Complex polyps require the use of advanced endoscopic techniques or minimally invasive surgery for their approach. In rectal polyps it is of special relevance to reach a consensus on the best approach to avoid under- or overtreatment that increases unnecessary morbidity and mortality.
MethodsWe describe a prospective, multicenter, pilot clinical trial with a first-in-human medical device. It is hypothesized that UNI-VEC® facilitates transanal laparoendoscopic surgery for the removal of early rectal tumors. The primary objective is to evaluate that it is safe and meets the established functional requirements. Secondary objectives are to evaluate results, complications and level of satisfaction.
Results16 patients were recruited in 12 months with a minimum follow-up of 2 months. The mean size was 3.4 cm with the largest polyp being 6 cm. Regarding location, the mean was 6.6 cm from the anal margin. Endoscopic Mucosal Resection (EMR) (6.3%), Endoscopic Submucosal Dissection ESD (43.8%), REC (6.3%) and TAMIS (43.8%) were performed. The mean time was 73.25 min. The 56.3% used a 30° camera and 43.8% used the flexible endoscope as a viewing instrument. The 56.3% were benign lesions and 43.8% malignant. Complete resection is achieved in 87.5%. Regarding complications, mild bleeding (Clavien I) occurred in 25%, 6.3% and 21.4% at 24 h, 48 h and 7 days respectively. Continence was assessed according to the Wexner scale. At 7 days, 60% showed perfect continence, 26.7% mild FI and 13.3% moderate FI. At 30 days, 66.7% had perfect continence, 20% mild FI and 13.3% moderate FI. At 2 months, 4 patients were reviewed who at 30 days had a Wexner's degree higher than preoperative and perfect continence was demonstrated in 25% of the patients, 50% mild and 25% moderate. In no case did rectal perforation or major complications requiring urgent reintervention occur. As for the level of reproducibility, safety, level of satisfaction with the device and evaluation of the blister, the evaluation on a scale of 0–10 (9.43, 9.71, 9.29 and 9.50 respectively). All the investigators have previous experience with transanal devices.
ConclusionsThe study demonstrates the efficacy and safety of UNI-VEC® for the treatment of rectal lesions. It will facilitate the implementation of hybrid procedures that seek to solve the limitations of pure endoscopic techniques by allowing the concomitant use of conventional laparoscopic and robotic instrumentation with the flexible endoscope.
Los pólipos complejos requieren el uso de técnicas endoscópicas avanzadas o la cirugía mínimamente invasiva para su abordaje. En los pólipos rectales es de especial relevancia llegar a un consenso de cuál es el mejor abordaje de éstos para evitar infratratamientos o sobretratamientos que incrementen una morbimortalidad innecesaria.
MétodosSe describe un ensayo clínico piloto con un producto sanitario de primer uso en humanos multicéntrico y prospectivo. Se plantea la hipótesis que UNI-VEC® facilita la cirugía laparoendoscópica transanal para la extirpación de tumores rectales precoces. El objetivo principal es evaluar que es seguro y cumple los requisitos funcionales establecidos. Los secundarios son evaluar resultados, complicaciones y nivel de satisfacción.
ResultadosSe reclutaron 16 pacientes en 12 meses con un seguimiento mínimo de 2 meses. El tamaño medio han sido 3,4 cm siendo el pólipo mayor de 6 cm. Respecto a la localización, la media se encontraba a 6,6 cm del margen anal. Se realizaron Resección Endoscópica Mucosa (REM) (6,3%), Disección Submucosa Endoscópica (DSE) (43,8%), Resección Espesor Completo (REC) (6,3%) y Transanal Minimally Invasive Surgery (TAMIS) (43,8%). El tiempo medio fueron 73,25 min. El 56,3% utiliza una cámara de 30º y el 43,8% el endoscopio flexible como instrumento de visión. El 56,3% son lesiones benignas y 43,8% malignos. En el 87,5% se consigue resección completa. En cuanto a las complicaciones, se presenta sangrado leve (Clavien I) en un 25%, 6,3% y 21,4% a las 24 h, 48 h y 7 días respectivamente. La continencia se valora según la Escala de Wexner. A los 7 días, el 60% presentan continencia perfecta, 26,7% IF leve y 13,3% IF moderada. A los 30 días, el 66,7% continencia perfecta, 20% IF leve y 13,3% IF moderada. A los 2 meses se revisan 4 de los pacientes que a los 30 días presentaban un Wexner superior al preoperatorio y se demuestra continencia perfecta en el 25% de los pacientes, 50% leve y 25% moderada. En ningún caso se presenta perforación rectal o complicaciones mayores que requieran reintervención urgente. En cuanto al nivel de reproducibilidad, seguridad, nivel de satisfacción del dispositivo y evaluación del blíster la valoración en la escala de 0 a 10 (9,43, 9,71, 9,29 y 9,50 respectivamente). Todos los investigadores tienen experiencia previa con dispositivos transanales.
