Extramammary perianal Paget's disease (EMPPD) accounts for <1% of perianal and anal lesions, and 6.5% of all cases of Paget's disease; typically affecting Caucasian and Asian population aged 60–80 years.1,2
Due to nonspecific related symptomatology combined with the equivocal cutaneous appearance leads to a high rate of misdiagnosis and therapeutic delay.3
We herein report two cases of EMPPD in whom several critical pathological and therapeutic features were found, representing a challenge in both diagnosis and management.
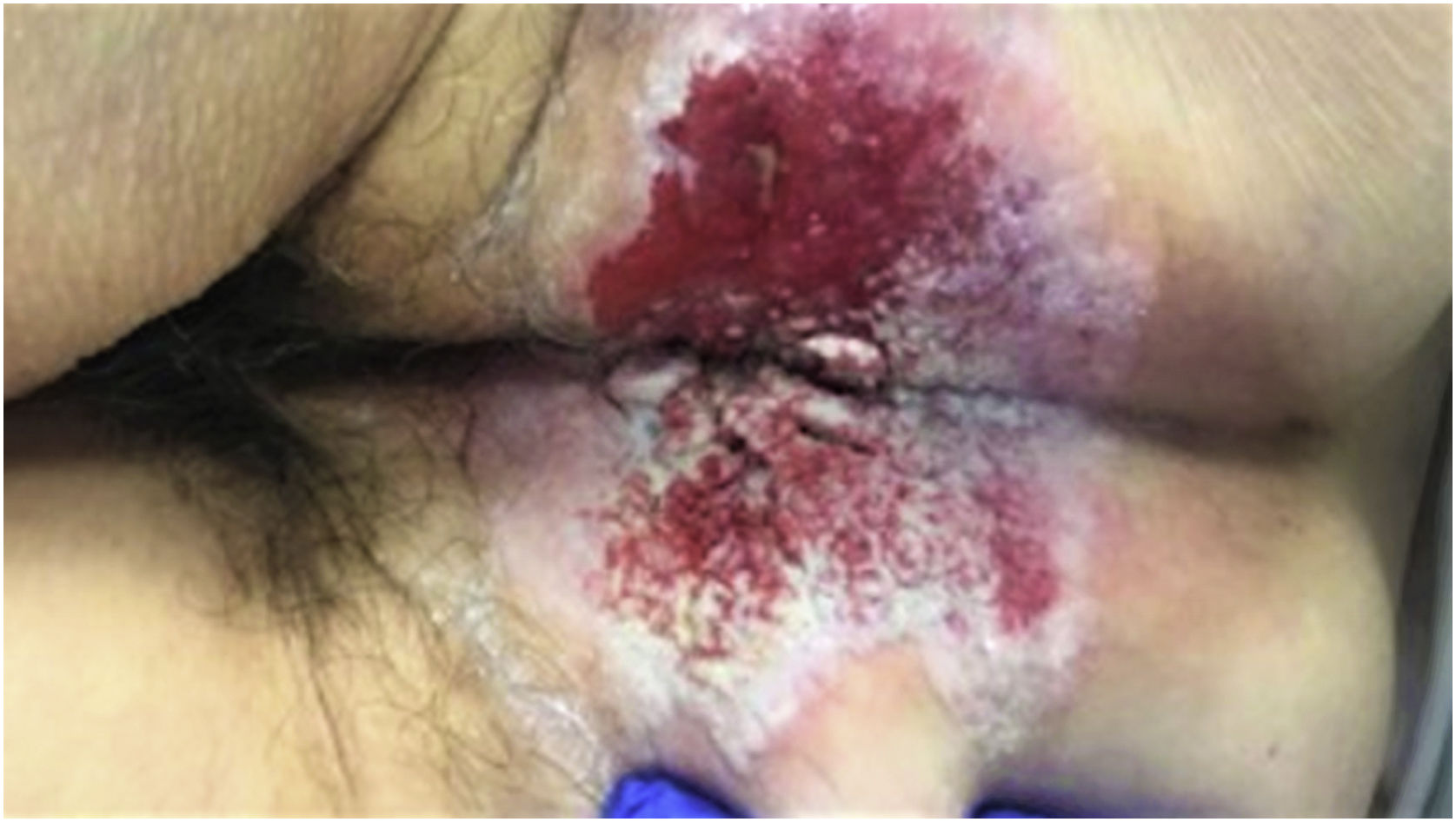
A 70 years old female with a background of right colon adenocarcinoma (pT3N0 (0/16) M0), treated with adjuvant chemotherapy, was referred to our outpatient's colorectal clinic complaining of long-standing perianal irritation along with refractory anal pruritus. The patient had followed the institutional oncological follow-up program and was disease-free at the time of presentation. Physical examination revealed a large verrucous, exfoliative well-demarcated perianal patch (Fig. 1) and presence of palpable left inguinal lymph nodes.
