The number of lung metastases (M1) of colorectal carcinoma (CRC) in relation to the findings of computed tomography (CT) is the object of study.
MethodsProspective and multicenter study of the Spanish Group for Surgery of CRC lung metastases (GECMP-CCR). The role of CT in the detection of pulmonary M1 is evaluated in 522 patients who underwent a pulmonary metastasectomy for CRC. We define M1/CT as the ratio between metastatic nodules and those found on preoperative CT. Disease-specific survival (DSS), disease-free survival (DFS), and surgical approach were analyzed using the Kaplan–Meier method.
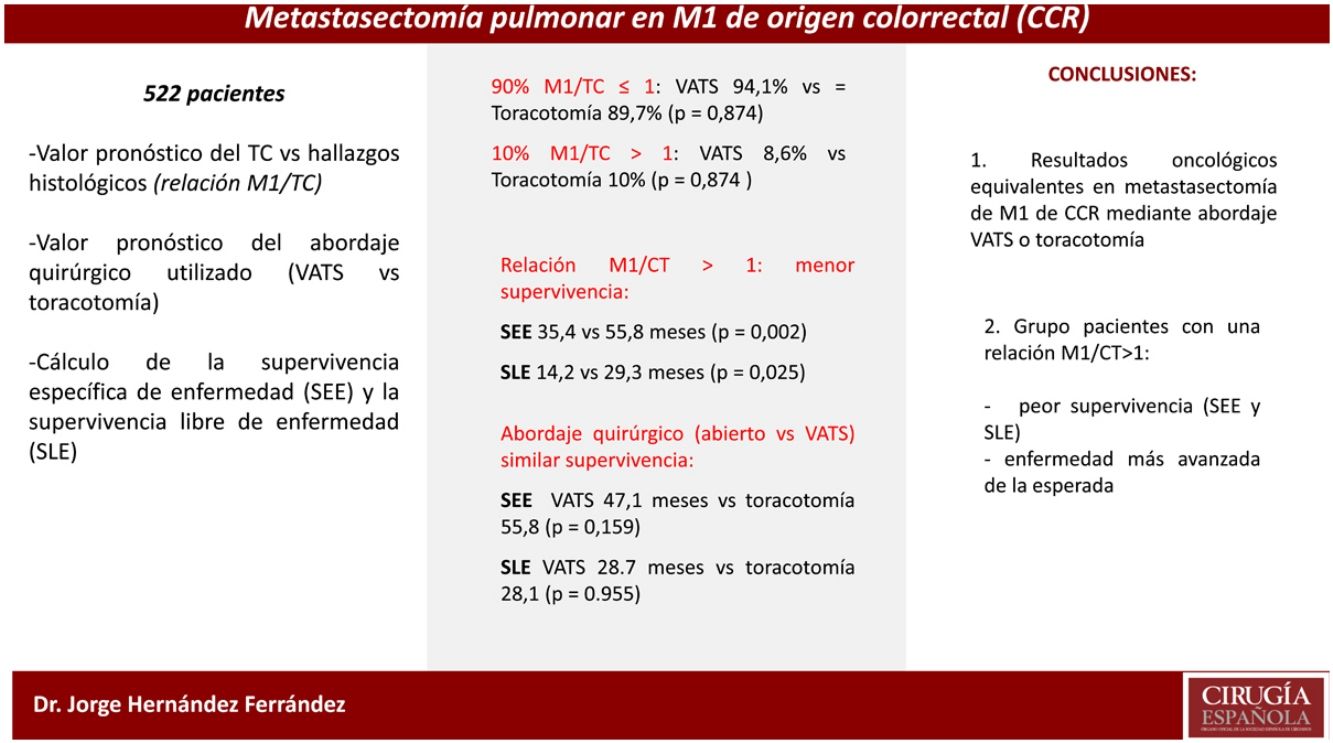
Results93 patients were performed by video-assisted surgery (VATS) and 429 by thoracotomy. In 90%, the M1/CT ratio was ≤1, with no differences between VATS and thoracotomy (94.1% vs 89.7%, p=0.874). In the remaining 10% there were more M1s than those predicted by CT (M1/CT>1), with no differences between approaches (8.6% vs 10%, p=0.874). 51 patients with M1/CT>1, showed a lower median DSS (35.4 months vs 55.8; p=0.002) and DFS (14.2 months vs 29.3; p=0.025) compared to 470 with M1/CT≤1. No differences were observed in DSS and DFS according to VATS or thoracotomy.
ConclusionsOur study shows equivalent oncological results in the resection of M1 of CRC using VATS or thoracotomy approach. The group of patients with an M1/CT ratio >1 have a worse DSS and DFS, which may mean a more advanced disease than predicted preoperatively.
El número de metástasis pulmonares (M1) de carcinoma colorrectal (CCR) en relación con los hallazgos de la tomografía computarizada (TC), es objeto de estudio.
MétodosEstudio prospectivo y multicéntrico del Grupo Español de Cirugía de las metástasis pulmonares del CCR (GCMP-CCR). Se evalúa el papel de la TC en la detección de M1 pulmonares en 522 pacientes intervenidos de una metastasectomía pulmonar por CCR. Definimos como M1/CT al cociente entre los nódulos metastásicos y los hallados en la TC preoperatoria. Se analizó la supervivencia específica de enfermedad (SEE), la supervivencia libre de enfermedad (SLE) y el abordaje quirúrgico mediante el método de Kaplan-Meier.
ResultadosEn 93 pacientes se utilizó la cirugía videoasistida (VATS) y 429 toracotomías. En un 90% el cociente M1/TC fue ≤1, sin diferencias entre VATS y toracotomía (94,1 vs. 89,7%; p=0,874). En el 10% restante existían más M1 que las predichas por la TC (M1/CT>1), sin diferencias entre abordajes (8,6 vs. 10%; p=0,874). Cincuenta y un pacientes con M1/CT>1, mostraron una menor mediana de SEE (35,4 vs. 55,8 meses; p=0,002) y SLE (14,2 vs. 29,3 meses; p=0,025) en comparación con 470 con M1/CT≤1. No se observaron diferencias en la SEE y la SLE según VATS o toracotomía.
ConclusionesNuestro estudio muestra unos resultados oncológicos equivalentes en la resección de M1 de CCR mediante abordaje VATS o toracotomía. El grupo de pacientes con un cociente M1/CT>1 presentan una peor SEE y SLE, pudiendo significar una enfermedad más avanzada de la predicha preoperatoriamente.