ConclusionesEl estudio demuestra la eficacia y seguridad de UNI-VEC® para el tratamiento de lesiones rectales. Facilitará la implementación de procedimientos híbridos que buscan resolver las limitaciones propias de las técnicas endoscópicas puras al permitir el empleo concomitante de instrumentación laparoscópica convencional y robótica con el endoscopio flexible.
The treatment of complex polyps (CP) requires the use of advanced endoscopic techniques1, such as endoscopic mucosal resection (EMR)2, endoscopic submucosal dissection (ESD)3, endoscopic full-thickness resection (EFTR)4 or minimally invasive surgery. In rectal polyps, it is especially important to reach a consensus on the best approach to avoid undertreatment or overtreatment, which unnecessarily increase morbidity and mortality.
EMR is used for lesions <20−30 mm in size. For lesions >2 cm, advanced endoscopic techniques such as ESD or EFTR are indicated for en bloc resections, and local transanal surgery is used for lesions that cannot be approached endoscopically, thereby preserving the rectum. In most cases, the use of rigid or flexible platforms is required to perform a quality transanal procedure. Since the transanal excision of Parks5 with conventional retractors, the technology has evolved, and more precise dissections are now possible. The two main rigid platforms are transanal endoscopic microsurgery (TEM)6 and transanal endoscopic operation (TEO)7. With either technique, localized lesions up to 20 cm can be resected, although this requires a learning curve, specific equipment, and changing the position of the patient so that the tumor is positioned at the bottom of the rectoscope. Transanal minimally invasive surgery (TAMIS)8,9 is a flexible platform resulting from the combination of single-port laparoscopic surgery and transanal endoscopic microsurgery. It provides access to the middle and upper rectum (12−14 cm) for complete resection of the rectal wall and the adjacent mesorectum. Standard laparoscopic material is used with single-port devices (SILS®, Medtronic) or specific devices for transanal surgery (GelPOINT Patch®, Applied). It is not necessary to change the position of the patient. With the development of robotic surgery, the first cases of robotic TAMIS (rTAMIS) were reported10. The advantages of this technique are the 3-dimensional vision, elimination of tremor, greater ergonomics and the 7 degrees of movement of the robotic arms. These characteristics facilitate the access of large lesions without depending on the position of the patient or the location of the polyp, which is a great benefit when working in a reduced surgical field like the anal canal11.
We describe the results of a multicenter clinical trial for the resection of rectal polyps using the UNI-VEC® multichannel access device, which can be used with the flexible endoscope but with the support of conventional laparoscopic instruments. Cooperation between surgeons and endoscopists, as well as prior experimental training, will facilitate the implementation of these hybrid procedures that seek to resolve the limitations of pure endoscopic techniques12,13.
MethodsA pilot clinical trial was carried out with a medical device used for the first time in humans. As the objective of our study was to evaluate whether the device is effective and safe for the patient and meets established functional requirements, with no need to compare it to other devices, the study design was multicenter with a prospective follow-up and no control group. The following medical centers have participated: Complexo Hospitalario Universitario A Coruña (CHUAC), HU Ortega River (Valladolid), Lucus Augusti University Hospital (HULA), HU Mutua Terrasa (Barcelona), HU La Paz (Madrid) and Hospital QuironSalud A Coruña. Before the trial, the teams participated in specific training in animal models at the Technological Training Center (CTF-XXIAC).
Target populationPatients with lesions in the rectum or distal colon (up to 20 cm from the anal margin), who required excision as a therapeutic or diagnostic option. After the study subject was assessed by the lead researcher at each hospital, the inclusion/exclusion criteria (Table 1) and pre-/post-operative Wexner continence scale (Table 2) were checked. When consent was obtained and the patient was included in the study, the research coordinator and promotor were informed. The subject was anonymized and received an identification code for follow-up of the UNI-VEC® results.
Inclusion and exclusion criteria of the UNI-VEC® clinical trial.
| Inclusion criteria |
| • Adults ≥ 18 years |
| • Gave specific informed consent for the study |
| • All polyps included in the Paris Classification: Polypoid (0-I): Sessile type (Is), Pedunculated type (Ip) Non-polypoid (0-II, III): Elevated type (IIa), Flat type (IIb), Depressed type (IIc), Excavated (III) |
| • Lesions located within 20 cm of the anal margin |
| • Extension of 2 cm2 (polyps 2 cm in diameter or EMR or ESD 2 × 2 cm) |
| • Well or moderately well differentiated tumors. T1: Tumors limited to the mucosa (Tis) and submucosa (T1) |
| • No positive lymph nodes |
| • No lymphatic, vascular or perineural involvement |
| Exclusion criteria |
| • Vulnerable subjects: patients who participate under a condition which the researchers believe could entail significant risk, confound or significantly interfere with the study results, or who cannot give their consent (cultural, linguistic or neurological factors) |
| • Patients with blood disorders or coagulation alterations (including pharmacologically induced; the use of 100 mg/day of acetylsalicylic acid is permitted) |
| • Patients with rectal lesions on the pectineal line or extending toward the anal canal |
| • Local or distant metastasis |
| • Score >2 points in the Wexner fecal incontinence (FI) scale (Table 2) |
Wexner incontinence scale.
