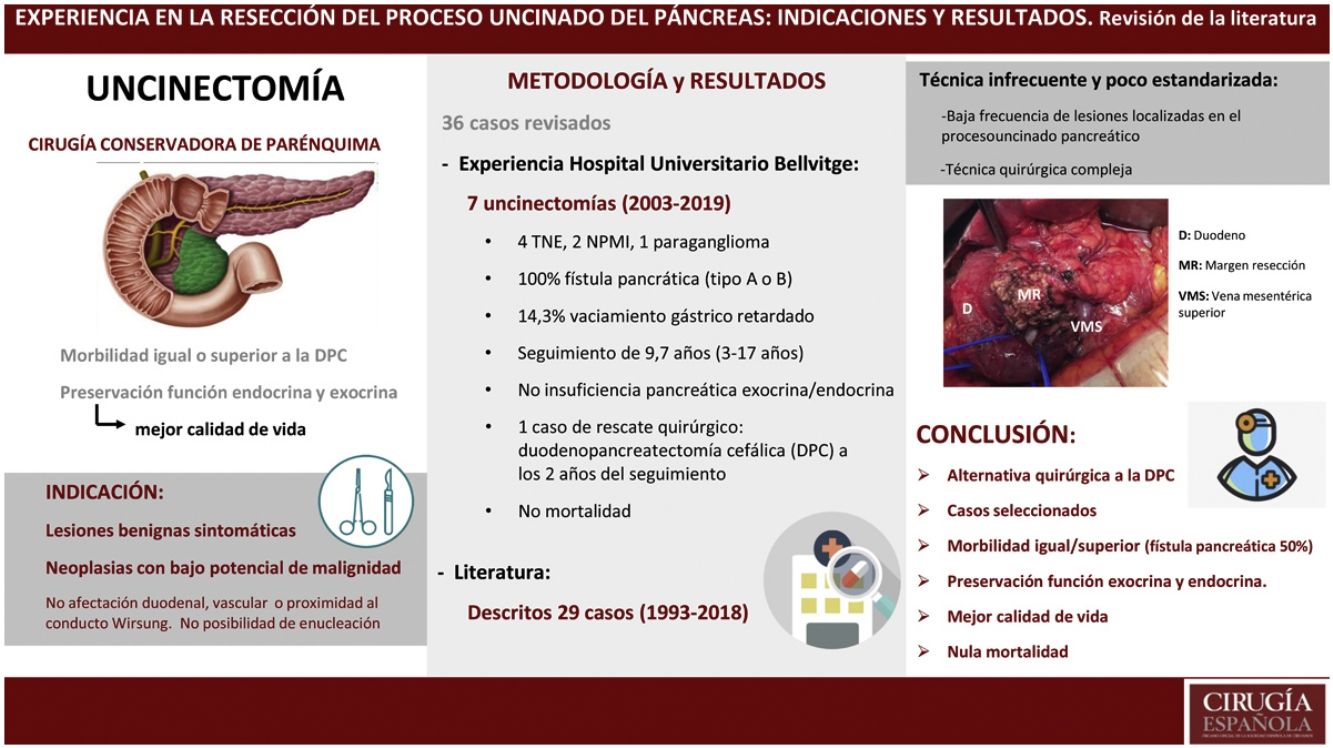
The aim of our study is to assess the accumulated experience in the use of uncinatectomy (UC) as a parenchymal-sparing pancreatectomy technique.
MethodWe have carried out a observational and descriptive study including restrospectively all the patients undergoing UC at Hospital Universitary de Bellvitge (HUB) and an exhaustive review of the cases described in the english literature.
ResultsFrom 2003 to 2019, seven patients have been operated by UC in the HUB with a diagnostic orientation of pancreatic lesion considered premalignant. All patients have presented morbidity, mainly in the form of postoperative pancreatic fistula, and none of them have presented endocrine or exocrine pancreatic insufficiency. Currently, all patients are alive and without recurrence of neoplastic disease. Another 29 cases have been described in the literature. Of all the cases (36 patients), the approach was minimally invasive (laparoscopic or robotic) in 6 patients (16.7%), leading to a shorter hospital stay. The global incidence of pancreatic fistula is 50%, with a re-admission rate of less than 10%, but without requiring re-intervention.
