Simultaneous pancreas-kidney (SPK) transplant is a proven option of treatment for patients with type 1 diabetes mellitus and related end-stage renal disease, who are candidates for kidney transplantation. The results from the beginning of SPK transplant program in Comunidad Valenciana are presented.
MethodsDescriptive, retrospective, and single-center study of the pancreas transplant performed at the Hospital Universitari i Politècnic La Fe, from September 2002 to December 2015. Clinical variables from donors and recipients, peri-operative variables, patient survival, and pancreatic graft survival were collected.
ResultsEighty-one patients with type 1 diabetes mellitus (48 males and 33 females, mean age 37.4 ± 5.7 years, mean BMI 24.1 ± 3.4 kg/m2, mean duration of diabetes 25.5 ± 6.5 years) received SPK transplantation. The overall patient survival at one, 3, and 5 years were 91.3%, 91.3% and 89.5%, respectively. However, patient survival in the periods 2002−2008 and 2009−2015 were 88.2% and 93.6% at one year, 88.2% and 93.7% at 3 years, and 85.3% and 93.7% at 5 years, respectively (P = 1). The overall pancreatic graft survival at one, 3, and 5 years were 75.2%, 69.1% and 63.2%, respectively. On the other hand, pancreatic graft survival in the periods 2002−2008 and 2009−2015 were 67.5% and 80.6% at one year, 64.7% and 71.8% at 3 years, and 58.8% and 65.3% at 5 years, respectively (P = .0109). Post-transplant complications were: graft rejection 8.6%, venous graft thrombosis 7.4%, graft pancreatitis 4.9%.
ConclusionsIn 13 years’ experience of SPK transplantation, patient and pancreatic graft survival and the rate of complications after pancreas transplantation were similar to those of other larger series. The medical-surgical team experience improves pancreatic graft survival without influencing patient survival.
El trasplante simultáneo de páncreas-riñón (SPK, por simultaneous pancreas kidney) es una opción terapéutica válida en pacientes afectos de diabetes mellitus tipo 1 con enfermedad renal crónica terminal que son candidatos a trasplante renal. Se presentan los resultados desde el inicio del programa de trasplante SPK en la Comunidad Valenciana.
MétodosEstudio descriptivo, retrospectivo y unicéntrico de los trasplantes de páncreas realizados en el Hospital Universitari i Politècnic La Fe, desde septiembre de 2002 a diciembre de 2015. Se recogieron variables clínicas de los donantes y receptores, variables peri-operatorias y supervivencia del paciente y del injerto pancreático.
ResultadosOchenta y un pacientes con diabetes mellitus tipo 1 (48 hombres y 33 mujeres, de 37,4 ± 5,7 años, IMC de 24,1 ± 3,4 kg/m2, con una duración de su diabetes de 25,5 ± 6,5 años) recibieron un trasplante SPK. La supervivencia global del paciente a uno, 3 y 5 años fue del 91,3, el 91,3 y el 89,5%, respectivamente. Sin embargo, la supervivencia del paciente en los periodos 2002−2008 y 2009−2015 fue del 88,2 y el 93,6% al año, del 88,2 y el 93,7% a los 3 años, y del 85,3 y el 93,7% a los 5 años, respectivamente (P = 1). La supervivencia global del injerto pancreático a uno, 3 y 5 años fue del 75,2, el 69,1 y el 63,2%, respectivamente. Por otra parte, la supervivencia del injerto pancreático en los periodos 2002−2008 y 2009−2015 fue del 67,5 y el 80,6% al año, del 64,7 y el 71,8% a los 3 años, y del 58,8 y el 65,3% a los 5 años, respectivamente (P = ,0109). Las complicaciones postrasplante fueron: rechazo del injerto en un 8,6%, trombosis venosa del injerto en un 7,4% y pancreatitis del injerto en un 4,9%.
ConclusionesEn 13 años de experiencia en trasplante SPK, la supervivencia del paciente y del injerto pancreático y la tasa de complicaciones tras el trasplante de páncreas son similares a las de otras series de mayor tamaño. La experiencia del equipo médico-quirúrgico mejora la supervivencia del injerto pancreático, sin influir en la supervivencia del paciente.
Simultaneous pancreas-kidney (SPK) transplantation is a therapeutic alternative in patients with type 1 diabetes mellitus (T1DM) and end-stage chronic kidney disease who are candidates for kidney transplantation. Successful SPK transplantation followed by good patient progress achieves insulin independence and long-term normoglycemia, which makes it possible to evaluate the effect of prolonged normalization of glucose metabolism on the course of diabetic complications, such as retinopathy and neuropathy1. It has also been shown to reduce cardiovascular risk factors (such as dyslipidemia and arterial hypertension), improve cardiac function and reduce mortality, thereby increasing the quality of life of these patients2.