Distant metastasis, especially located in liver and lung, are nearly the most important prognostic factor for colorectal cancer (CRC). It is estimated that between 10% and 20% of patients with CRC will develop pulmonary metastasis. Pulmonary metastasectomy can only be performed in a small group of patients, due to the high incidence of extrathoracic disease at the moment of the diagnosis, or the significant pulmonary involvement.1,2 Whenever feasible, surgery is considered an effective therapeutic option, with reported 5-year overall survival rates after radical lung resection, ranging from 41% to 68%.3,4 In properly selected patients, pulmonary metastasectomy has become widely accepted as a potential curative option for lung metastasis from CRC.5,6
Open thoracotomy and video-assisted thoracic surgery (VATS) are both accepted techniques to perform pulmonary metastasectomy, but, since few years ago, VATS has gained popularity, and right now, numerous surgeons chose VATS as the first surgical approach.7–9 A survey done to members of the European Society of Thoracic Surgeons (ESTS) reported; 60% of surgeons considered VATS an acceptable surgical approach for selected cases. 40% of them considered this approach the first choice for unilateral disease.10 Although VATS approach seems to be accepted by most thoracic surgeons, there are not prospective randomized studies comparing VATS with the standard thoracotomy for CRC lung metastases resection. The crux lies on the undetected M1 by preoperative chest CT, which could be lost during VATS procedures, compared with pulmonary nodules that might be found by careful manual palpation.11
A prospective multicenter study was designed to assess the prognostic value of preoperative CT imaging compared to histological findings, regarding surgical approach comparing VATS versus conventional thoracotomy.
Material and methodsStudy designThe prospective multicenter registry of the Spanish Group for Colorectal Carcinoma Lung Metastases Surgery (GECMP-CCR) was established by The Spanish Society of Pneumology and Thoracic Surgery (SEPAR) in January 2008. All patients with one or more histologically proven and surgery removed of CRC lung metastasis between March 2008 and February 2010 by the Thoracic Services of 32 public hospitals were included in the database. The purpose of the GECMP-CCR-SEPAR registry was to obtain nationwide high-quality data in a short and defined period of time, in order to document the current practice of the Spanish health care system, regarding at least the 60% of all patients with this pathology controlled in Spain. Details of the GECMP-CCR-SEPAR database were previously described.12
PatientsAvailable data corresponded to 543 patients undergoing their first CRC pulmonary metastasectomy during two consecutive years (March 2008–March 2010) with a follow-up period completed in March 2013, ensuring a minimum three year follow-up. Lung resection must be carried out with radical intention, that is, without leaving apparent macroscopic disease, and at least one excised lesion must be consistent with histologically confirmed CRC metastasis. The study was approved by the Ethics Committee of each participating hospitals as well as the regional Ethics Committee for Scientific and Medical Research of the different Autonomous Communities when necessary. Written informed consent was obtained from all patients.
Because of the study purpose, patients were divided in two groups according to the surgical approach; VATS surgery or conventional open thoracotomy. VATS was defined by the non-use of rib separation by using a rib retractor, making manual pulmonary palpation impossible by introducing one or two hands in full. The selection of the approach was decided by the primary surgeon based on his own criteria and experience.
Data collectionThe following variables were taken into account; age, sex, disease-free interval (DFI) (defined as; time elapsed from the last disease episode, regardless of location, until the date of pulmonary metastasis diagnosis); preoperative data including serum carcinoembryonic antigen (CEA) level, CT findings (number and nodules size as well as bilateral lesions), lymph node status according to CT, and when done, positron emission tomography (PET) and PET/CT findings were also recorded, surgical approach (VATS, open surgery), type and extend of surgical resection, postoperative data including; number of lung removed nodules, size of metastatic nodules, histologic lymph node involvement, other structures involvement, postoperative morbidity, postoperative mortality, adjuvant treatment, local lung recurrence and recurrent metastatic disease after the first pulmonary metastasectomy.
M1/CT ratio corresponded to the quotient between the actual number of metastatic lung nodes identified by histopathologic report after surgery and the number of nodes identified by preoperative CT scan. The M1/CT ratio was defined as ≤ 1 or >1.
Statistical analysisSurvival analysis was carried out from the first pulmonary metastasectomy date. In bilateral disease cases requiring bilateral procedures, the date of the second surgery was considered. Such survival analysis was specific for disease [disease-specific survival (DSS)], excluding patients who died in early postoperative period (first 30 days or during hospitalization), and considered censored those who died from other causes different than their cancer disease. Disease-free survival (DFS) was defined as the time elapsed from the first pulmonary metastasectomy until the first metastatic lesion registration date. Patients who were lost to follow-up were also censored, recording the time until the last date of known follow-up period.
The relationship between the clinical and oncological variables of the CRC episode and the type of surgical approach was analyzed by bivariate analysis, using the chi-square test or the Fisher's exact test for the comparison of categorical variables, and the Student's T test or the Mann–Whitney U test for the comparison of continuous variables. DSS and DFS were assessed with the Kaplan–Meier method and differences between curves were analyzed with the log-rank test. Factors independently associated with DSS and DFS were assessed using a Cox regression model in which all variables with a p value of <0.2 in the bivariate analysis were included. All hypothesis tests were bilateral and statistical significance was set at p<0.05. Analyses were carried out using the Statistical Package for the Social Sciences (SPSS) version 18.0 for Windows.
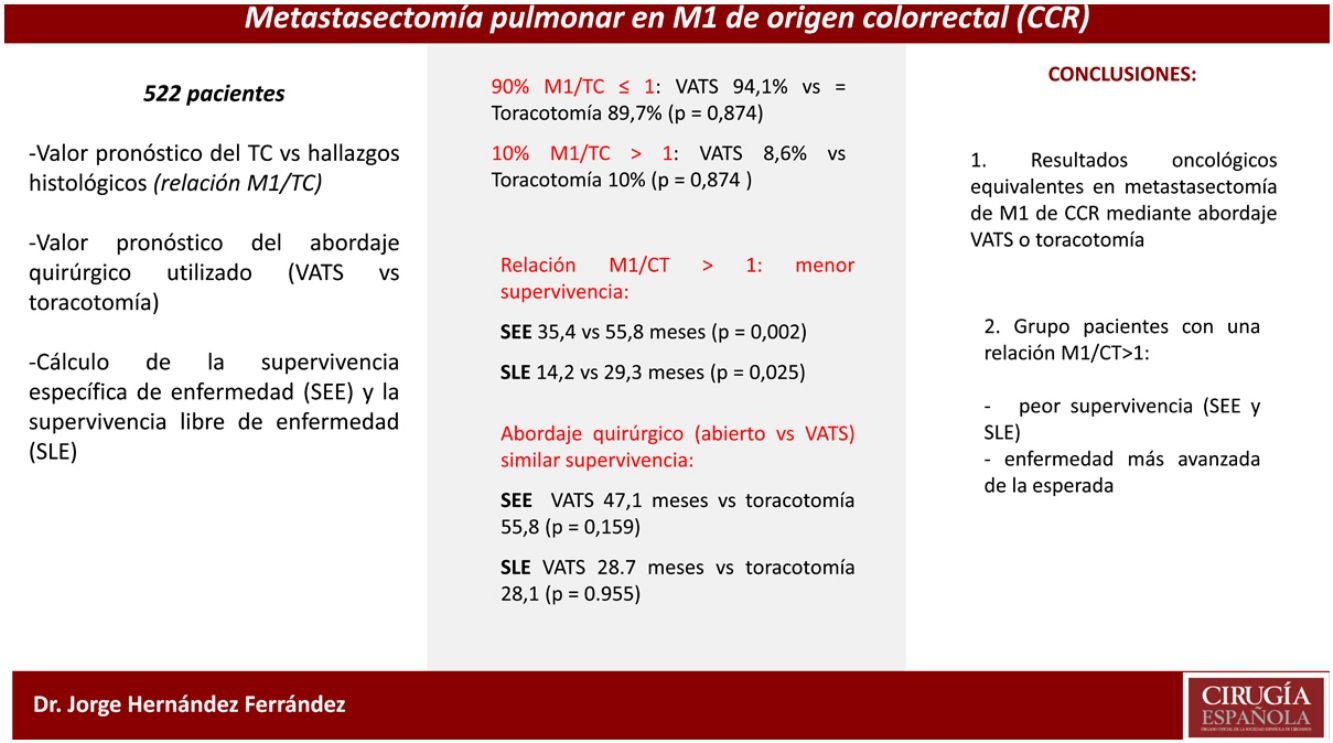
ResultsFrom the 543 initially included patients, 21 were excluded due to different reasons, including duplicate patient's information in 1 patient, lack of data on pulmonary metastasectomy in 7, and metastasectomy performed before March 1, 2008 in 8. Therefore, the study population consisted of 522 patients, 335 men (64.2%), with a mean (standard deviation, SD) age of 64.7 (10.2) years old. Surgery procedures were carried out by VATS in 93 (17.8%) patients and by open thoracotomy in 429 (82.2%). VATS procedure was not performed in 11 (34.4%) of the 32 participating hospitals. Resections classification according to the surgical approach is shown in Table 1. Major resection frequency was significantly different, being much more frequent in the thoracotomy group than in the VATS group (22.8% vs. 6.5%; p<0.001).