| Type of leak | Never | Rare (<1/month) | Sometimes >1/month <1/week | Generally >1/week <1/day | Always >1/day |
|---|---|---|---|---|---|
| Solid | 0 | 1 | 2 | 3 | 4 |
| Liquid | 0 | 1 | 2 | 3 | 4 |
| Gas | 0 | 1 | 2 | 3 | 4 |
| Use of absorbent pads | 0 | 1 | 2 | 3 | 4 |
| Altered lifestyle | 0 | 1 | 2 | 3 | 4 |
| Wexner scale score | |||||
| Mild FI | 0−8 points | ||||
| Moderate FI | 9−16 points | ||||
| Severe FI | >17 points | ||||
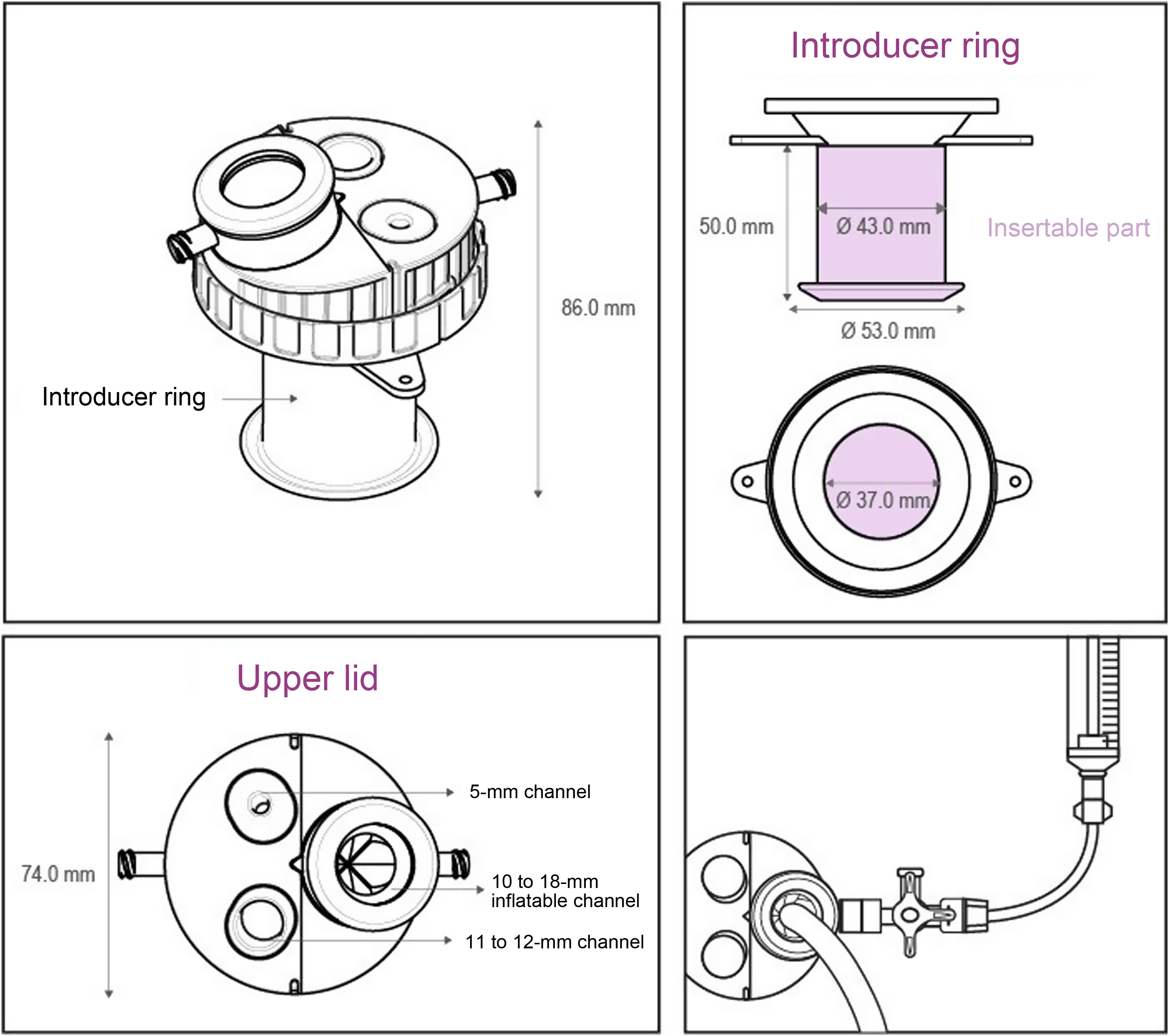
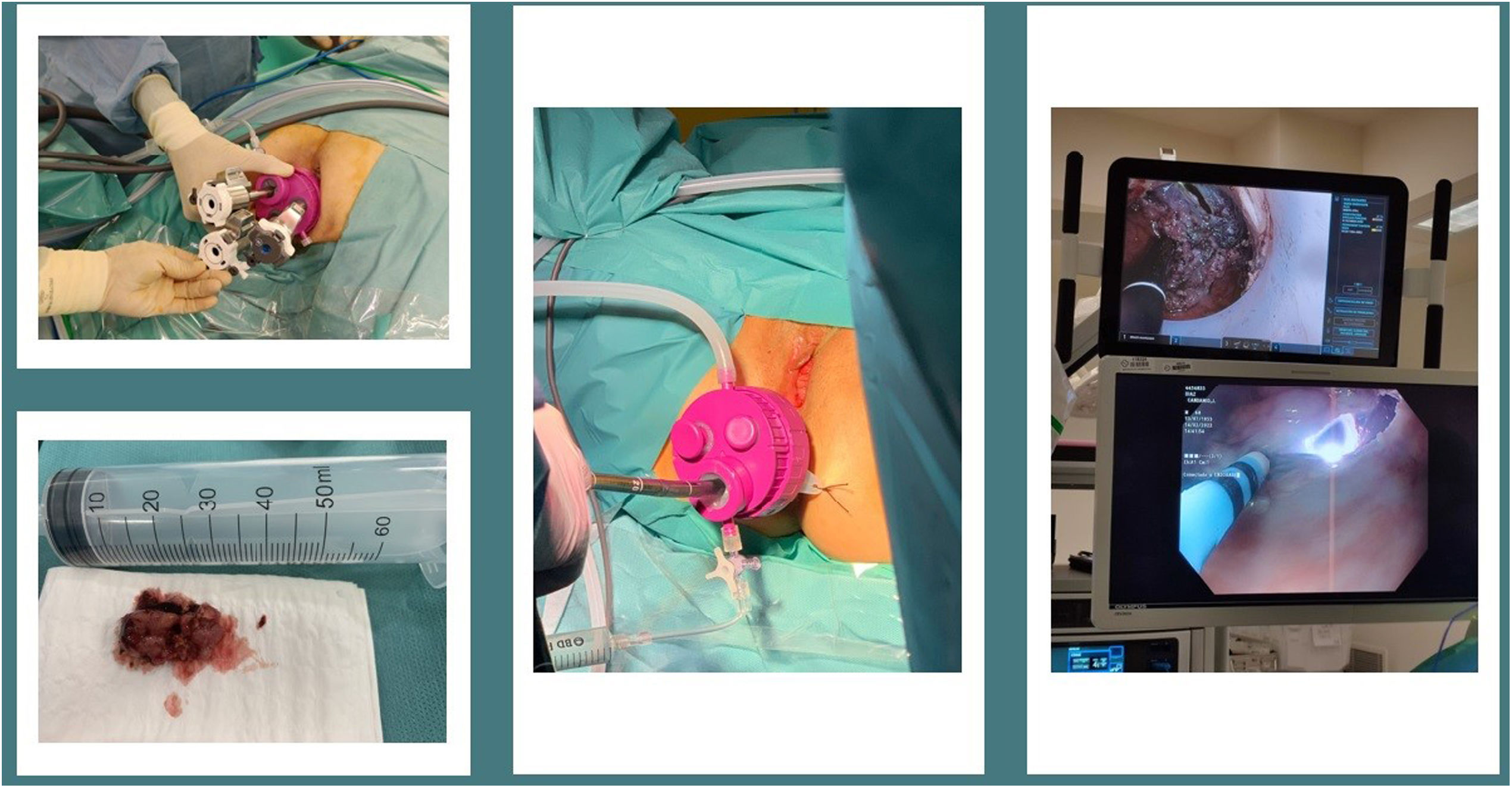
The procedures were performed under general anesthesia. The lead researcher decided the bowel preparation. Patient placement was in lithotomy with high stirrups, except in one robotic case that was placed prone to avoid arm collision. The UNI-VEC® device (Fig. 1) was used in all cases: it is a multichannel transanal access platform manufactured by VECMEDICAL in collaboration with the Valencia Biomechanics Institute (IBV). It consists of:
- •
A 5-cm long silicone introducer ring which is attached to the upper cover by means of an intermediate piece. It is inserted through the rectum and has two tabs with central holes to be able to affix the lid with a suture.
- •
A closure piece between the upper lid and the introducer ring, which allows one piece to be attached to the other.
- •
A 5-cm wide rigid plastic upper lid that contains the 3 working channels: the main channel with a pneumatic system and 2 secondary silicone channels for 5-mm and 12-mm trocars. The upper cover has 2 lateral ports, one for standard laparoscopic insufflation that allows for the creation of pneumorectum and another for insufflation of the main pneumatic system. Using high flow, the pneumorectum is maintained at 10−12 mmHg until appropriate rectal distension is achieved. If there is pushing, the device is compatible with the use of intelligent insufflation systems.
UNI-VEC® presents a main channel with an innovative sealing system unlike others on the market. The system consists of a channel with a balloon that allows for the adaptation of 12−18 mm instruments. This makes it easier to seal the flexible endoscope or optical camera without damaging them and without losing the pneumorectum. It has a luer-lock connection to which the 3-way key with extension can be attached and inflated with air using a syringe. The no-return valve reduces CO2 leakage when removing the endoscope (Fig. 2).