ConclusionsUC is an infrequent and poorly standardized technique for the resection of benign lesions or those with low potential for malignancy located in the uncinate process of the pancreas. Although it is associated with equal or greater morbidity than standardized resection techniques, it offers excellent preservation of endocrine and exocrine pancreatic function, with the consequent long-term benefit in the patients life quality.
El objetivo de nuestro trabajo es evaluar la experiencia acumulada en el empleo de la uncinectomía (UC) como técnica de pancreatectomía conservadora de parénquima.
MétodoEstudio observacional y descriptivo que incluye retrospectivamente todos los pacientes intervenidos mediante la técnica de UC en Hospital Universitari de Bellvitge (HUB), y revisión exhaustiva de los casos descritos en la literatura inglesa hasta la actualidad.
ResultadosDesde el 2003 hasta el 2019 han sido intervenidos 7 pacientes mediante UC en el HUB con orientación diagnóstica de lesión pancreática considerada premaligna. Todos los pacientes han presentado morbilidad, fundamentalmente en forma de fístula pancreática postoperatoria y ninguno de ellos ha presentado insuficiencia pancreática endocrina ni exocrina. Actualmente todos los pacientes se encuentran vivos y sin recidiva de enfermedad neoplásica. Otros 29 casos han sido descritos en la literatura. Del total de los casos (36 pacientes), el abordaje ha sido mínimamente invasivo (laparoscópico o robotizado) en 6 pacientes (16,7%) conllevando una estancia hospitalaria inferior. La incidencia global de fístula pancreática es del 50% comportando una tasa de re-ingreso inferior al 10% pero sin necesitar re-intervención.
ConclusiónLa UC es una técnica infrecuente y poco estandarizada para la resección de lesiones benignas o de bajo potencial de malignidad localizadas en el proceso uncinado del páncreas. Aunque se asocia a una morbilidad igual o superior a las técnicas de resección estandarizadas, ofrece una preservación excelente de la función endocrina y exocrina pancreática, con el consiguiente beneficio en la calidad de vida de los pacientes a largo plazo.
Pancreatoduodenectomy (PD) of the Whipple or Traverso-Longmaire type is considered an oncological surgical technique for the treatment of cancer of the head of the pancreas and periampullary area.1–3 These two techniques, along with distal pancreatectomy for lesions located in the left pancreas as well as total pancreatectomy, are considered standard techniques in pancreatic surgery. In our setting, morbidity rates of 30%–50% for PD and 9%–31% for left pancreatectomies, as well as mortality rates lower than 3%–5% and 1%, respectively, are considered acceptable.
The knowledge acquired in recent years about the anatomy of the pancreas has led to the development of conservative pancreas surgery, known as parenchyma-sparing pancreatectomy (PSP). The popularity of this technique has grown among surgeons, and its indication has been expanded to include symptomatic benign lesions or neoplasms with low malignant potential.
This type of PSP includes various surgical techniques, such as duodenum-preserving pancreaticoduodenectomy, medial or central pancreatectomy, and enucleation, depending on the type of lesion and its location.
The resection of the uncinate process (UP) or uncinatectomy (UC) is also considered an alternative to PD by some authors and is accepted as a PSP technique.4–21
The objective of the present study is to evaluate the accumulated experience in the use of the UC technique as a PSP technique at the Hospital Universitari de Bellvitge (HUB).
MethodsWe designed an observational and descriptive study, which was conducted in the General Surgery Service of the HUB. The study included all patients who underwent the UC technique at our hospital have been included retrospectively, with prior approval by the Research Ethics Committee of said medical center (PR413/21).