Type of resection and surgical approach.
| Pulmonary metastasectomy | VATSNo. (%) | Open surgeryNo. (%) | TotalNo. (%) |
|---|---|---|---|
| Number of patients | 93 | 429 | 522 |
| Type of resection (p<0.001) | |||
| Pneumonectomy | 0 | 4 (0.9) | 4 (0.8) |
| Lobectomy | 6 (6.5) | 94 (22.1) | 100 (19.3) |
| Anatomical segmentectomy | 1 (1.1) | 18 (4.2) | 19 (3.7) |
| Wedge | 85 (92.4) | 309 (72.7) | 394 (76.2) |
| Total | 92 | 425 | 517a |
| Extended resection (p>0.99) | |||
| No | 92 (98.9) | 123 (98.6) | 394 (76.2) |
| Yes | 1 (1.1) | 6 (1.4) | 7 (1.3) |
| Total | 93 | 429 | 522 |
| Major resection (p<0.001) | |||
| No | 87 (93.5) | 331 (77.2) | 418 (80.1) |
| Yes | 6 (6.5) | 98 (22.8) | 104 (19.9) |
| Total | 93 | 429 | 522 |
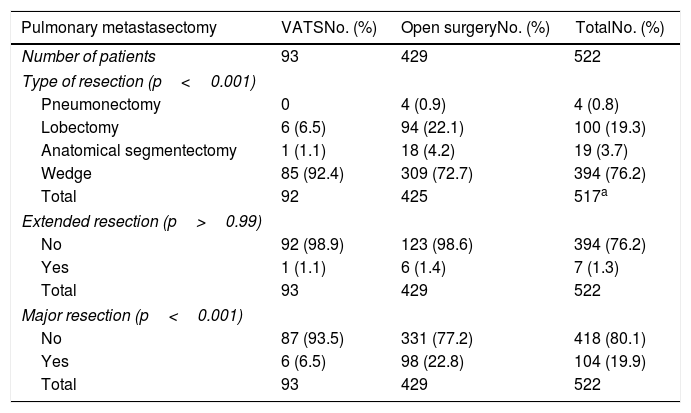
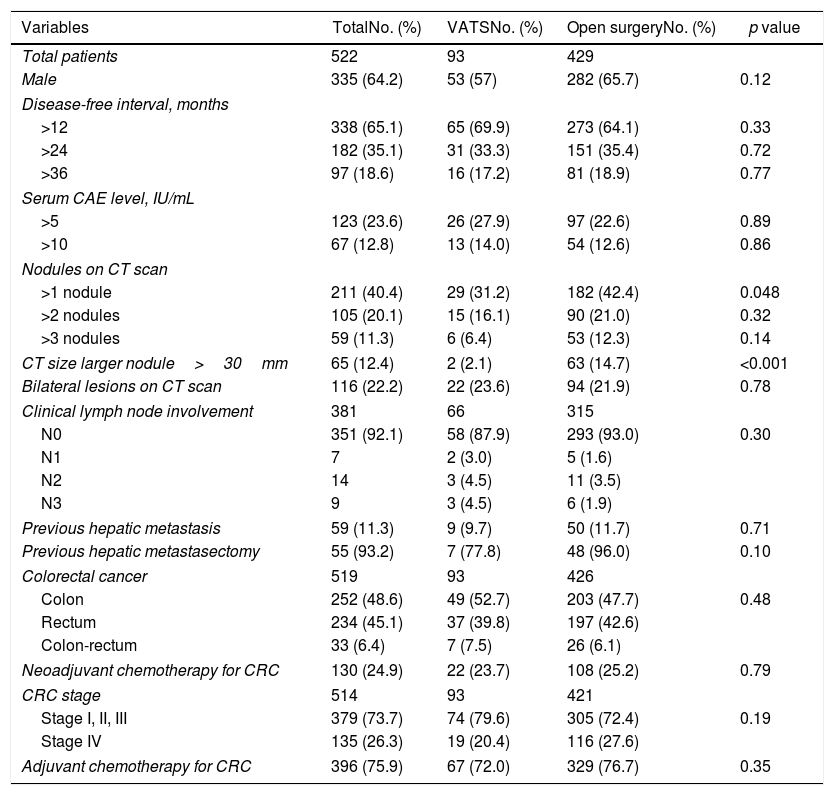
Results of bivariate analysis of preoperative variables are shown in Table 2. There were statistically significant differences between VATS and open surgery groups, when >1 nodule was found on the CT scan (p=0.048) and when the size of the largest nodule on CT scan was >30mm (p<0.001), with higher percentages in open surgery group. Regarding postoperative variables (Table 3), significant differences were found; percentage of nodules >10mm (p=0.015), mean number of lymph nodes removed (p=0.022) and lymph node involvement (N0, N1, N2, and NX) (p<0.001). All the previous differences were found with a higher percentage in the open surgery group when compared with VATS. Although the postoperative morbidity was significantly lower in the VATS group (5.4%) than in the open surgery group (17.9%) (p=0.002), the percentage of patients with pulmonary recurrence and metastatic disease was similar in both groups (pulmonary recurrence 32.7% vs. 34.2%, p=0.810; metastatic disease 71.4% vs. 67.5%, p=0.730). The postoperative mortality rate was 0.4%, corresponding to two metastasectomies by open approach because of ventricular fibrillation in one patient and sepsis in the other patient.