The first SPK transplantations were performed at the University of Minnesota (USA) in the 1960s3. However, the morbidity and mortality rates associated with these procedures at that time was high4,5. It was not until the 1980s that they reached Europe6, and the first SPK transplantation in Spain was performed in 1983 at the Hospital Clínic in Barcelona6,7. Since then and until 2015, a total of 1632 pancreas transplantations have been performed in Spain, and SPK transplantation is the most frequently performed8.
Several variables have been shown to be essential to achieve good transplantation results and to minimize complications. These include donor selection, the need for prior dialysis or the experience of the medical-surgical team in charge of performing and managing SPK transplantations9.
The objective of this study is to describe the results of SPK transplantation in the Comunidad Valenciana region of Spain, measured in survival of the patient and of pancreatic and renal grafts, globally and in the time periods from 2002 to 2008 and from 2009 to 2015, while also describing the related complications.
MethodsWe conducted a retrospective, descriptive and single-center study of all patients undergoing pancreas transplantation consecutively from September 2002 to December 2015 in the Comunidad Valenciana.
The donor selection criteria were those from 20158: age between 10 and 45 years; weight greater than 30 kg; compatible blood group and hemodynamic stability; no personal or family history of diabetes mellitus; no previous pancreatic trauma, history of acute or chronic pancreatitis, previous pancreatic surgery or splenectomy; no history of chronic alcoholism; no malignant, infectious or communicable disease; and negative serology for human immunodeficiency virus (HIV) and hepatitis B and C viruses.
The criteria for selecting recipients for SPK transplantation were also those of 2015: diagnosis of T1DM; age under 50 years (with individual assessment of patients above that age); absence of confirmed pancreatic reserve with peptide values C < 0.5 ng/mL; absence of severe psychiatric or psychological disorders; and ability to understand what a pancreas transplant entails in terms of collaboration in the postoperative period (complications that may arise and in the follow-up of treatment). Recipient exclusion criteria were: positive HIV infection or active infection; uncontrollable coagulation abnormalities; positive T-cell crossmatch with current serum; active drug or alcohol addiction; incapacitating psychiatric illness; personal history of cancer (last 5 years); recent myocardial infarction (last 6 months); coronary angiography with non-correctable lesions; cardiac ejection fraction less than 50%; body mass index (BMI) greater than 30 kg/m2; non-compliance with previous treatments or recent retinal hemorrhage.
Clinical data were collected for donors and recipients, both systematically and retrospectively by consulting the patient files. Baseline laboratory data were collected for donors and recipients; in addition, post-transplant progress data were recorded for recipients up until the end of the study.
The protocol used in pancreas transplantation has been previously described9,10. The same surgical technique, immunosuppression regimen, and antithrombotic and antibiotic prophylaxes were used for all patients.
All pancreas and kidney implants, as well as all graft explants, were performed by the same surgical team, using the same standardized surgical technique in all cases9,10. All pancreatic and kidney grafts came from cadaver donors.
The duodenum-pancreas graft was extracted en bloc with the liver graft, and the duodenum and pancreas were subsequently separated. For graft preservation, Wisconsin solution was used for the initial transplants until 2009, later followed by the Celsior solution. The maximum accepted cold ischemia time was 12 h.
During bench surgery, the arterial vascular reconstruction of the pancreas was performed by means of a ‘Y’ graft from the donor’s common iliac; the external iliac artery was anastomosed to the superior mesenteric artery and the internal iliac artery to the splenic artery of the graft. Systemic venous drainage was performed with an end-to-side anastomosis between the graft portal vein and the inferior vena cava. The pancreatic graft was placed intraperitoneally in the right iliac fossa, and the kidney graft was placed extraperitoneally in the left iliac fossa, both through the same midline incision. For the diversion of the pancreatic exocrine secretion, enteric drainage was performed by means of a side-to-side intestinal anastomosis between the graft duodenum and the recipient’s ileum, with double continuous suture in the intestinal serous and submucosal layers. The exocrine secretion was not diverted to the bladder.
Either antithymocyte globulin or basiliximab was used as induction immunosuppressive therapy10. As maintenance immunosuppressive therapy, the most common regimen consisted of a combination of tacrolimus, mycophenolate mofetil or sirolimus, and prednisone.