The following procedures have been performed:
- •
Laparoscopy-assisted ESD: The endoscopist performs ESD with the Endoscopic HybridKnife® scalpel (ERBE). To facilitate dissection, the surgeon performs traction and hemostasis with laparoscopic instruments that are inserted through the secondary channels.
- •
Robot-assisted ESD: This technique offers a greater degree of movement and facilitates wall suturing in the event of perforation. It requires placement of 3 robotic trocars, including the 30° robotic camera, which is unavoidable.
- •
TAMIS–“flexible”: In these cases, the flexible endoscope is used as a camera and inserted through the channel with a pneumatic seal. The endoscope provides self-washing and vision without collision with the instruments, which translates into a reduction in intervention time.
We hypothesize that UNI-VEC® could facilitate transanal laparoendoscopic surgery and would be a reproducible, feasible, safe technique for the removal of early rectal tumors. With the device, said approach could be performed in the operating room and in endoscopy rooms by either gastroenterologists or surgeons after specific training. The main objectives of this study are to evaluate whether the device can be used in patients safely and whether it meets established functional requirements:
- •
Enables the specialist to remove pedunculated and sessile polyps or perform EMR and ESD with a minimum size of 2 cm in the rectum in less than 60 min.
- •
Maintains pneumorectum with minimal CO2 leakage through the device.
The secondary objectives include:
- -
Evaluate side effects or unexpected adverse events, giving special importance to hemorrhagic anorectal events, injury to the anorectal canal and perianal region, and anorectal continence evaluated early after performing the procedure using the Wexner Scale.
- -
Evaluate the device in terms of ergonomics:
- o
The shape, size and appearance of the cover-ring closure and the upper cover, allowing the user to open and close the top lid with ease.
- o
The shape, size and appearance of the plugs, allowing the user to easily insert and remove them from the accessory working channels.
- o
The length of the extension is adequate so as not to interfere with the mobility of the user.
- o
The shape, size and hardness of the accessory working channels is adequate to easily insert and withdraw the cannulae.
- o
- -
Evaluate the blister of the device for utility and functionality.
The number of patients recruited was 16 in 12 months (April 2021–2022). During the study, the variables were recorded in an electronic Data Collection Notebook (CRDe) using OpenClinica software managed by SAIL SL (Table 3). This technology collects and transmits data in a secure manner, which can be monitored or managed in real time. Interim analyses were performed after 24 h, 48 h, 1 week, 30 days and 2 months. The variables were analyzed using the SPSS system (version 26.0).
Data collection notebook (CRDe).
| Previous evaluation | Age | Sex | BMI | Employment status | Physical activity | Comorbidity | Coagulation/blood disorders | ASA | Concomitant medication |
|---|---|---|---|---|---|---|---|---|---|
| Preoperative data | N lesions | Location (cm versus margin) | Paris Classification | Date evaluation lesions | TNM | Date evaluation FI | |||
| Lesion type | Location | Wexner | |||||||
| Polyp size (cm) | Wexner severity | ||||||||
| Procedure | Procedure date | Complete? | Where performed | Time | Optical instruments | Instruments for execution | Events related with UNI-VEC | Events related with the procedure | Complications (Clavien) |
| Procedure type | Anesthesia type | CO2 liters | |||||||
| Previous procedure | Bowel preparation | CO2 consumption (L/min) | |||||||
| Specialist | |||||||||
| Hospitalization | Hospitalization time | Hospitalization type | |||||||
| 24 h visit | Date | Complications | |||||||
| 48 h visit | Date | Complications | |||||||
| 7-day visit | Date | Complications | Wexner FI score | Severity | |||||
| 30-day visit | Date | Complications | Wexner FI score | Severity | |||||
| 2-month visit | |||||||||
| Results | Sample size | Date evaluation lesions | TNM | Resectability (margins) | Residual tumor | Multidisciplinary committee assessment | |||
| PA | PA | ||||||||
| C. Haggitt | |||||||||
| UNIVEC evaluation | Reproducibility (0−10) | Safety (0−10) | Satisfaction with UNIVEC | Blister evaluation | Previous experience with similar devices | Is UNI-VEC better? |
Our sample of 16 patients had a mean age of 68 years (Table 4); 56.3% were male and 43.8% female, with a mean BMI of 26.82 and mostly sedentary lifestyle (62.5%). Some 50% were ASA III, and HTN was the most frequent comorbidity (75%). Regarding the preoperative data, 93.8% of the cases presented with a single polyp, while one case (6.3%) had 3 polyps. The types of initial lesion were: 68.8% benign, 18.8% potentially malignant (atypia); and 12.5% carcinoma in situ. The average size was 3.4 cm, and the largest polyp was 6 cm. In terms of location, the average was 6.6 cm from the anal margin (range: 3 cm–10 cm); 31.1% were located on the anterior side, 43.8% on the posterior side, 6.3% right lateral, 12.5% left lateral and 6.3% indeterminate. The Paris Classification categories were: 75% Is, 6.3% IIa, 6.3% IIb, and 12.5% III. Initial continence: 56.3% perfect continence, and 43.8% mild FI. All transanal procedures were performed with the UNI-VEC® device, with prior colon preparation in the operating room and under general anesthesia. One patient had a history of previous endoscopic resection. In 93.8% of treatments, the person responsible was the surgeon and in 6.3% the endoscopist. The procedures included: EMR (6.3%), ESD (43.8%), EFTR (6.3%) and TAMIS (43.8%). The average intervention time was 73.25 min (range: 25–165), with an average CO2 consumption of 96.64 L. In 56.3% of cases, a rigid 30° camera was used, while in 43.8% the flexible endoscope was the vision instrument. Instruments for the execution of the intervention included: hook-knife (43.8%), loop diathermy (6.3%), harmonic scalpel (6.3%), endoscopic scalpel (12.5%), harmonic scalpel and hook (6.3%), endoscopic scalpel and hook (6.3%), hook with robotic assistance (6.3%), and endoscopic scalpel with robotic assistance (6.3%). The procedure was completed in 100% of cases.