Bivariate analysis of preoperative variables.
| Variables | TotalNo. (%) | VATSNo. (%) | Open surgeryNo. (%) | p value |
|---|---|---|---|---|
| Total patients | 522 | 93 | 429 | |
| Male | 335 (64.2) | 53 (57) | 282 (65.7) | 0.12 |
| Disease-free interval, months | ||||
| >12 | 338 (65.1) | 65 (69.9) | 273 (64.1) | 0.33 |
| >24 | 182 (35.1) | 31 (33.3) | 151 (35.4) | 0.72 |
| >36 | 97 (18.6) | 16 (17.2) | 81 (18.9) | 0.77 |
| Serum CAE level, IU/mL | ||||
| >5 | 123 (23.6) | 26 (27.9) | 97 (22.6) | 0.89 |
| >10 | 67 (12.8) | 13 (14.0) | 54 (12.6) | 0.86 |
| Nodules on CT scan | ||||
| >1 nodule | 211 (40.4) | 29 (31.2) | 182 (42.4) | 0.048 |
| >2 nodules | 105 (20.1) | 15 (16.1) | 90 (21.0) | 0.32 |
| >3 nodules | 59 (11.3) | 6 (6.4) | 53 (12.3) | 0.14 |
| CT size larger nodule>30mm | 65 (12.4) | 2 (2.1) | 63 (14.7) | <0.001 |
| Bilateral lesions on CT scan | 116 (22.2) | 22 (23.6) | 94 (21.9) | 0.78 |
| Clinical lymph node involvement | 381 | 66 | 315 | |
| N0 | 351 (92.1) | 58 (87.9) | 293 (93.0) | 0.30 |
| N1 | 7 | 2 (3.0) | 5 (1.6) | |
| N2 | 14 | 3 (4.5) | 11 (3.5) | |
| N3 | 9 | 3 (4.5) | 6 (1.9) | |
| Previous hepatic metastasis | 59 (11.3) | 9 (9.7) | 50 (11.7) | 0.71 |
| Previous hepatic metastasectomy | 55 (93.2) | 7 (77.8) | 48 (96.0) | 0.10 |
| Colorectal cancer | 519 | 93 | 426 | |
| Colon | 252 (48.6) | 49 (52.7) | 203 (47.7) | 0.48 |
| Rectum | 234 (45.1) | 37 (39.8) | 197 (42.6) | |
| Colon-rectum | 33 (6.4) | 7 (7.5) | 26 (6.1) | |
| Neoadjuvant chemotherapy for CRC | 130 (24.9) | 22 (23.7) | 108 (25.2) | 0.79 |
| CRC stage | 514 | 93 | 421 | |
| Stage I, II, III | 379 (73.7) | 74 (79.6) | 305 (72.4) | 0.19 |
| Stage IV | 135 (26.3) | 19 (20.4) | 116 (27.6) | |
| Adjuvant chemotherapy for CRC | 396 (75.9) | 67 (72.0) | 329 (76.7) | 0.35 |
CAE: carcinoembryonic antigen; CRC: colorectal cancer.
Bivariate analysis of postoperative variables.
| Variables | TotalNo. (%) | VATSNo. (%) | Open surgeryNo. (%) | p value |
|---|---|---|---|---|
| Total patients | 522 | 93 | 429 | |
| Bilateral surgical approach | 81 (15.5) | 19 (20.4) | 62 (14.5) | 0.15 |
| Extended resections | 7 (1.3) | 1 (1.1) | 6 (1.4) | 0.99 |
| Number of metastasis | ||||
| >1 | 179 (34.3) | 29 (31.2) | 150 (35.0) | 0.54 |
| >2 | 83 (15.9) | 14 (15.1) | 69 (16.1) | 0.87 |
| >3 | 42 (8.0) | 5 (5.4) | 37 (8.6) | 0.4 |
| Larger size histological nodules | ||||
| >10mm | 359 (70.9) | 54 (58.0) | 305 (73.3) | 0.015 |
| >30mm | 70 (13.8) | 7 (7.8) | 63 (15.1) | 0.09 |
| Histological lymph node involvement | ||||
| N0 | 119 (22.8) | 3 (3.2) | 116 (27.0) | <0.001 |
| N1 | 8 (1.5) | 0 | 8 (1.9) | |
| N2 | 18 (3.4) | 2 (2.1) | 16 (3.7) | |
| Nx | 374 (71.6) | 87 (93.5) | 287 (66.9) | |
| N1–2–3 | 26 (5.0) | 2 (2.1) | 24 (5.6) | |
| Lymph nodes removed, mean | 5.5 | 2.9 | 5.6 | 0.022 |
| Involvement of other structures | 27 (5.2) | 6 (6.4) | 21 (4.9) | 0.6 |
| Postoperative morbidity | 82 (15.7) | 5 (5.4) | 77 (17.9) | 0.002 |
| Postoperative mortality | 2 (0.4) | 0 | 2 (0.5) | >0.9 |
| Adjuvant chemotherapy after pulmonary metastasectomy | 321(61.5) | 56 (60.2) | 265 (61.8) | 0.81 |
Data expressed as frequencies and percentages in parenthesis unless otherwise stated.
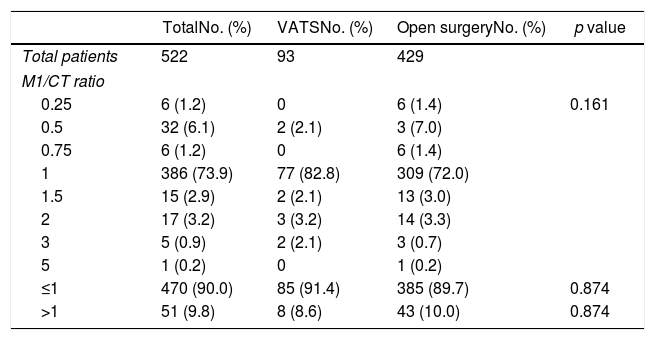
The different values of the M1/CT ratio were similar (Table 4) regardless the surgical approach, either VATS or thoracotomy. CT and histological findings (M1/CT ratio≤1) agreed in 90% of cases, without differences between VATS and open thoracotomy (94.1% vs. 89.7%). For the remaining 10% disagreement cases (M1/CT ratio> 1), differences between VATS and open thoracotomy were either not found (8.6% vs. 10%) (Table 4).
Percentages of patients undergoing pulmonary metastasectomy with VATS or open surgery according to results of the M1/CT ratio.
| TotalNo. (%) | VATSNo. (%) | Open surgeryNo. (%) | p value | |
|---|---|---|---|---|
| Total patients | 522 | 93 | 429 | |
| M1/CT ratio | ||||
| 0.25 | 6 (1.2) | 0 | 6 (1.4) | 0.161 |
| 0.5 | 32 (6.1) | 2 (2.1) | 3 (7.0) | |
| 0.75 | 6 (1.2) | 0 | 6 (1.4) | |
| 1 | 386 (73.9) | 77 (82.8) | 309 (72.0) | |
| 1.5 | 15 (2.9) | 2 (2.1) | 13 (3.0) | |
| 2 | 17 (3.2) | 3 (3.2) | 14 (3.3) | |
| 3 | 5 (0.9) | 2 (2.1) | 3 (0.7) | |
| 5 | 1 (0.2) | 0 | 1 (0.2) | |
| ≤1 | 470 (90.0) | 85 (91.4) | 385 (89.7) | 0.874 |
| >1 | 51 (9.8) | 8 (8.6) | 43 (10.0) | 0.874 |
M1/CT ratio: quotient between the actual number of metastatic lymph nodes identified by histopathologic examination of the surgical specimen and the number identified by preoperative CT scan.