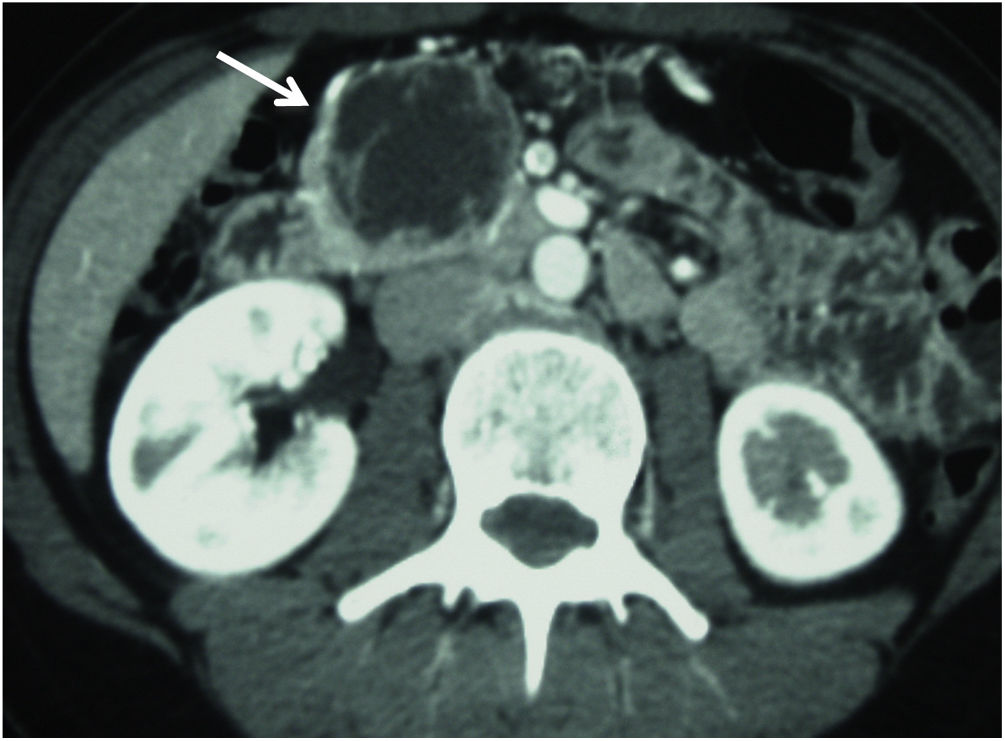
The preoperative study of the patients was carried out with imaging techniques, such as abdominal computed tomography (CT) scans (Fig. 1) and magnetic resonance cholangiopancreatography (MRCP)/magnetic resonance imaging (MRI) of the pancreas and octreotide scan in cases of neuroendocrine tumor (NET). In some patients, endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS) were necessary to obtain a preoperative biopsy. Duodenal and vascular involvement, both venous and arterial, as well as proximity of the lesion to the Wirsung duct, were ruled out preoperatively.
The surgical indications for these patients were agreed upon by a multidisciplinary committee specialized in biliopancreatic pathology, including surgeons, pathologists, radiologists and gastroenterologists.
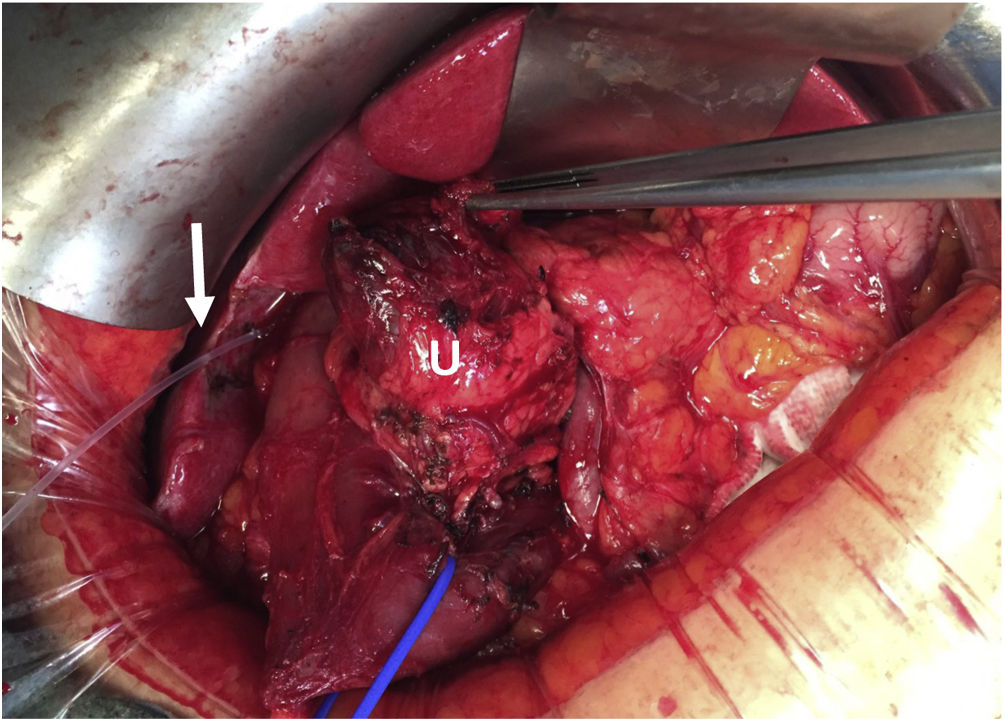
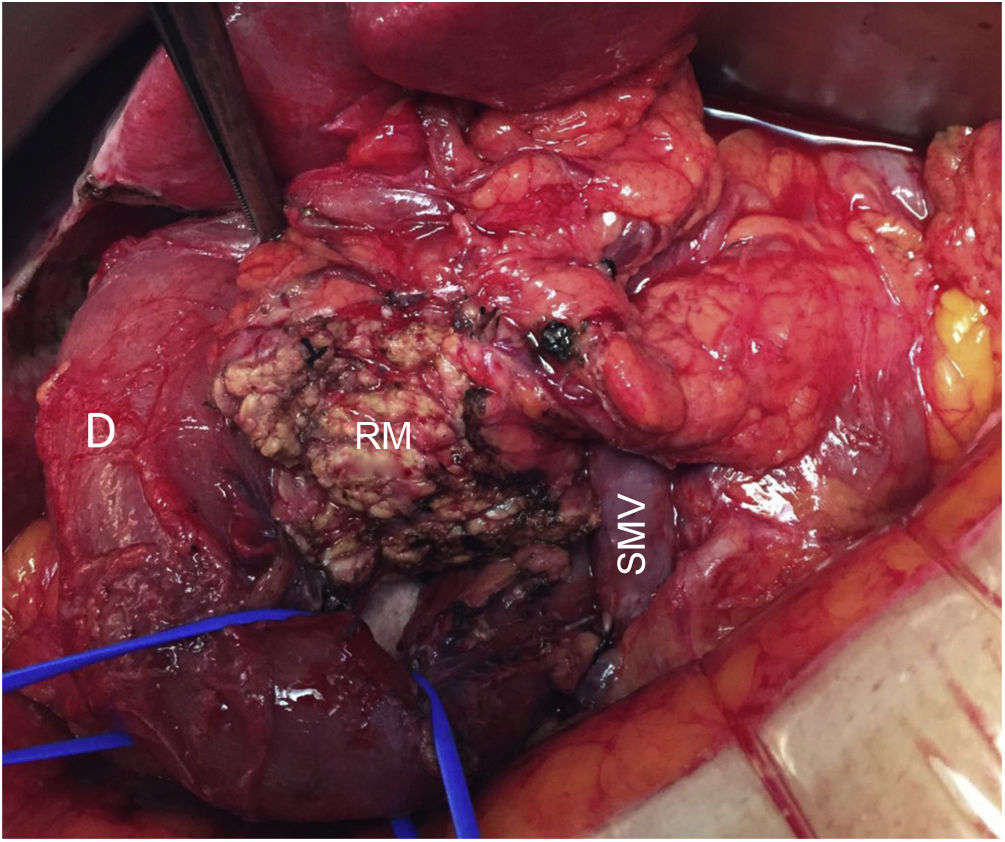
In all cases, intraoperative prophylactic antibiotic therapy was administered, and the surgical approach was via laparotomy. Cholecystectomy and cannulation of the bile duct were performed up to the ampulla of Vater through the cystic duct to locate the intrapancreatic bile duct, thereby preventing its injury (Fig. 2). After performing the Kocher maneuver, the affected pancreatic parenchyma was resected at the UP after dissection of the superior mesenteric vein and following a line parallel to the probable location of the Wirsung duct with great care to avoid injury (Fig. 3). In all cases, a closed suction drainage was placed.
All resection specimens underwent intraoperative pathological study to rule out malignancy or involvement of the pancreatic resection margin. Margins greater than 1 mm were considered ‘clear’ for solid lesions, and moderate dysplasia in the resection margin of the secondary branch IPMN.22
Study variables included age, sex, indication for surgery, preoperative study, surgical technique, intraoperative and definitive studies of the specimen, overall hospital stay, re-admission, follow-up time, and morbidity/mortality.