The infection prophylaxis protocol was: antibacterial, with amoxicillin-clavulanate 1 g/8 h (iv) (if allergy: ciprofloxacin 200 mg/12 h) during the first 3 postoperative days; antifungal, with fluconazole at a dose of 4 mg/kg/day (iv or oral) for 2 months; against Pneumocystis jirovecii, with trimethoprim/sulfamethoxazole at a dose of 80/400 mg, one tablet per day as the patient begins oral intake, for 6 months; and antiviral against cytomegalovirus, with ganciclovir 5 mg/kg/12 h (iv) for 7 days (in cases of: positive donor and recipient, positive donor with negative recipient, and negative donor with positive recipient). It was not administered in the case of a negative donor and recipient. Starting on the 7th day, oral valganciclovir was administered at a dose adjusted to renal function for a minimum of 3 months.
Vascular thrombosis prophylaxis was carried out with low-molecular-weight heparin (enoxaparin 4000 IU/day), starting 24 h after surgery, and with acetylsalicylic acid at a dose of 100 mg every 24 h starting day 5 or 6 post-transplant.
Glycemic, lipid and blood pressure control goals were defined in accordance with the recommendations of the American Diabetes Association11. Maintained pancreatic graft function12 was defined as an HbA1c < 7.0%, without insulin or with a daily insulin dose <50% of the baseline needs prior to transplantation and <0.5 IU/kg/day, together with C-peptide levels higher than the baseline level and without presenting severe hypoglycemia. Pancreas graft loss was defined as a situation when, after transplantation, the graft presented partial function that required the use of exogenous insulin at a dose of >50% of baseline needs12, there was rejection, T1DM recurred10, or pancreatectomy was conducted due to surgical complications. Maintained kidney graft function was defined as achieving complete independence from dialysis, and loss of kidney graft was considered the need for dialysis after transplantation. Acute pancreas graft rejection was suspected when serum amylase and/or lipase levels and/or serum glucose levels increased, along with a marked drop in serum C-peptide levels and/or abdominal pain. However, since serum amylase and lipase measurements tend to be relatively nonspecific, when acute rejection of the pancreas was suspected, the diagnosis was confirmed by ultrasound-guided percutaneous biopsy of the pancreas graft. The tail of the pancreas was the area chosen for biopsy, since it generally provides better histological support because it has the highest density of beta cells. Nonetheless, while most cases of rejection of the renal and pancreatic grafts usually coexisted simultaneously, only biopsy of the renal graft was performed for confirmation. In the event that the biopsy result was inconclusive, a pancreatic graft biopsy was performed exclusively. The Banff criteria were followed for the classification of pancreatic graft rejection13.
This study was approved by the Clinical Research Ethics Committee of the Instituto de Investigación de Salud La Fe in Valencia (Spain). All patients gave their written informed consent prior to inclusion in the study.
Statistical analysisContinuous variables are reported as median and interquartile range, both at baseline and during follow-up. Categorical variables are described as proportions. As for the statistical analysis of the continuous variables, the chi-squared test was used to verify the normality of the data, the Student’s t test for paired data, and the Wilcoxon test for unpaired data. For categorical variables, the Pearson’s test was used; however, for comparisons with an N less than 10 (GL = 9), Fisher’s exact test was used instead of Pearson’s. Comparisons were made between the baseline period and the year of follow-up and also according to the period of the intervention: 2002−2008 and 2009−2015. This division was established since they are each 7-year periods, which is an equitable division of the years of evolution of the transplant program in the Comunidad Valenciana.
Survival of the patient, pancreatic graft, and kidney graft are described using the Kaplan-Meier method to estimate survival function. Total functions and for each period are described. The statistical comparison between periods was carried out using the non-parametric Mann-Whitney function and over equivalent follow-up periods (5 years). In all the tests, a P value <.05 was considered statistically significant.
The statistical analysis was completed with SPSS® Statistics (version 20) and Matlab R2019a.
ResultsA total of 104 patients were evaluated as possible candidates for pancreas transplantation, 22 of which (21.2%) were excluded because they did not meet the inclusion criteria.
The other 82 patients (78.8%) underwent pancreatic transplantation: 81 SPK transplantation, and one isolated pancreas transplantation in a patient who had previously received a renal transplant (PAK, or ‘pancreas after kidney’). No isolated pancreas transplantation (PTA, or ‘pancreas transplantation alone’) was performed in our center.