Descriptive analysis of the results.
| N | Minimum | Maximum | Mean | SD | |
|---|---|---|---|---|---|
| Age | 16 | 42 | 83 | 68.13 | 10.732 |
| BMI | 16 | 19.6 | 34.7 | 26.825 | 4.5034 |
| Initial polyp size (cm) | 16 | 1.0 | 6.0 | 3.444 | 1.4733 |
| Location in cm from the anal margin | 16 | 3 | 10 | 6.63 | 2.872 |
| Wexner scale | 16 | 0 | 2 | 0.63 | 0.806 |
| Procedure time (min) | 16 | 25 | 165 | 73.25 | 41.932 |
| Liters of CO2 | 11 | 20 | 235 | 96.64 | 85.335 |
| CO2 consumption rate | 11 | 6.400 | 7344.000 | 1436.21818 | 2150.294483 |
| Hospitalization (days) | 16 | 1 | 4 | 2.31 | 0.873 |
| Wexner scale (1 week) | 15 | 0 | 14 | 2.13 | 4.658 |
| Wexner (30 days) | 15 | 0 | 15 | 2.33 | 4.608 |
| Wexner (2 months) | 4 | 0 | 12 | 5.00 | 5.598 |
| Specimen size (mm) | 16 | 15 | 70 | 34.81 | 15.433 |
| Reproducibility | 14 | 6 | 10 | 9.43 | 1.089 |
| Safety | 14 | 8 | 10 | 9.71 | 0.726 |
| Level of satisfaction with device | 14 | 7 | 10 | 9.29 | 1.069 |
| Evaluation of blister funcionality | 14 | 7 | 10 | 9.50 | 1.019 |
| N valid (by list) | 2 |
Regarding the events related to the UNI-VEC® device, 37.5% did not present any difficulty, 18.8% described difficulties in placing the device, one case presented difficulty in freedom of movement (6.35%), in another there was a rupture of the pneumatic seal due to technical difficulties related to the size and location of the polyp and the impossibility to create the pneumorectum (6.3%). Regarding events related to the procedure, 56.3% did not present difficulties, in 2 cases there were difficulties in navigation with the endoscope and incorrect triangulation to exert traction and countertraction with tissues (12.5%), and one case a collision of instruments inside the rectum (6.3%). Patients were admitted in all cases, and mean hospital stay was 2.31 days. Postoperative complications were recorded according to the Clavien classification (I-IV) as: 0 = No complications; Tears: 1 = Mild, 2 = Moderate (fever, IV antibiotic), 3 = Severe (injury or perforation of the rectal wall requiring repair in the operating room); Bleeding: 4 = Mild (watch & wait), 5 = Moderate (blood transfusion), 6 = Severe (reoperation), 7 = post-polypectomy syndrome, 8 = Stenosis. Follow-up visits were 24 h, 48 h, 7 days, 30 days and 2 months after surgery. The assessments after the first 24 h, 48 h and 7 days were in person. The lead researcher determined whether the following consultations were in person or by telephone, which were generally by telephone after 30 days and 2 months. There were no postoperative complications in 75% after 24 h, 93.8% after 48 h, and 78.6% after 7 days. Mild bleeding (Clavien I) occurred in 25%, 6.3% and 21.4% after 24 h, 48 h and 7 days, respectively. Continence was assessed according to the Wexner scale. After 7 days, 60% presented perfect continence, 26.7% mild FI, and 13.3% moderate FI. After 30 days, 66.7% had perfect continence, 20% mild FI, and 13.3% moderate FI. After 2 months, 4 of the patients were reviewed who, after 30 days, presented Wexner scores higher than preoperatively and perfect continence was demonstrated in 25% of the patients, 50% mild FI and 25% moderate FI. At the final follow-up visit of each patient, perfect continence was reported by 75% of the patients, mild incontinence 6.25%, and moderate incontinence 18.75%. The follow-up of patients with moderate incontinence was extended to observe their evolution. In the 16 patients treated, no cases of severe incontinence, lesions or perforation of the rectal wall requiring major surgical intervention were recorded.