Statistical analysisFor the analysis of the variables, the IBM SPSS-25 statistical program (SPSS Inc., an IBM Company) was used. In the descriptive analysis, the data are presented as mean and standard deviation in the case of continuous variables with normal distribution, median and interquartile range if the continuous variable does not follow the normal law, and as a percentage in the case of categorical variables. When extrapolations are made from the descriptive data to the general population, they are given using a 95% confidence interval.
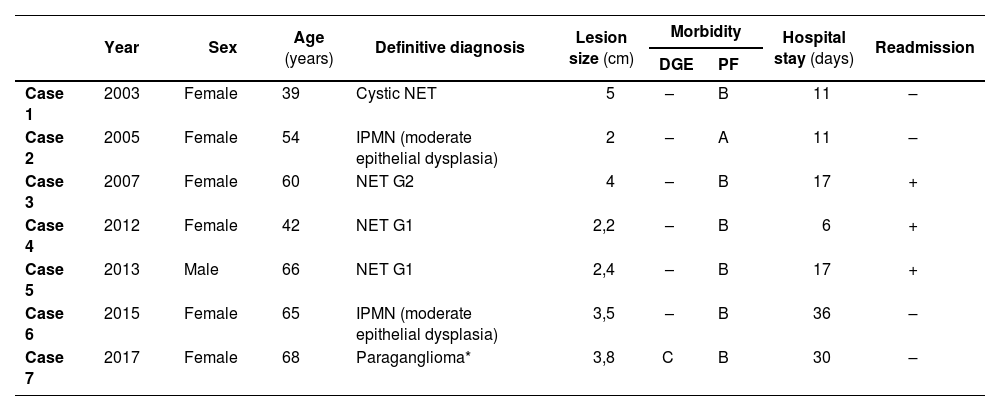
ResultsFrom 2003 to 2019, the HUB group has operated on 7 patients using the uncinatectomy technique. These patients represent only 0.6% of patients undergoing pancreatic resection (1078 patients) and 8.1% of patients treated with PSP (86 patients). Table 1 summarizes the case data.
Data of patients who underwent uncinatectomy at the HUB.
| Year | Sex | Age (years) | Definitive diagnosis | Lesion size (cm) | Morbidity | Hospital stay (days) | Readmission | ||
|---|---|---|---|---|---|---|---|---|---|
| DGE | PF | ||||||||
| Case 1 | 2003 | Female | 39 | Cystic NET | 5 | – | B | 11 | – |
| Case 2 | 2005 | Female | 54 | IPMN (moderate epithelial dysplasia) | 2 | – | A | 11 | – |
| Case 3 | 2007 | Female | 60 | NET G2 | 4 | – | B | 17 | + |
| Case 4 | 2012 | Female | 42 | NET G1 | 2,2 | – | B | 6 | + |
| Case 5 | 2013 | Male | 66 | NET G1 | 2,4 | – | B | 17 | + |
| Case 6 | 2015 | Female | 65 | IPMN (moderate epithelial dysplasia) | 3,5 | – | B | 36 | – |
| Case 7 | 2017 | Female | 68 | Paraganglioma* | 3,8 | C | B | 30 | – |
DGE: delayed gastric emptying, PF: pancreatic fistula; NET: neuroendocrine tumor; IPMN: intraductal papillary mucinous neoplasm.
Most of these patients are women (85.7%), with a mean age of 56 years (range 39–68 years).
The indication for PSP was due to the diagnostic orientation of a pancreatic lesion considered premalignant (intraductal papillary mucinous neoplasm [IPMN], solid pseudopapillary neoplasm, or NET). The preoperative presumptive studies correlated with the postoperative studies in all patients except in case 7, since the definitive pathological result of the suspected solid pseudopapillary neoplasm was conclusive for paraganglioma. In all cases, malignancy and/or involvement of the resection margin were ruled out. Likewise, all the lesions treated had a size equal to or less than 5 cm.