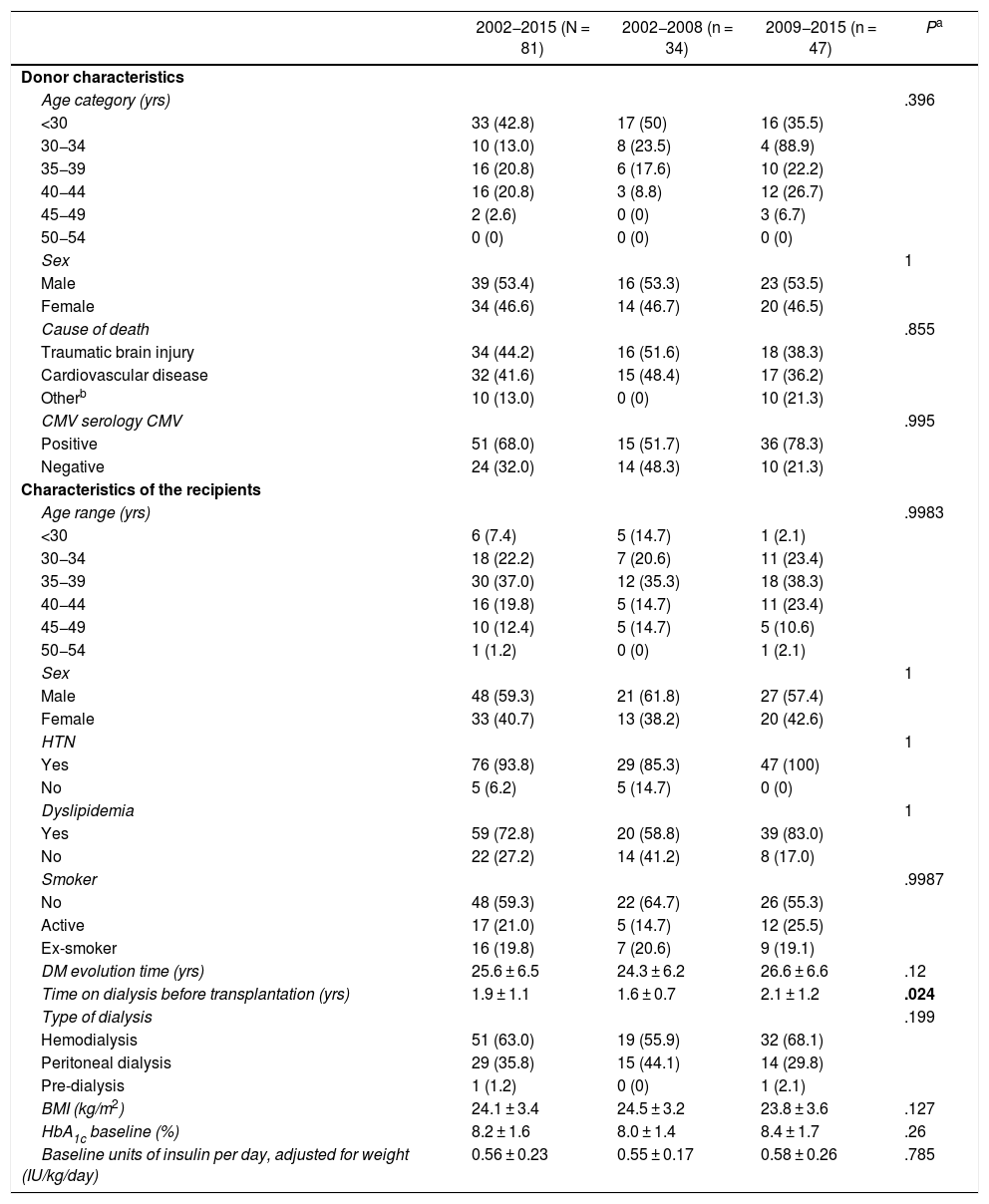
For the present study, patients who had undergone SPK transplantation were considered. The characteristics of the 81 donors and the baseline characteristics of the 81 recipient patients are shown in Table 1. Wisconsin preservation solution was used in 18 grafts and the Celsior solution in the remaining 63.