According to the histological results, mean specimen size was 34.81 mm; 56.3% were benign lesions (3 non-neoplastic [18.8%], 3 low-grade dysplasia [18.8%], 3 high-grade dysplasia [18.8%]), and 43.8% were malignant (one adenoma [6.3%], 4 intramucosal carcinoma [25%] and 2 invasive adenocarcinoma [12.5%]). In 87.5% there was no residual lesion, while one case presented microscopic residual tumor (6.3%) and another macroscopic residual tumor (6.3%). In the latter case, the multidisciplinary committee decided to administer RTx, and the remainder stayed under surveillance.
As for the reproducibility, safety, satisfaction with the device and evaluation of the blister, the scores from 0 to 10 were 9.43, 9.71, 9.29 and 9.50, respectively. All researchers had prior experience with transanal devices.
DiscussionScreening for colorectal cancer (CRC) has resulted in the increased diagnosis of earlier-stage lesions. These lesions are a diagnostic challenge and require complex clinical decision-making to avoid overtreatment with unnecessary mortality and morbidity on the one hand, or undertreatment on the other. This dilemma is even more significant in the rectum, where radical surgical resections are associated with higher rates of serious morbidity and mortality (permanent stoma, sexual dysfunction, etc), which affect quality of life and can be avoided with endoscopic and/or minimally invasive techniques14. Some 10%–15% of polyps are “complex” and are therefore considered unsuitable for conventional endoscopic removal. Although the experience of the endoscopist plays a fundamental role, some common characteristics are defined by the SMSA score15: size (>20 mm), shape (sessile), difficult to locate (right side, ileocecal valve, peridiverticular) or difficult to access, such as polyps in previously treated areas. CP carry a higher risk of CRC, so en bloc resection is essential. In the effort to increase organ preservation strategies, local excision is a valid option for the treatment of CP and early rectal cancer, as long as the clinical and pathological characteristics are favorable16.
Endoscopic techniques that are usually used include EMR and ESD. EMR is not appropriate for en bloc resection of laterally spreading tumors (LST) >20 mm, as it is associated with high rates of specimen fragmentation, positive margins and, therefore, local recurrence1. ESD has a higher en bloc excision rate and low recurrence rate (2%). However, it is associated with a longer procedure time, a greater learning curve and risk of complications (perforation is detected in 5% of cases)2.
Beck et al.17 described laparoscopic-assisted endoscopic polypectomy in 1993. Since then, combined endo-laparoscopic surgery (CELS) and combined endoscopic robotic surgery (CERS), which includes robotic support, have been used to treat PC in different locations to avoid the morbidity and mortality associated with segmental oncological surgery18.
Local rectal surgery can be performed using conventional transanal excision (TAE)5, rigid access platforms like transanal endoscopic microsurgery (TEM)19 and transanal endoscopic operation (TEO)7, or flexible platforms for performing TAMIS8,9 (transanal minimally invasive surgery), which requires the use of single-port devices: the SILSport® (Covidien), GelPOINT Path (AppliedMedical), PAT® (Developia)20. The Da Vinci® system offers its Si® and SP® robotic platforms, the Flex® Colorectal system (Medrobotics) and, in the development phase, STRAS® (Single-Access Transluminal Robotic Assistant for Surgeons), etc.21,22.
The choice of platform for transanal minimally invasive surgery is still a matter of debate. In the search for the ideal platform to facilitate laparoendoscopic surgery, a new prototype called UNI-VEC® has emerged (Fig. 2). It is a sterile, single-use, multichannel device that allows surgeons to work in the anorectal canal, facilitating the hybridization of laparoscopic and flexible endoscopic instruments. Although its current indicated use is transanal, the device can potentially be used for abdominal and vaginal surgery. The first prototype was developed at the IBV. A previous experimental preclinical study was carried out with 36 porcine models, and the European patent was applied for. To continue the development process, a public Innovation (Code 100) contract was applied for, and the VECMEDICAL® company was hired with the aim to industrialize the device and obtain the CE mark to be able to market the product. UNI-VEC® facilitates working with the flexible endoscope, but with the support of surgical instruments. Standard laparoscopic instruments can be used with the secondary channels, which favor the removal of lesions of the shape and size that endoscopic instruments alone could not resect: they offer greater traction with grasping forceps, greater hemostasis and in situ repair of the wall rectal in case of injury. The ability to use standard instruments also lowers costs compared to other platforms.