All patients in the series have presented morbidity, mainly in the form of postoperative pancreatic fistula (PF). Following the current classification of the International Study Group for Pancreatic Surgery (ISGPS),23 6 out of the 7 patients presented grade B PF. This complication led to the readmission of 3 patients for treatment. Among the patients who presented clinically relevant PF (grade B), 1 patient required surgical drainage for the following 29 postoperative days (case 1), 2 patients also required broad-spectrum intravenous antibiotics (cases 3 and 6), and the other 3 patients required percutaneous drain tube placement in addition to intravenous antibiotic treatment (cases 4, 5 and 7). One patient (14.3%) presented delayed gastric emptying (DGE) caused by an intra-abdominal collection secondary to a poorly drained PF (case 7), which was classified as grade C according to the ISGPS24 as it had had not resolved by postoperative day 14th.
Mean hospital stay was 18 days (range 6−36 days).
None of the patients has presented endocrine or exocrine pancreatic insufficiency during their evolution, and mean patient follow-up was 9.7 years (3–17 years). However, it is worth mentioning that case 6 required rescue surgery by performing a PD 2 years after the first surgery due to recurrence of the IPMN of the secondary branch at the resection margin. The intraoperative study of the margin of the UC specimen demonstrated low-grade epithelial dysplasia, which was also observed in the definitive study of the PD specimen. Currently, all patients are alive and have had no recurrence of neoplastic disease.
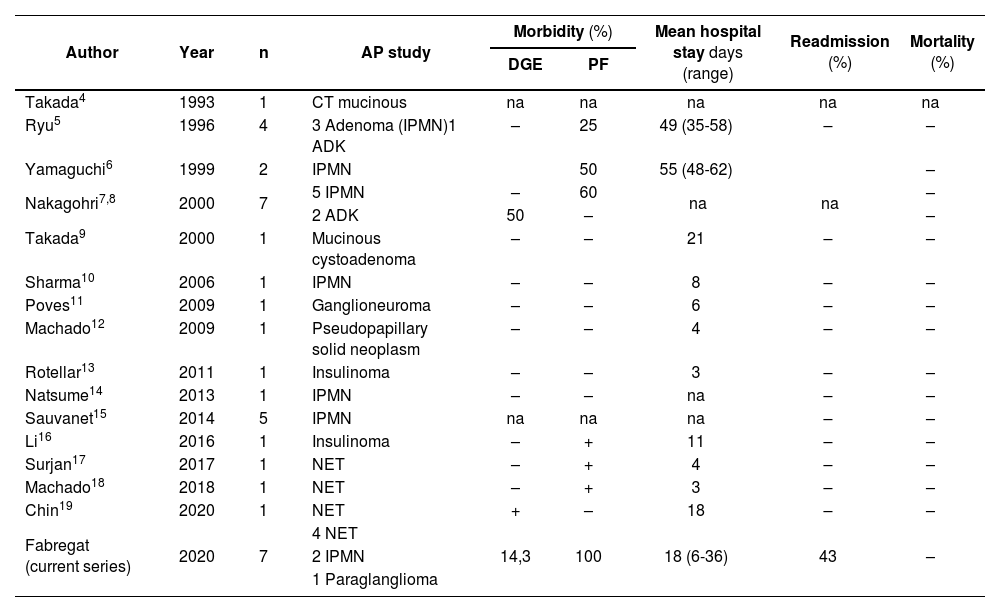
DiscussionAfter a thorough review of the English-language literature regarding UC as a PSP technique, we have only found 29 published cases and one previous series of 7 patients. The main characteristics from these cases are summarized in Table 2, along with the data from the HUB series presented in this study.
Case data from HUB and other published UC series.