Characteristics of donors and recipients of the 81 simultaneous pancreas-kidney transplants and comparisons between both periods (2002–2008 and 2009–2015).
| 2002−2015 (N = 81) | 2002−2008 (n = 34) | 2009−2015 (n = 47) | Pa | |
|---|---|---|---|---|
| Donor characteristics | ||||
| Age category (yrs) | .396 | |||
| <30 | 33 (42.8) | 17 (50) | 16 (35.5) | |
| 30−34 | 10 (13.0) | 8 (23.5) | 4 (88.9) | |
| 35−39 | 16 (20.8) | 6 (17.6) | 10 (22.2) | |
| 40−44 | 16 (20.8) | 3 (8.8) | 12 (26.7) | |
| 45−49 | 2 (2.6) | 0 (0) | 3 (6.7) | |
| 50−54 | 0 (0) | 0 (0) | 0 (0) | |
| Sex | 1 | |||
| Male | 39 (53.4) | 16 (53.3) | 23 (53.5) | |
| Female | 34 (46.6) | 14 (46.7) | 20 (46.5) | |
| Cause of death | .855 | |||
| Traumatic brain injury | 34 (44.2) | 16 (51.6) | 18 (38.3) | |
| Cardiovascular disease | 32 (41.6) | 15 (48.4) | 17 (36.2) | |
| Otherb | 10 (13.0) | 0 (0) | 10 (21.3) | |
| CMV serology CMV | .995 | |||
| Positive | 51 (68.0) | 15 (51.7) | 36 (78.3) | |
| Negative | 24 (32.0) | 14 (48.3) | 10 (21.3) | |
| Characteristics of the recipients | ||||
| Age range (yrs) | .9983 | |||
| <30 | 6 (7.4) | 5 (14.7) | 1 (2.1) | |
| 30−34 | 18 (22.2) | 7 (20.6) | 11 (23.4) | |
| 35−39 | 30 (37.0) | 12 (35.3) | 18 (38.3) | |
| 40−44 | 16 (19.8) | 5 (14.7) | 11 (23.4) | |
| 45−49 | 10 (12.4) | 5 (14.7) | 5 (10.6) | |
| 50−54 | 1 (1.2) | 0 (0) | 1 (2.1) | |
| Sex | 1 | |||
| Male | 48 (59.3) | 21 (61.8) | 27 (57.4) | |
| Female | 33 (40.7) | 13 (38.2) | 20 (42.6) | |
| HTN | 1 | |||
| Yes | 76 (93.8) | 29 (85.3) | 47 (100) | |
| No | 5 (6.2) | 5 (14.7) | 0 (0) | |
| Dyslipidemia | 1 | |||
| Yes | 59 (72.8) | 20 (58.8) | 39 (83.0) | |
| No | 22 (27.2) | 14 (41.2) | 8 (17.0) | |
| Smoker | .9987 | |||
| No | 48 (59.3) | 22 (64.7) | 26 (55.3) | |
| Active | 17 (21.0) | 5 (14.7) | 12 (25.5) | |
| Ex-smoker | 16 (19.8) | 7 (20.6) | 9 (19.1) | |
| DM evolution time (yrs) | 25.6 ± 6.5 | 24.3 ± 6.2 | 26.6 ± 6.6 | .12 |
| Time on dialysis before transplantation (yrs) | 1.9 ± 1.1 | 1.6 ± 0.7 | 2.1 ± 1.2 | .024 |
| Type of dialysis | .199 | |||
| Hemodialysis | 51 (63.0) | 19 (55.9) | 32 (68.1) | |
| Peritoneal dialysis | 29 (35.8) | 15 (44.1) | 14 (29.8) | |
| Pre-dialysis | 1 (1.2) | 0 (0) | 1 (2.1) | |
| BMI (kg/m2) | 24.1 ± 3.4 | 24.5 ± 3.2 | 23.8 ± 3.6 | .127 |
| HbA1c baseline (%) | 8.2 ± 1.6 | 8.0 ± 1.4 | 8.4 ± 1.7 | .26 |
| Baseline units of insulin per day, adjusted for weight (IU/kg/day) | 0.56 ± 0.23 | 0.55 ± 0.17 | 0.58 ± 0.26 | .785 |
CMV: cytomegalovirus; DM: diabetes mellitus; HbA1c: glycosylated hemoglobin; HTN: arterial hypertension; BMI: body mass index.
The results have been calculated based on the data available and expressed as number (percentage) or as mean ± standard deviation.
After pancreas transplantation, 8 out of the 81 initial patients died between 2002 and 2015 (Fig. 1), obtaining an overall mortality rate of 9.9%. Six of these patients died within the first 3 months after transplantation, with a median and interquartile range of 1.5 [0.00–6.75] months after transplantation. The causes of mortality were:
- -
Infectious, in 4 cases (50% of total deaths): 2 abdominal surgical infections, one hospital-acquired pneumonia, and one urinary infection that led to septic shock.