Preoperative work up included full blood tests with specific serum tumor markers (i.e. CEA), pelvic MRI, full-colonoscopy and chest-abdomen-pelvis CT. Preoperative biopsy of both the primary lesion and left inguinal lymph nodes was performed, confirming the presence of an invasive primary EMPPD (CEA+, CK 7+, CK20−, GCDFP-15+ and S100−) with lymph node metastasis.
After multidisciplinary discussion, abdominal–perineal resection (APR) with left inguinal lymphadenectomy (LDN) was indicated. The patient was discharge at 6th postoperative days with no postoperative complications. At 11 months of follow-up, no local recurrence has been detected.
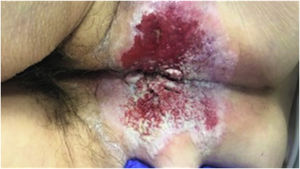
A 69 years old female patient, with no remarkable medical records, was referred to our outpatients’ clinic due to 2 years long refractory anal pruritus. Physical examination revealed an exfoliative, well-demarcated perineal patch located close to the vaginal introit. An identical preoperative work-up was performed including a preoperative biopsy, reporting the presence of a noninvasive primary EMPPD (CEA+/−, CK 7+, CK20−, GCDFP-15+, CDX2− and S100−) (Fig. 2).
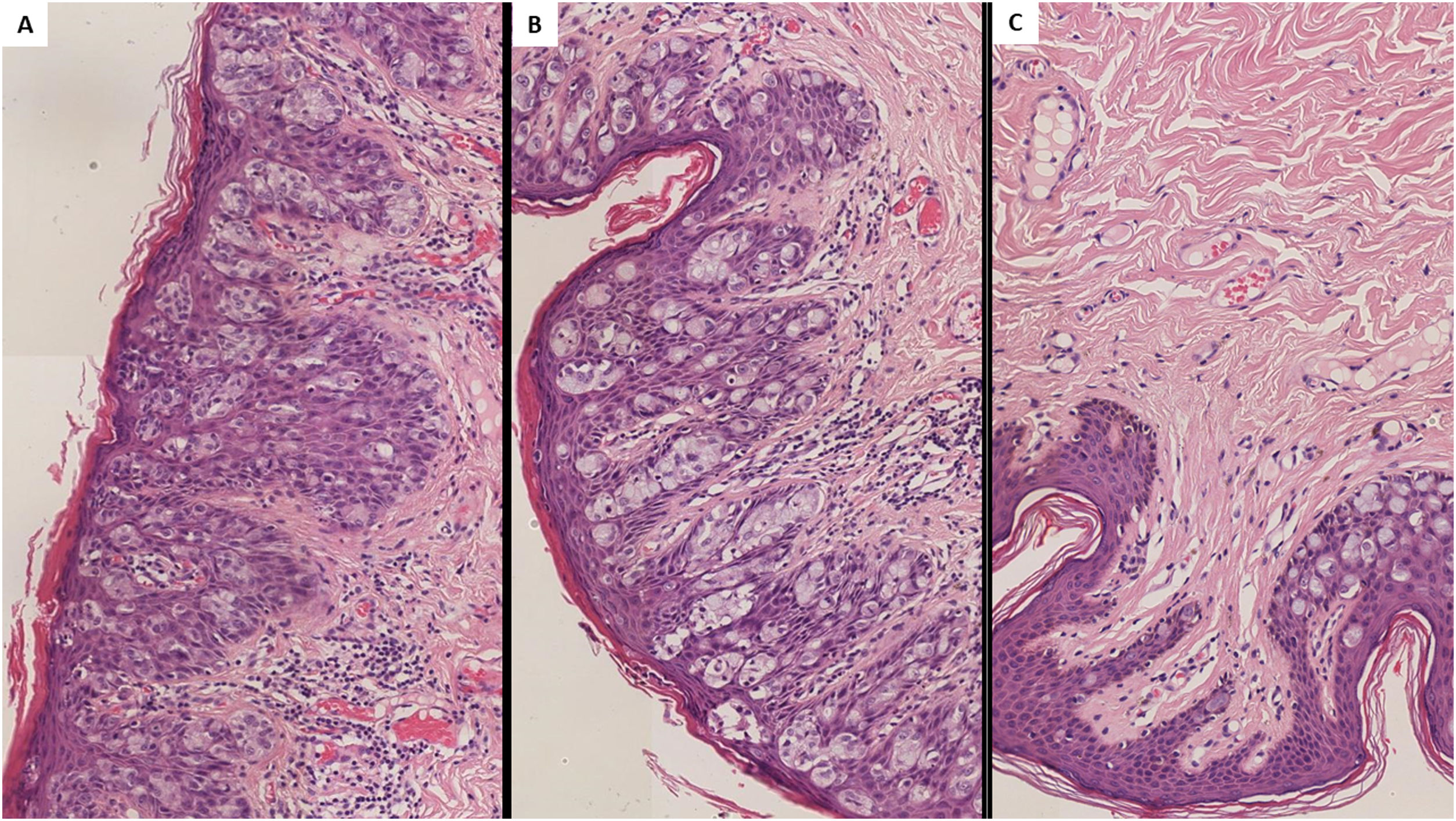
Histological findings in extramammary perianal primary Paget's disease. Caption: Hematoxylin–eosin staining 400× magnification. Characteristic intraepithelial Paget's cells with a large, pale-staining vacuolated cytoplasm and prominent nuclei are present within the epidermis, showing a cranial spread. No invasion of the basement membrane is present.