| Author | Year | n | AP study | Morbidity (%) | Mean hospital stay days (range) | Readmission (%) | Mortality (%) | |
|---|---|---|---|---|---|---|---|---|
| DGE | PF | |||||||
| Takada4 | 1993 | 1 | CT mucinous | na | na | na | na | na |
| Ryu5 | 1996 | 4 | 3 Adenoma (IPMN)1 ADK | – | 25 | 49 (35-58) | – | – |
| Yamaguchi6 | 1999 | 2 | IPMN | 50 | 55 (48-62) | – | ||
| Nakagohri7,8 | 2000 | 7 | 5 IPMN | – | 60 | na | na | – |
| 2 ADK | 50 | – | – | |||||
| Takada9 | 2000 | 1 | Mucinous cystoadenoma | – | – | 21 | – | – |
| Sharma10 | 2006 | 1 | IPMN | – | – | 8 | – | – |
| Poves11 | 2009 | 1 | Ganglioneuroma | – | – | 6 | – | – |
| Machado12 | 2009 | 1 | Pseudopapillary solid neoplasm | – | – | 4 | – | – |
| Rotellar13 | 2011 | 1 | Insulinoma | – | – | 3 | – | – |
| Natsume14 | 2013 | 1 | IPMN | – | – | na | – | – |
| Sauvanet15 | 2014 | 5 | IPMN | na | na | na | – | – |
| Li16 | 2016 | 1 | Insulinoma | – | + | 11 | – | – |
| Surjan17 | 2017 | 1 | NET | – | + | 4 | – | – |
| Machado18 | 2018 | 1 | NET | – | + | 3 | – | – |
| Chin19 | 2020 | 1 | NET | + | – | 18 | – | – |
| Fabregat (current series) | 2020 | 7 | 4 NET | |||||
| 2 IPMN | 14,3 | 100 | 18 (6-36) | 43 | – | |||
| 1 Paraglanglioma | ||||||||
AP: anatomopathological; DGE: delayed gastric emptying; PF: pancreatic fistula; CT: cystic tumor; na: not available; ADK: adenocarcinoma; NET: neuroendocrine tumor; IPMN: intraductal papillary mucinous neoplasm.
The UC technique, or resection of the ventral pancreas, was first described by Takada in 1993 for the resection of a cystic lesion in a patient with pancreas divisum.4 In 2009, the first national case was published by the Poves group.11 Machado reported the first laparoscopic UC in 2009,12 and Rotellar published the second case of laparoscopic resection described in the literature in 2011, which was the first described in our country.13 In 2018, the first robot-assisted UC was reported, also by the Machado group.18
Out of the total of 36 cases, including those of the Fabregat group, the approach has been minimally invasive (laparoscopic or robotic) in 6 of the patients (16.7%), which has led to shorter hospital stays in these patients. Regarding postoperative complications, and by analyzing the cases for which information is available, we conclude that postoperative biliary fistula has not been described in any case. However, PF has been the main complication, with a frequency of 50%, which has frequently led to patients requiring drains even after hospital discharge and a readmission rate of less than 10%, although without requiring reoperation. The incidence of DGE has been less than 10%, and no case of postoperative hemorrhage has been described. The absence of postoperative mortality and the preservation of endocrine and exocrine pancreatic function should be noted.
PSP techniques have emerged and evolved as alternatives to standard pancreatic resection techniques to preserve the anatomy of the upper digestive tract, mainly the duodenum and the main bile duct. Thus, the long-term morbidity associated with a more extensive pancreatic resection is reduced, and the probability of presenting endocrine and exocrine pancreatic insufficiency in the postoperative period is likewise lower. However, although these techniques are associated with high postoperative morbidity, especially PF, they present almost zero postoperative mortality. If we also add that sometimes the technical complexity can be greater than that of standard surgery, we understand that the risk/benefit of this type of resection is poorly established.25,26
Currently, lesions that are considered benign or have low malignant potential are candidates for pancreatic resection by UC. The most frequent are IPMN and well-differentiated NET, as long as they do not present signs suggestive of malignancy or invasion, and enucleation is not possible.10,15 In the resection of these lesions, the authors recommend performing an intraoperative anatomopathological study to rule out invasion by adenocarcinoma and evaluation of the resection margin; a margin >1 mm was considered free for solid lesions and moderate dysplasia in the resection margin for secondary branch IPMN.22 In addition, it is recommended to precisely locate the ductal branch on which the lesion depends in order to seal it, thereby avoiding the dreaded postoperative PF.5,14,15 In our opinion, the size of the lesion does not limit the execution of this technique; the most important factor is the relationship of the lesion with the Wirsung duct, while also ruling out malignancy or that the lesion has a high probability of developing metastasis.
