able to confirm this aspect through studies with a greater number of patients.
Multivisceral resection has been implicated with an increase in postoperative complications. In the literature, 16.4% of patients suffered a serious complication after surgery (Clavien-Dindo≥3), 10.5% required reoperation, and 1.8% died 30 days after surgery.25 Our data are close to those published in the international literature. In fact, in our series, the most frequent complications were those classified as Clavien-Dindo 1, occurred in 13 (26%) patients, while Clavien-Dindo≥3 were 6 (12%) patients. No deaths were recorded 30 days after surgery and reoperation due to postoperative complications was in 4 (8%) patients. We have not observed that postoperative complications influence overall survival or disease-free survival. In the survival analysis, an interesting result is given by the ASA classification. A patient with ASA≥3 has a worse prognosis in both overall and disease-free survival. From this we can deduce that the worse the patient is from a preoperative point of view, the more his survival expectation is reduced. This aspect is not considered in other international works published. No difference has been observed with respect to the preoperative ECOG classification.
Our results show a paradoxical result with respect to the results presented in other publications, where it has been seen that tumor grade is an independent predictor of postoperative survival time. Specifically, a better prognosis is described in patients with low-grade tumors compared to the group with high-grade tumors.1,22 In our series, low-grade tumors show better overall survival compared to high-grade tumors, and surprisingly, this trend is reversed, and high-grade tumors have better disease-free survival. This aspect, clearly in contrast to the international literature,26 can find its explanation in that the sample presented is relatively small and not very consistent and that the surgery could have been incomplete due to the impossibility of differentiating pathological tissue from healthy tissue. When the pathological residue is not identified in the first evolutionary imaging tests until its growth makes it evident early in time. These are the reasons that lead to the enactment of compartmental or multivisceral surgery.27
Although in the work of Singer et al.8 it is shown that the histological subtype of liposarcoma represents an independent predictor of postoperative survival time, our data does not allow us to draw the same type of conclusions. The most frequent histological subtype of the sample was well differentiated liposarcoma and was compared with the rest of the histological subtypes and there were no statistically significant differences in terms of survival. The retroperitoneal space allows these tumors to grow excessively before presenting clinical symptoms. This phenomenon was also observed in our sample, where 23 (46%) patients presented a tumor with a diameter ≥20cm at the time of diagnosis. In the international literature there is controversy regarding this variable. Some author states that larger tumors could have a worse prognosis, because large dimensions could make complete resections difficult and cause residual microscopic residual disease.28,29 However, others have contradicted these findings by showing that there is no significant correlation between tumor size and survival prognosis.30,31 In the analysis of our data, tumor size has not shown a statistically significant difference with respect to overall survival (p=0.08) and disease free survival (p=0.88). Makelä et al.31 have suggested that instead of size, the inaccessible and deep location of retroperitoneal liposarcomas may influence postoperative survival time. We have not found significant differences regarding the location of the tumors, classified in right hemi-abdomen, left hemi-abdomen and pelvis. Although the study by Lou et al. demonstrated a significant association between laterality, tumor histology and resection of contiguous organs, in our study the laterality of the tumor does not appear to be related to survival.32
The characteristics of the territory in which this work has been carried out make the hospital a reference center, although it does not manage to collect a large number of cases. It is important to contextualize observed results because this is a monocentric study with a limited number of patients. The low incidence and prevalence of these cancers and the complexity of the treatment make it necessary to centralize this pathology in large-volume centers with multidisciplinary experience in order to obtain the best results in terms of survival.33,34 For this reason, we strongly believe in the need to facilitate multi-institutional collaborations is essential to improve understanding of these neoplasms and advance in their management.26,35
Finally, this work has important limitations. Mainly the different surgical teams that have treated the patients presented, the different perioperative and postoperative management and the different surgical indications. All these aspects have their reason in the temporal extension of the series.
In conclusion, in our study compartment surgery has not shown benefits in terms of overall survival and disease-free survival. The ASA classification (≥3) predicts a poor prognosis in both overall survival and disease-free survival. Resection with free margins, described on the pathological results and defined in this work as R0, show better disease-free survival. For this reason, it is appropriate to deduce that complete resection remains the most effective method of treating liposarcoma.
Authors’ contributionAPP and AB were the main surgeons. JAMC, AOS and MJS helped in literature search and maintained database. PLF followed the evolution of patients in the outpatient clinic. RRA performed the pathological studies of all the patients analyzed. NPC, APP and AB planned the study and performed the statistical study. AB wrote the article that was corrected by XFGA and PLF. All authors read and approved the final manuscript.
Compliance with ethical standardsAll the phases of the work have been carried out with the approval of the ethics committee and under the edge of a written consensus.
FundingNo funding sources.
Conflict of interestNone declared.