Due to presence of noninvasive EMPPD, a wide-local excision (WLE) with Singapore myocutaneous flap reconstruction was performed. The patient was discharged at 5th postoperative day with no postoperative complications. After 5 months of follow-up no LR has been detected.
Although the precise pathogenesis of EMPPD has not been precisely studied, nowadays is accepted that it can present as two forms of different carcinogenesis. Primary EMPPD is considered a in situ adenocarcinoma arising from local epidermal apocrine glands.2,3 Conversely, secondary EMPPD develop as a result of a locoregional intraepithelial spread of an underlying primary colorectal neoplasm.2,3 Although more rarely, secondary EMPPD patients might not present synchronous neoplasms but develop a metachronic proximal adenocarcinoma.4,5
This biological distinction is important since primary EMPPD is not associated with proximal malignancies. Nevertheless, up to 40% of primary EMPPD might experience an invasive transformation, infiltrating the basement membrane.3 Reported incidence of additional malignancies in secondary EMPPD range widely from 33% to 86%,1 probably due to previous studies not discriminating between primary and secondary EMPPD.1,3 According to recent data from Surveillance, Epidemiology and End Results (SEER) database proximal invasive neoplasm rate is approximately 18.5%, 3.7% metachronous.4
Symptomatology is usually simmering being presence of refractor long-lasting anal pruritus one of most frequent manifestations.1,3 These are accompanied with exfoliative, well-demarcated, verrucous and erythematous or hypopigmented perianal dermic patches.1–3 Considering the vague clinical course patients are usually misdiagnosed with more common perianal conditions.3
At diagnosis work-up a thorough perianal, anorectal and vulvar examination are mandatory along with colonoscopy.2,3 As in other perianal premalignant and malignant lesions, biopsies are imperative.
The essential histological finding is that of Paget's cells, characterized by a round, pale staining vacuolated cytoplasm, along with a prominent hyperchromatic excentric nuclei.1–3 Specific immunohistochemical (IHQ) stains are useful for discriminating primary and secondary EMPPD and also exclude additional differential diagnoses such as Bowens’ disease.1,2
Surgery is considered the mainstay treatment of EMPPD.2–4 EMPPD tends to display a multifocal growth with asymmetric local invasion.2,3 Wide local excision (WLE) is the preferred option in non-invasive EMPPD.3,4,6 High LR rates have been reported regardless of margins completeness.4,7 The combination of WLE and scouting biopsies has been suggested to improve WLE efficacy, as first described by Beck and Fazio.8 Nevertheless, scouting biopsies might not be necessary in well-defined EMPPD patients provided 2cm surgical margin are achievable.2 Moreover, APR is mandated in those with invasive EMPPD or presence of proximal anorectal synchronous adenocarcinoma.6,7,9
Local lymph nodes status has proven to influence EMPPD patients’ prognosis.2,5 Recent evidence advocate for sentinel lymph node biopsy (SNLB) when invasive disease is suspected.10 However, real efficacy of SNLB for EMPPD is yet to be elucidated. Therapeutic local lymph node dissection (LND) is advised in proven lymph node metastasis.7,9
The presence of invasive EMPPD along with lymph node metastases have proved to determine prognosis of EMPDD.2 Overall 5-years survival range 59.7–71.7%,6,7,9 being both overall survival and disease-specific survival significantly shorter in patients with invasive disease.9 LR remains the main concern as rates for noninvasive EMPPD reach 30%, although in patients with invasive EMPPD LR these accrue up to 56–60%.2,9
In conclusion, these cases underline the importance of an early diagnosis of EMPPD. Understanding the pathophysiology and histological key features is crucial to offer patients and early and individualized treatment.