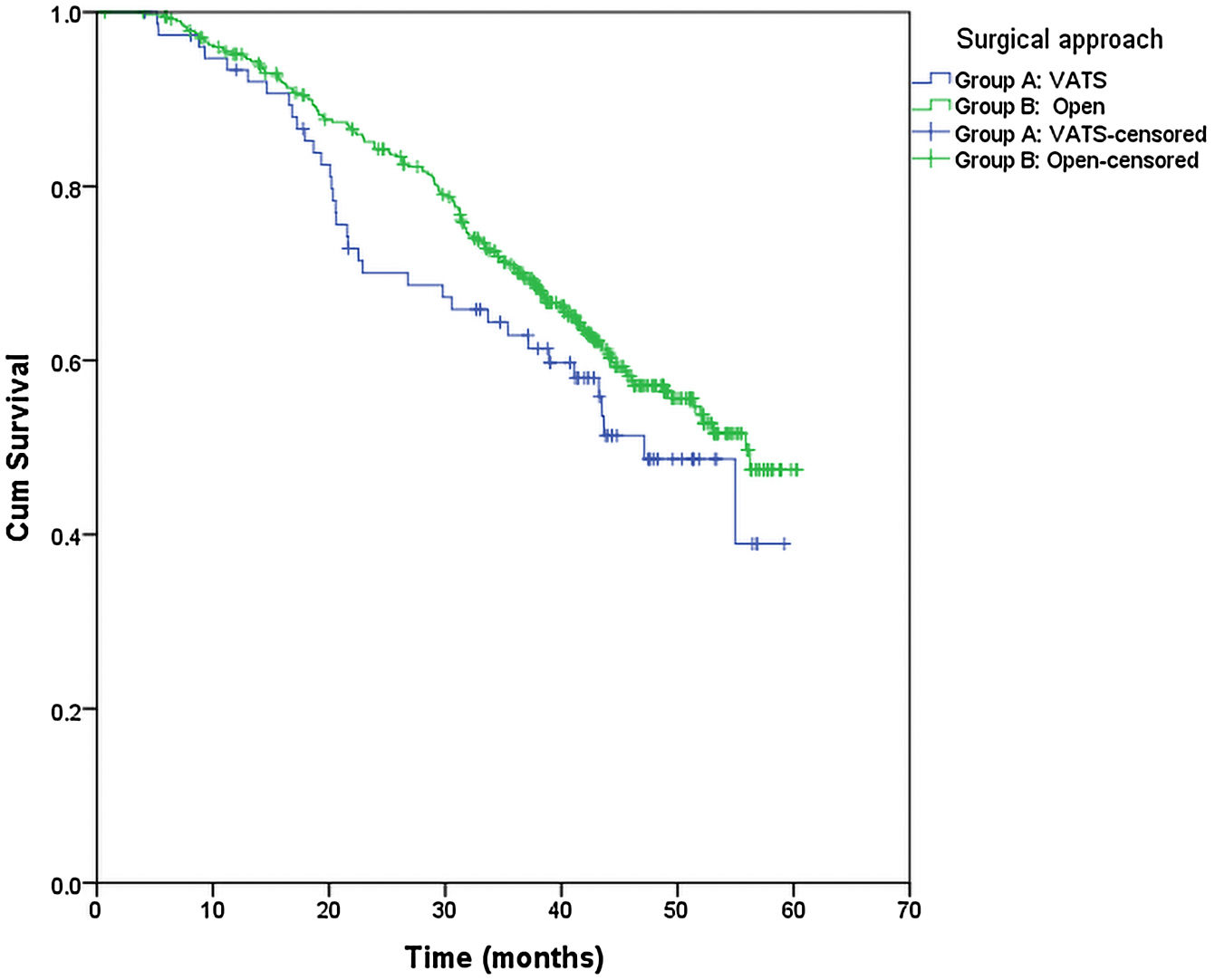
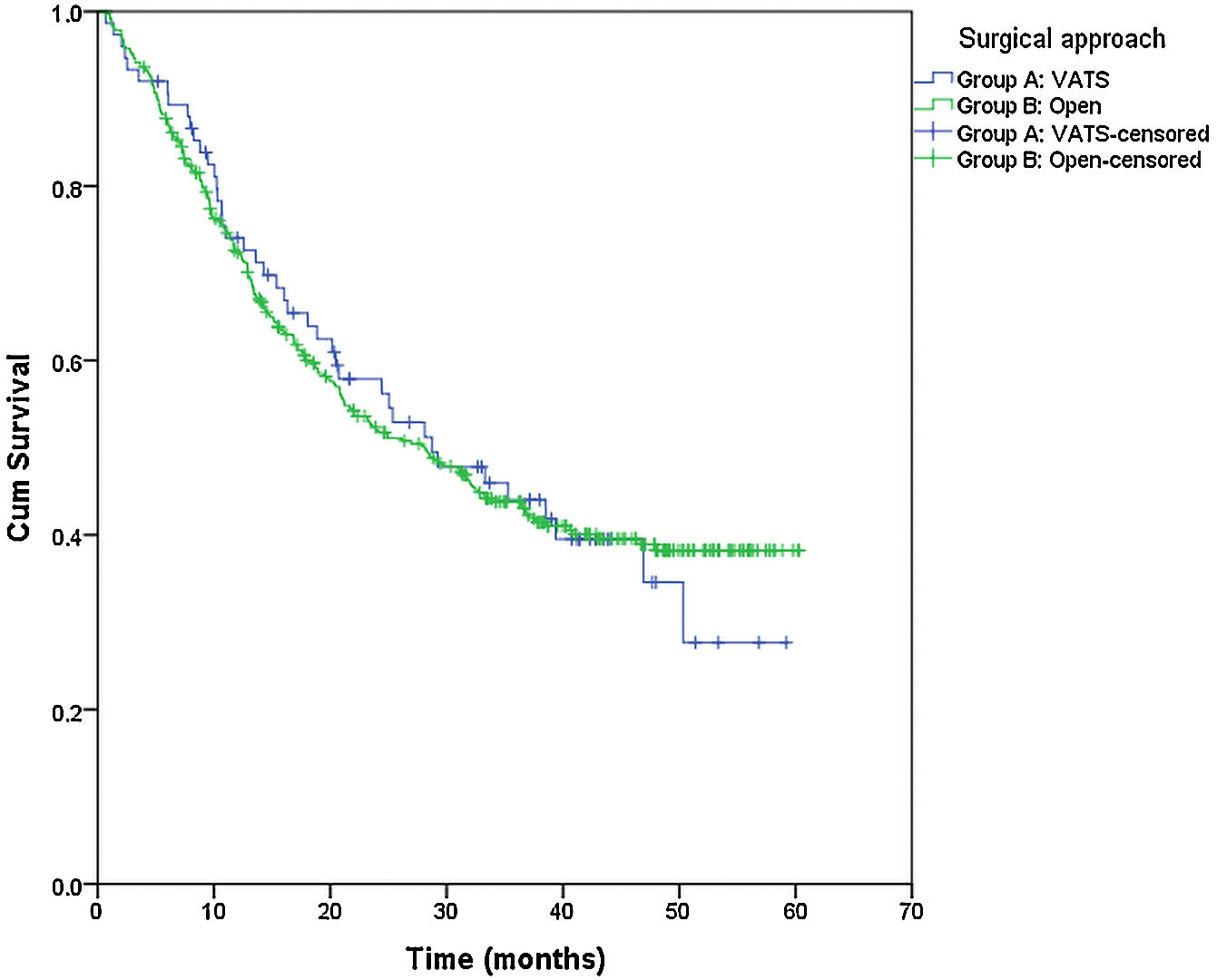
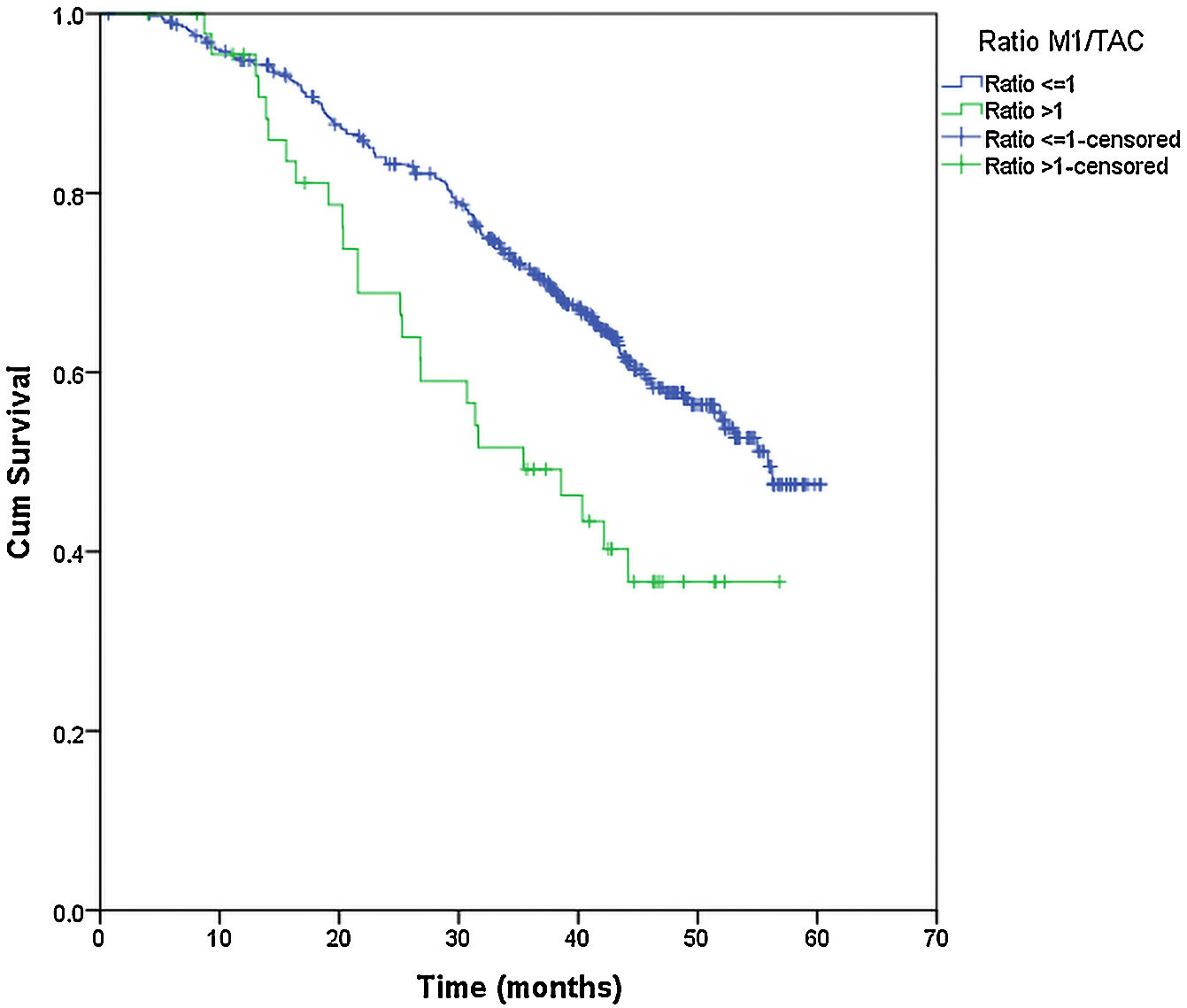
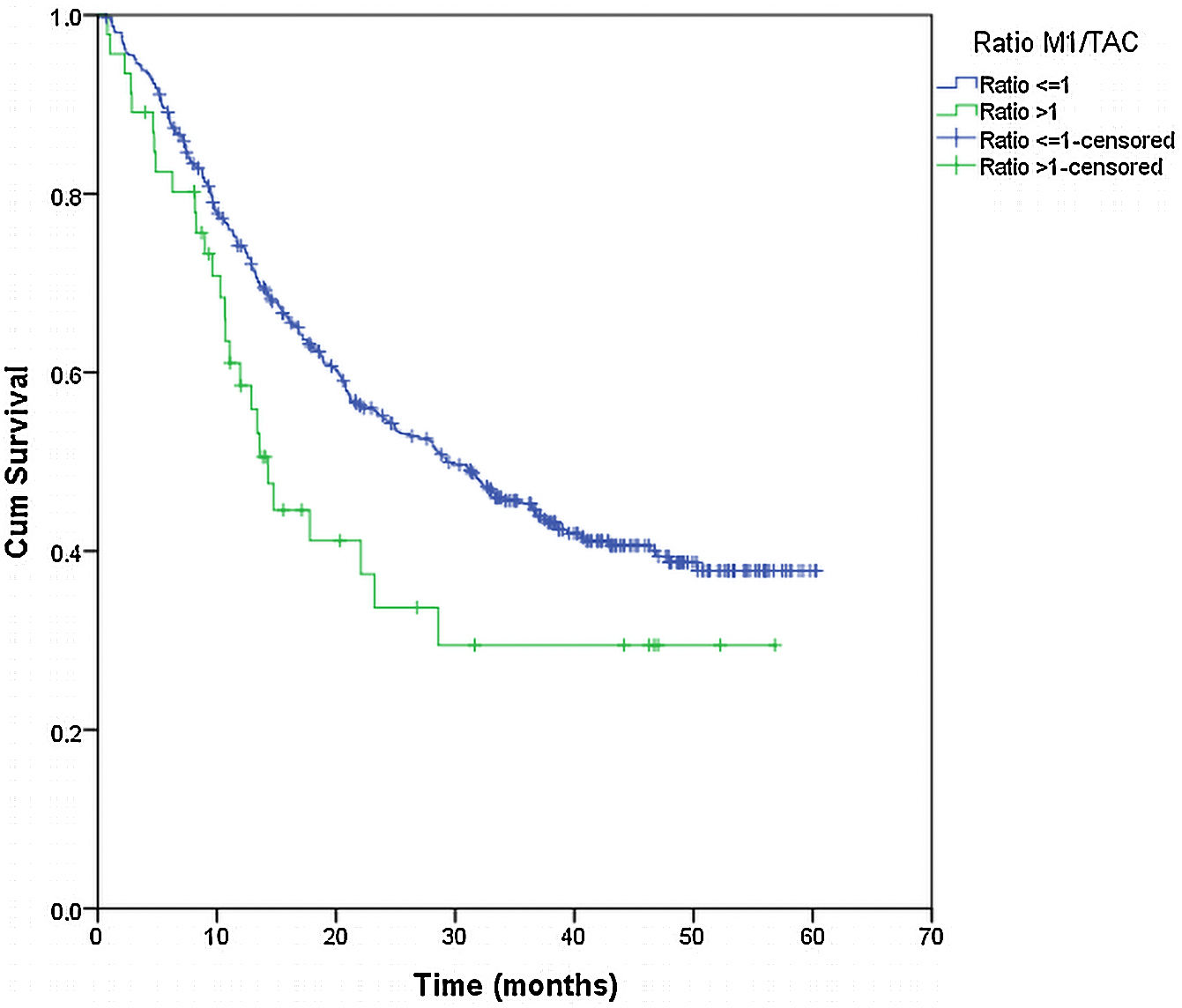
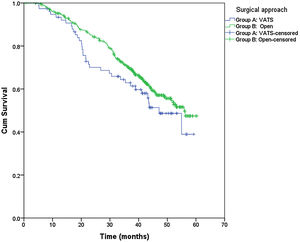
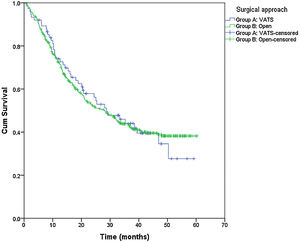
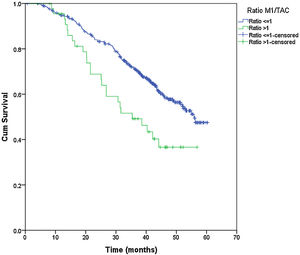
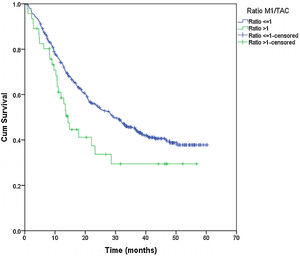
From the overall study population (522 patients), 2 patients died after surgery and 64 were lost to follow-up; therefore, a total of 456 patients were included for the survival analysis. The median follow-up was 38.7 months (range 0.7–60.3 months). The median overall survival was 55 months. The surgical approach (VAT vs. open surgery) did no effect the outcome. The median DSS was 47.1 months (95% confidence interval [CI] 38.2–56.0) for VATS and 55.8 months (95% CI 18.0–39.4) for open surgery (p=0.159) (Fig. 1). The median DFS was 28.7 months (95% CI 18.0–39.4) for VATS and 28.1 months (95% CI 22.2–34.0) for open surgery (p=0.955) (Fig. 2). However, differences in survival according to the M1/CT ratio value for both approaches were observed, with a median DSS of 55.8 months (95% CI unknown) for patients with M1/CT≤1 compared to 35.4 months (95% CI 24.1–46.6) for those with M1/CT>1 (p=0.002) (Fig. 3). The median DFS was 29.3 months (95% CI 22.9–35.7) for the group of patients with M1/CT≤1 compared to 14.2 months (95% CI 11.7–16.8) for the group of patients with M1/CT>1 (p=0.025) (Fig. 4).