- -
Venous thrombosis of the pancreatic graft, in 2 cases (25%): in one, coexisting with renal graft thrombosis.
- -
Iliac-mesenteric fistula, only one case (12.5%).
- -
Postoperative hemorrhage, only one case (12.5%).
Evolution of SPK transplants.
T1DM: type 1 diabetes mellitus; HbA1c: glycosylated hemoglobin; SPK: simultaneous pancreas kidney transplantation.
*Pancreas graft functionality was maintained in accordance with Igls criteria12.
After SPK transplantation, 52 patients had optimal or good pancreatic graft function according to Igls criteria12, including 41 patients who did not require insulin and the remaining 11 had low requirements, at a rate of 0.20 [0.16−0.24] IU/kg/day 6 months after transplantation (Fig. 1). Demographic, clinical, and biochemical data were collected from these 52 patients during their evolution after SPK transplantation. These 52 patients with a normally functioning pancreatic grafts achieved good control of their blood pressure and lipid profile during post-transplant follow-up, with or without antihypertensive or lipid-lowering treatment, respectively (Fig. 2).
Baseline and evolution of HbA1c (A), LDL and patients receiving cholesterol-lowering treatment (B), SBP and DBP levels and patients with antihypertensive treatment (C) post-SPK transplantation of patients with normal functioning pancreas and kidney grafts. The results are expressed as median and percentage.
HbA1c: glycosylated hemoglobin; LDL: low-density lipoprotein cholesterol; SPK: simultaneous pancreas kidney transplantation; DBP: diastolic blood pressure; SBP: systolic blood pressure.
Out of the 29 remaining patients, 8 died, 17 lost the pancreatic graft due to graft rejection, recurrence of T1DM or surgical graft explant, and the remaining 4 presented functional failure of the pancreatic graft with HbA1c levels >7% or with insulin requirements greater than baseline (Fig. 1).
Overall patient survival rates one, 3, and 5 years after SPK transplantation were 91.3%, 91.3%, and 89.5%, respectively. However, patient survival rates for the periods 2002−2008 and 2009−2015 were 88.2% and 93.6% after one year, 88.2% and 93.7% after 3 years, and 85.3% and 93.7% after 5 years, respectively, although the differences between the two periods were not statistically significant (P = 1) (Fig. 3A).
Overall pancreatic graft survival rates one, 3 and 5 years after transplantation were 75.2%, 69.1% and 63.2%, respectively, while the kidney graft survival rates were 95.1% in the 3 cases (Figs. 3B and C). However, survival rates of the pancreatic graft in the periods 2002−2008 and 2009−2015 were 67.5% and 80.6% after one year, 64.7% and 71.8% after 3 years, and 58.8% and 65.3% after 5 years, respectively, which was statistically significant (P = .0109) between the 2 evaluated periods.
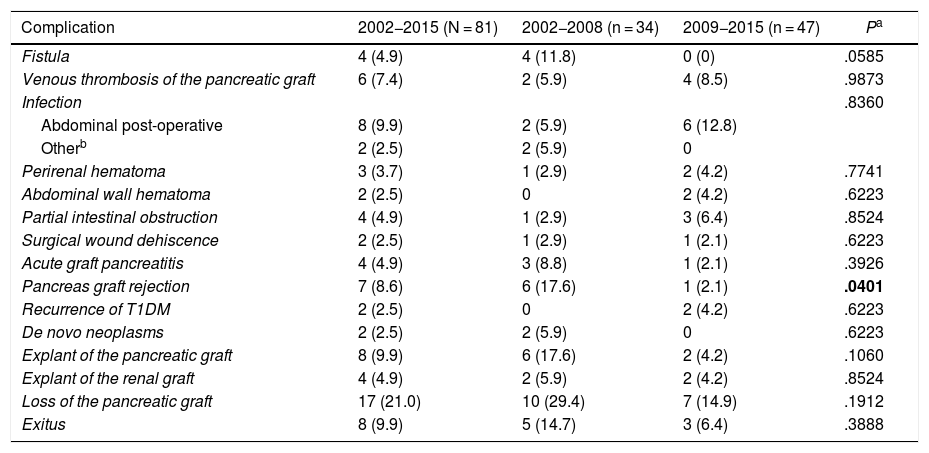
The complications that appeared after SPK transplants are shown in Table 2.