Given that UC is not considered oncological surgery, the main challenge of this surgical strategy is meticulous patient selection to rule out lesions with a high probability of having invasive behavior. Therefore, precise preoperative studies should include abdominal CT scan and pancreatic MRCP/MRI for the characterization of these lesions and assessment of their relationship with surrounding structures. ERCP and EUS, which are associated with bleeding, are considered second-line techniques for the diagnosis of lesions located in the UP, the latter being essential if biopsies are required.
Regarding the details of the surgical technique, the importance of the anatomical limits, which are not easily identifiable, should be highlighted. One of the main difficulties when performing UP resection of the pancreas is identifying its upper limit in order to preserve the bile duct and the main pancreatic duct. The use of intraoperative ultrasound can assist in the identification of both main ducts (biliary and pancreatic).18 If the patient presents the gallbladder in situ, we believe that cannulation of the bile duct through the cystic duct is justified due to its localization, but performing a choledochotomy is not; this was described in only one of the cases, which is why the patient required T-tube drainage for the first 6 weeks after surgery.10 Nor do we currently justify preoperative transpapillary biliary cannulation, since the possibility of complications, especially in the form of pancreatitis, could complicate the surgery. These procedures should only be carried out in highly selected cases due to the risk involved.19 On the other hand, only one of the series in the bibliography consulted refers to partial resection of the Wirsung at the level of the pancreatic head, requiring its reconstruction.7
In addition, the UP has the superior mesenteric vein on its left side, where venous branches can be found that can cause massive bleeding if the dissection plane is incorrect. In the lower and right margins, there is an arterial arcade, formed by the inferior pancreatic-duodenal artery, which is responsible for the perfusion of the duodenum and must be preserved. This dissection plane must preserve said arch and at the same time control the branches that irrigate the UP.10,18 Therefore, to ensure adequate blood supply to the duodenum after UC, the lower pancreaticoduodenal arcade and the retroperitoneal vessels must be preserved, which is why some authors recommend not performing the Kocher maneuver in order to maintain the integrity of the mesoduodenum.4,5,7 However, and similar to reports by other authors, we have performed this maneuver and observed optimal duodenal vascularization after resection, with no cases of postoperative duodenal ischemia.
PF remains the main concern after UP resection. To date, there has been no study of its incidence after this complex technique due to the few publications on the subject. After completing this study, we can confirm that its incidence is 50%, a figure comparable to the rates of standard pancreatic surgery (30%–50%). Specifically, the HUB series has the highest rate regarding this complication, since all the patients presented PF. On the other hand, we believe it is very important that grade C PF has not been reported in any case. Since the potential to present postoperative PF is considered high, all authors leave surgical drains in the resection site for its diagnosis and treatment, with the exception of the Rotellar group.13 It should be noted that even patients who did not present PF have had surgical drain tubes for more than one week after surgery, which were then removed on an outpatient basis in most cases. The application of sealing substances could reduce the incidence of PF, but there is no clear evidence in this regard.
The incidence of DGE is low, and, when it has occurred, it has been related to the presence of PF and intra-abdominal collections requiring drainage. In no case was re-operation required.
The minimally invasive approach (laparoscopic/robotic) is associated with shorter hospital stays, as is the case with standard pancreatic surgery.
Recurrence of neoplastic disease has been described in 2 patients. One of the patients with adenocarcinoma identified in the resection piece presented peritoneal carcinomatosis,7 and another patient in the Fabregat series presented recurrence of IPMN after 2 years of follow-up, despite free resection margins (low-grade epithelial dysplasia), which required PD as salvage surgery and provided a definitive result of low-grade epithelial dysplasia. This fact reinforces the need for routine follow-up in these patients through imaging tests, at least during the first years after surgery.
Lastly, an important fact: no alterations in pancreatic endocrine or exocrine function have been detected during the follow-up of patients who underwent UC, while the rate of pancreatic insufficiency in patients undergoing standard resections is 45%.27
Therefore, we believe that UC should be considered a surgical alternative to PD as an option for the resection of benign lesions and those with low-malignant potential located in the UP of the pancreas. Although it is associated with equal or even higher morbidity than PD, mainly due to the appearance of PF, it offers excellent preservation of pancreatic endocrine and exocrine function, with the resulting long-term benefits in the quality of life of these patients.
Conflicts of interestsThe authors have no conflicts of interests to declare.
FundingThis research project has received no specific funding from public, commercial or non-profit sectors.