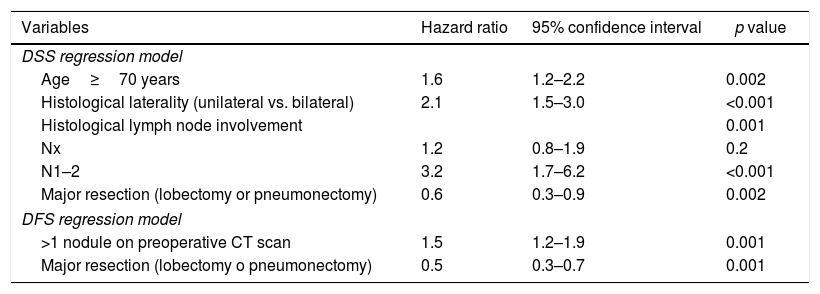
In the multivariate analysis, major resection appeared to be a protective factor in DSS (hazard ratio [HR] 0.6, 95% CI 0.3–09, p=0.002) and DFS (HR 0.5, 95% CI 0.3–0.8, p=0.001), whereas age of 70 years old or more, bilateral histological involvement, and histological lymph node involvement were independent predictors of DSS. More than 1 nodule reported by the preoperative CT was a significant predictor of DFS (Table 5).
Cox regression analysis of disease-specific survival (DSS) and disease-free survival (DFS).
| Variables | Hazard ratio | 95% confidence interval | p value |
|---|---|---|---|
| DSS regression model | |||
| Age≥70 years | 1.6 | 1.2–2.2 | 0.002 |
| Histological laterality (unilateral vs. bilateral) | 2.1 | 1.5–3.0 | <0.001 |
| Histological lymph node involvement | 0.001 | ||
| Nx | 1.2 | 0.8–1.9 | 0.2 |
| N1–2 | 3.2 | 1.7–6.2 | <0.001 |
| Major resection (lobectomy or pneumonectomy) | 0.6 | 0.3–0.9 | 0.002 |
| DFS regression model | |||
| >1 nodule on preoperative CT scan | 1.5 | 1.2–1.9 | 0.001 |
| Major resection (lobectomy o pneumonectomy) | 0.5 | 0.3–0.7 | 0.001 |
The present study shows two main findings. On one hand, it demonstrates that CRC pulmonary metastases resection can be effectively and safely performed using either VATS or a conventional thoracotomy approach. On the other hand, it proves that open approach is not superior to VATS detecting hidden lung metastases during the preoperative radioimaging studies.
After at least 3 years’ follow-up, we did not observe survival differences between patients undergoing metastasectomy by VATS or open surgery. However, VATS was associated with a significantly lower postoperative morbidity and, in addition, no patient from the VATS group, died. These results are consistent with a previous analysis of morbidity and mortality of data collected from the GECMP-CCR-SEPAR registry, in which VATS vs. thoracotomy showed an independent protective effect (OR 0.3, 95% CI 0.1–0.8, p=0.01).13 The advantages of the minimally invasive route (including smaller and esthetic wounds, less blood loss, minor postoperative pain, lower immunosuppression consequences, fewer postoperative complications and shorted hospital stay), as compared with more invasive approaches, have been highlighted in different studies.14–16 All these factors contribute to a faster recovery and prompt use of adjuvant chemotherapy.