Surgical complications after the simultaneous pancreas-kidney transplantation: global and by time period.
| Complication | 2002−2015 (N = 81) | 2002−2008 (n = 34) | 2009−2015 (n = 47) | Pa |
|---|---|---|---|---|
| Fistula | 4 (4.9) | 4 (11.8) | 0 (0) | .0585 |
| Venous thrombosis of the pancreatic graft | 6 (7.4) | 2 (5.9) | 4 (8.5) | .9873 |
| Infection | .8360 | |||
| Abdominal post-operative | 8 (9.9) | 2 (5.9) | 6 (12.8) | |
| Otherb | 2 (2.5) | 2 (5.9) | 0 | |
| Perirenal hematoma | 3 (3.7) | 1 (2.9) | 2 (4.2) | .7741 |
| Abdominal wall hematoma | 2 (2.5) | 0 | 2 (4.2) | .6223 |
| Partial intestinal obstruction | 4 (4.9) | 1 (2.9) | 3 (6.4) | .8524 |
| Surgical wound dehiscence | 2 (2.5) | 1 (2.9) | 1 (2.1) | .6223 |
| Acute graft pancreatitis | 4 (4.9) | 3 (8.8) | 1 (2.1) | .3926 |
| Pancreas graft rejection | 7 (8.6) | 6 (17.6) | 1 (2.1) | .0401 |
| Recurrence of T1DM | 2 (2.5) | 0 | 2 (4.2) | .6223 |
| De novo neoplasms | 2 (2.5) | 2 (5.9) | 0 | .6223 |
| Explant of the pancreatic graft | 8 (9.9) | 6 (17.6) | 2 (4.2) | .1060 |
| Explant of the renal graft | 4 (4.9) | 2 (5.9) | 2 (4.2) | .8524 |
| Loss of the pancreatic graft | 17 (21.0) | 10 (29.4) | 7 (14.9) | .1912 |
| Exitus | 8 (9.9) | 5 (14.7) | 3 (6.4) | .3888 |
T1DM: type 1 diabetes mellitus.
The results are expressed as number (percentage).
Among the SPK transplants, 8 pancreatic graft explants (9.9%) were necessary, all of them due to surgical complications: 3 venous thromboses of the pancreatic graft, 2 acute pancreatitis of the pancreatic graft secondary to surgery, one duodenal-ileal anastomotic suture dehiscence, one fistula, and in another case the appearance of a fistula coexisted with venous thrombosis. Furthermore, these surgical complications caused the loss of functionality of the pancreatic graft in 47% of the cases.
As for the kidney graft, out of the 81 SPK transplant patients, 4 (4.9%) required renal graft removal after loss of the kidney graft. Three (75%) of the explants were in the immediate post-transplant period and were due to surgical complications: 2 renal graft thrombosis, and one postoperative hematoma. In the fourth patient (25%), the explant was performed 9 months after transplantation due to chronic rejection of the kidney graft.
Out of the 81 patients who received SPK transplants, 7 (8.6%) had pancreatic graft rejection: 2 acute, and 5 chronic. In all cases, high levels of amylase and lipase were found. Biopsies of the pancreatic grafts were taken, which confirmed rejection in 5 patients. In the other 2, renal and pancreatic graft rejection coexisted, and only renal graft biopsy was performed.
One of the cases was diagnosed with moderate acute cellular rejection (Banff II) and medical treatment with IV boluses of glucocorticoids allowed to maintain the function of the pancreatic graft. In the other 6 cases with rejection of the pancreatic graft, there was a loss of functionality, and one subsequently underwent PAK transplant, again developing chronic graft rejection with the consequent loss of the new pancreatic graft. Pancreatic graft rejection was responsible for 41.2% of all cases with loss of pancreatic graft functionality.
T1DM recurrence occurred in 2 patients who underwent SPK transplantation, causing the loss of functionality of both grafts10. T1DM recurrence accounted for 11.8% of pancreatic graft losses.
DiscussionAccording to data from the International Diabetes Federation, the incidence of T1DM is between 5 and 8 new cases per 100 000 inhabitants per year14. In the Comunidad Valenciana, T1DM has a prevalence of approximately 0.3% and an incidence of 11–15 cases per 100 000 inhabitants15.
Chronically poorly controlled diabetes mellitus leads to the appearance of macro and microvascular complications and is the main cause of acquired blindness, kidney failure and non-traumatic amputation of the lower limbs. Furthermore, diabetes mellitus is responsible for 8.5% of all deaths that occur in Europe before the age of 8014,16, it is the sixth leading cause of mortality in the Comunidad Valenciana, and it contributes to vascular causes and certain types of malignant neoplasms15.