The systematic review by Arezzo et al.23 compared ESD versus transanal surgery with the TEM device for the treatment of non-pedunculated rectal lesions. Twenty-one studies were analyzed: 11 ESD series and 10 TEM series, with a total of 1541 patients. Intervention time was 96 min in ESD versus 67 min in the TEM series. In our pilot study, 93.8% of the procedures were performed by the surgeon and 6.3% by the endoscopist. EMR (6.3%), ESD (43.8%), REC (6.3%) and TAMIS (43.8%) were performed. Mean procedure time was 73.25 min (range: 25–165). In 56.3%, a rigid 30° camera was used, while in 43.8% flexible endoscope was used as a viewing instrument. The surgeons in the study concluded that using the flexible endoscope decreases the intervention time since the endoscope itself facilitates self-cleaning and does not require withdrawal to clean the camera. In addition, the flexible endoscope offers 360° vision, without collision of the instruments. Also, when the endoscopist performs ESD, the intervention time is reduced by being able to introduce instruments through the accessory trocars to facilitate polyp traction, hemostasis or wall suture, if necessary. As for the histological results, the review by Arezzo described a mean polyp size of 35 mm in the ESD series versus 40 mm in TEM. The en bloc resection rate was 87.8% for ESD and 98.7% in the TEM series, and the R0 resection rate was 74.6% for ESD and 88.5% for TEM. The pooled estimation for the percentage of patients with invasive adenocarcinoma was 9.5% in the ESD series and 3.9% in the TEM series. In our series, the mean size of the specimen was 34.81 mm. Our results showed that 56.3% were benign lesions (3 non-neoplastic [18.8%], 3 low-grade dysplasia [18.8%], 3 high-grade [18.8%]), and 43.8% were malignant (1 adenoma [6.3%], 4 intramucosal carcinoma [25%] and 2 invasive adenocarcinoma [12.5%]). We observed no residual lesion in 87.5%, one case of microscopic residual tumor (6.3%) and another macroscopic residual tumor (6.3%). Regarding perioperative complications, Arezzo registered 8% complications for ESD (19 hemorrhages, 20 perforations) and 8.4% for TEM (30 hemorrhages, 7 fistulae, 43 suture leaks, 17 other). In the ESD group, 1.3% of patients required abdominal surgery and 1.6% TEM to resolve complications. In our series, mild bleeding (Clavien I) occurred in 25%, 6.3% and 21.4% after 24 h, 48 h, and 7 days, respectively. Bleeding in the first 24 h was the main reason for increased hospitalization time (2.31 days).
The TRIASSIC study24 is a European clinical trial with 15 participating centers comparing TAMIS surgery and ESD for en bloc resection of rectal lesions within <15 cm without pedicles >2 cm; follow-up studies 6 and 12 months after surgery include rectoscopy. The GelPOINT patch device is being used to ensure the greatest possible uniformity in the procedures. We are awaiting the publication of these results to compare them with our study20.
Cooperation between surgeons and endoscopists, as well as prior experimental training, will facilitate the implementation of these hybrid procedures that seek to resolve the limitations of pure endoscopic surgical techniques, such as the lack of effective retraction, adequate vision, and triangulation of instruments, which are basic elements for effective surgery. The advantages that the added robotic support has provided are three-dimensional vision, tremor elimination, greater ergonomics and the seven degrees of movement of the robotic arms, all of which make it easier to access large lesions without depending on the patient position or location of the polyp. These characteristics are a great benefit when working in a reduced surgical field such as the anorectal canal.
LimitationsThis is a pilot study in a sample of 16 patients. Different advanced endoscopic approaches were performed, such as ESD by the endoscopist with the collaboration of the surgeon, or as minimally invasive transanal surgery by the surgeon using the flexible endoscope as a camera. In June 2022, authorization was given to extend our research (requested 01/15/2021) to include 8 other medical centers:
- •
Hospital del Mar (Barcelona)
- •
Hospital QuironSalud “Sagrado Corazón” de Sevilla
- •
Hospital Universitario Reina Sofía (Murcia)
- •
Hospital Universitario de Cruces (Barakaldo)
- •
Hospital Universitario Virgen Macarena de Sevilla
- •
Complejo Hospitalario Universitario de Vigo (CHUVI)
- •
Clínica Rotger (Palma de Mallorca)
- •
Hospital Universitario de Cabueñes (Gijón)
- •
Hospital Universitario La Fe (Valencia)
This study will have an expected duration of 24 months. With the extension to 14 hospitals, the patient recruitment period will be 18 months, with competitive inclusion until a sample of 40 patients is reached. New variables will be added in the CRD to improve the final results of the research project:
- -
Use of dilators prior to placement of the device
- -
Intelligent insufflation systems to facilitate pneumorectum
- -
Preoperative studies carried out: endoscopic ultrasound, MRI, CT
- -
Number of cases recruited from the screening program
The results of this pilot clinical study with 16 patients have demonstrated the efficacy and safety of the UNI-VEC® device for the treatment of rectal lesions, proving it to be a device that optimizes the joint work of surgeons and endoscopists while allowing for the concomitant use of rigid laparoscopic and flexible endoscopic instruments. It has also been shown to be a safe and versatile transanal platform to be used in transanal robotic procedures.
FundingDuring the study we have been assisted by two different entities: In the experimental development part with proof of concept and redesign of prototypes, with help from SERGAS- ACIS (Servizo Galego de Saúde - Axencia Galega de Coñecemento en Saúde), thanks to the call for aid for innovation projects PRIS-2. The development of the randomized clinical trial has been supported by the company Vecmedical SL, Spain.
Conflicts of interestsThe authors have no conflicts of interests to declare.