The rate of recurrence after complete metastasectomy with bimanual palpation exceeds 50%, as exactly expected for a systemic disease. That is why, some authors suggest that open surgical resection of all palpable pulmonary nodules is not a complete biological resection of all metastatic deposits.17 However, similar long-term survival outcome for patients undergoing VATS pulmonary resection CRC lung metastases and those undergoing conventional open thoracotomy have been reported in numerous studies.2,7,15,18,19,8 In agreement with these results, we did not find differences in DSS and DFS, neither in the rate of recurrence of pulmonary disease between patients undergoing VATS or conventional thoracotomy. Although VATS is an acceptable alternative to thoracotomy for anatomic lung resection, there is still a large variability in its clinical application routine.20,21
CT is considered the gold standard to assess intrathoracic extension before performing metastasectomy, but despite technical improvements in the diagnostic accuracy of CT, the rate of non-imaged malignant pulmonary metastasis remains around 15–25%.22 Although the sensitivity of helical CT to correctly identify histologically proven metastases exceeds high-resolution CT (82% vs. 75%), preoperative radiological assessment of lung lesions smaller than 6mm is still limited.23 Some studies on the detection of lung metastases have shown correlation between radiological findings and resected lesions in the surgical specimen. Kang et al.24 reported a diagnostic accuracy of 97% for the 1-mm thin-section 16-channel multi-detector row CT (TSMDCT). Also, in highly selected groups, this technique allowed to detect the same metastatic nodules as manual palpation.24 In patients with a unilateral solitary lesion, the sensitivity of helical CT on the detection of pulmonary metastasis in patients with CRC can reach 95.5%.25 However, discrepancies grow as the nodule count increases.26 Therefore, open procedures theoretically may provide better identification and removal of occulted lung nodules compared to VATS.11,19 Complete removal of metastatic lesions should improve long-term survival since R0 lung resection is the major prognostic factor of survival after metastasectomy. Incomplete resection is a poor prognostic factor. Nonetheless, persistency of occult residual disease provides a better prognosis than incomplete excision with evidence of macroscopic disease.27 A review by Patel and DeCamp28 justified the unilateral approach since there was no evidence of improved survival after a bilateral approach.
Interestingly, disagreement in the detection of lung metastases by CT scan and histological examination (M1/CT>1) was found in only 10% of cases. Differences according to the surgical approach were not observed, but patients in the M1/CT>1 group, independent of undergoing VATS or open surgery, had a significantly worse DSS and DFS when compared with patients with consistent CT and histological findings. Therefore, the decision of the surgical approach used should be made by the surgeon responsible for the surgery based on his surgical experience in both procedures and with the premise of being able to resect the tumour “visible” in the preoperative imaging tests.
Regarding lymphadenectomy, a high percentage of patients lacked examination of lymph nodes (Nx 72%), being even significantly higher on VATS group compared to the open surgery group. In a previous analysis of the GECMP-CCR-SEPAR database,29 lymph node metastasis was detected in 10% of patients who had undergone lymphadenectomy. 5-year DSS, according to lymph node status, was 58.3% without of lymph nodes involvement vs. 24.8% when positive lymph node was confirmed, and 44% for uncertain lymph node status. Lymph node involvement increases the risk of late death and adequate lymph node resection is mandatory in all metastasectomies. The multivariate analysis of our study, shows that histological lymph node involvement and >1 nodule on preoperative CT scan are poor DSS and DFS predictors, respectively, constituting a group of patients with more disease than that detected in the preoperative study, so they should be evaluated more cautiously for a later, more strict monitoring or adjuvant treatment.
This study has several limitations to consider. First, the subjectivity of the radiologist in the number and characterization of suspicious nodules observed in the preoperative CT. On the other hand, this study began to collect patients in 2008. During these 12 years the use of the videothoracoscopic approach has been increasing and it seems safe to affirm that the number of pulmonary metastasectomies currently performed using VATS will exceed 17.8% of our study.
ConclusionsAccording to the results of our study, we can conclude that the surgical approach used is not related to an increase in the radiopathological discrepancy between the resected and expected pulmonary M1s, according to the preoperative study by thoracic CT, nor is it related with a variation in survival (DSS or DFS). Only in a small percentage of patients (10%) will we find a discrepancy with more pulmonary M1s than expected. However, in this group of patients, it is worth noting the worsening in both survivals. This worsening could suggest the existence of more tumor disease than initially expected, so it would seem reasonable to assess the possibility of closer postoperative monitoring or to assess its candidacy for adjuvant treatment. It would be advisable to carry out future studies aimed at clarifying the management of this specific group of patients.
FundingThe GECMP-CCR-SEPAR database was founded by Ethicon Endosurgery. This research has not received specific aid from agencies of the public sector, commercial sector or non-profit entities.
Conflicts of interestNone.
The authors thank María José Sánchez for help in the retrieval of references and Marta Pulido, MD, for editing the manuscript and editorial assistance.
Coordinators: Juan J. Rivas (Hospital Universitario Miguel Servet, Zaragoza) and Laureano Molins (Hospital Universitari del Sagrat Cor, Barcelona); Secretary: Raúl Embún (Hospital Universitario Miguel Servet, Zaragoza); Local Heads and Departments: Francisco Rivas (Hospital Universitari del Bellvitge, L’Hospitalet de Llobregat, Barcelona); Raúl Embún (Hospital Universitario Miguel Servet, Zaragoza); Jorge Hernández and José Manuel Mier (Hospital Universitari del Sagrat Cort, Barcelona); Félix Heras (Hospital General Universitario de Valladolid, Valladolid); Javier de la Cruz (Hospital Universitario Virgen del Rocío, Sevilla); Matilde Rubio (Hospital Universitari Josep Trueta, Girona); Esther Fernández (Hospital Universitari Germans Trias i Pujol, Badalona, Barcelona); Miguel Carbajo (Hospital Universitario Marqués de Valdecilla, Santander); Rafael Peñalver (Hospital Universitario Gregorio Marañón, Madrid); José Ramón Jarabo (Hospital Clínico San Carlos, Madrid); Diego González-Rivas (Complejo Hospitalario Universitario A Coruña, A Coruña);Sergio Bolufer (Hospital General Universitario de Alicante, Alicante); Carlos Pagés (Hospital General Universitario Carlos Haya, Málaga); Sergi Call (Hospital Universitari Mútua Terrassa, Terrassa, Barcelona); David Smith (Hospital Italiano, Buenos Aires, Argentina); Richard Wins (Hospital Clínico Universitario de Valencia, Valencia); Antonio Arnau (Hospital General Universitario de Valencia, Valencia); Andrés Arroyo (Hospital Universitario Virgen de la Arrixaca, Murcia); M. Carmen Marrón (Hospital Universitario 12 de Octubre, Madrid); Akiko Tamura (Clínica Universitaria de Navarra, Pamplona); Montse Blanco (Complejo Hospitalario Universitario de Vigo, Pontevedra); Beatriz de Olaiz (Hospital Universitario de Getafe, Getafe, Madrid); Gemma Muñoz (Hospital Universitario Ramón y Cajal, Madrid); José M. García Prim (Complejo Hospitalario Universitario de Santiago, Santiago de Compostela); Carlos Rombolá (Hospital General Universitario de Albacete, Albacete); Santiago García Barajas (Hospital Universitario Infanta Cristina, Badajoz); Alberto Rodríguez (Hospital Universitari del Mar, Barcelona); Jorge Freixinet (Hospital Universitario Dr. Negrín, Las Palmas de Gran Canaria); Javier Ruiz (Hospital Universitario Virgen de las Nieves, Granada); Guillermo Carriquiry (Hospital Maciel, Universidad de la República, Montevideo, Uruguay); Moisés Rosenberg (InstitutoOncológico Alexander Fleming, Buenos Aires, Argentina); and Emilio Canalís (Hospital Universitari. U. Juan XXIII, Tarragona).
The name of the members of the Spanish Group for Colo-Rectal Carcinoma Pulmonary Metastasis Surgery (GECMP-CCR) of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) are listed in Appendix A.