Long-term intensive insulin therapy has been shown to achieve normoglycemia in patients with T1DM, thereby preventing the appearance of chronic complications. However, intensive insulin therapy is not without risks as it increases the number of hypoglycemia episodes by two- or three-fold compared to the conventional insulin regimen17. In turn, hypoglycemia, especially severe hypoglycemia, is associated with higher mortality18,19. For this reason, SPK transplantation is a valid therapeutic option in patients who are candidates for kidney transplantation due to end-stage diabetic nephropathy, since it restores the euglycemic state without risk of hypoglycemia.
According to the International Pancreas Transplant Registry20, since 2001 more than 2000 pancreas transplantations have been performed annually worldwide, and SPK transplantation is the most frequent, with more than 1500 transplantations per year. The number of SPK transplantations in our country has been increasing progressively, especially since 20008, reaching its maximum figure in 2011 with 106 transplants. Subsequently, the number of transplants performed decreased but remained above 70 pancreas transplantations per year8. Similarly, since the first SPK transplantation was performed in the Comunidad Valenciana in 2002, there has been a progressive increase in this type of transplant, with 2008 and 2010 being the years with the highest number of SPK transplants: 108. In our series, we have focused on the SPK results, since the sample size of PAK transplantations was very small and no PTA transplantations were performed.
In the US series21, the largest reported to date, one-year and 3-year patient survival rates after SPK transplantation between 2001–2005 were 95.2% and 91%, respectively. These figures increased progressively as the years of transplantation progressed, reaching 96.1% and 92.8%, respectively, for SPK transplants performed from 2006 to 2010, and 97.6% and 94.6% for SPK transplants performed from 2011 to 201621.
Similarly, in Europe and Spain, other pancreas transplant series show similar patient survival rates one year after transplantation22–26. Evaluating the longer-term patient survival, optimal results have also been obtained, with 5-year and 10-year post-transplantation survival rates greater than 85%24–27.
In our series, the results obtained are similar to those reported in these series, with overall patient survival rates both one year and 5 years after SPK transplantation of 91.3% and 89.5%, respectively. In addition, there seems to be a trend towards greater patient survival in transplantations performed more recently, in the 2009−2015 period versus 2002−2008, as occurred in previous series. However, the differences between the two periods were not statistically significant.
On the other hand, overall pancreatic graft survival one year after SPK transplantation (75.2%) is somewhat lower than other published series, which report survival rates above 80%22–26. However, when we consider the survival of the pancreatic graft by time periods, we observe that the survival in the most recent period of time (2009−2015) is greater than 80% versus 67.5% in the first period, which is a statistically significant difference (P = .0109). Therefore, pancreatic graft survival increases progressively with the years of transplantation, and these figures are comparable to those of larger series21–24,26.
The improved pancreatic graft survival in the second time period compared to the first is probably due to greater experience of the medical-surgical team in SPK transplantation, among other causes. The immunosuppression regimen and surgical technique were the same in all patients, regardless of when the transplant was performed, so these variables would not influence the result. Also, no statistically significant differences were found in terms of donor characteristics between the study periods. Regarding recipients, there were only statistically significant differences in the time spent on dialysis before SPK transplantation, which was higher in patients from the second time period. These factors would indicate that the experience of the team is essential to achieve better results, without influencing patient survival.
As for the survival of the kidney graft, this is generally greater than the survival of the pancreatic graft. Our results are similar to previously described series21–27, with no statistically significant difference between the 2 time periods analyzed.
Mortality in patients with diabetes mellitus is attributed to kidney disease in 56% of cases and to cardiovascular disease in 44%28. It has been shown that pancreas transplantation, in any of its modalities (SPK, PAK and PTA), entails a notable increase in patient survival, of around 10 years, compared to patients who remain on the waiting list29.
In our hospital, in patients undergoing SPK transplantation with good function of the pancreatic and renal grafts, good blood pressure control was achieved30, which was maintained during the 10-year post-transplant follow-up. Fig. 2 shows how the number of patients requiring antihypertensive and lipid-lowering treatment after transplantation progressively decreased.
Thus, after SPK transplantation, improved glycemic control, normalized kidney function and controlled cardiovascular risk factors like blood pressure and dyslipidemia would contribute to the increased survival of transplanted patients with diabetes versus those not transplanted.
Nevertheless, pancreas transplantation is not without complications. Historically, surgical complications or “technical failures” have been the leading cause of death and loss of the pancreatic graft after SPK transplantation. A surgical complication rate of 15.3% was initially described after SPK transplantation and was the most common cause of pancreatic graft loss (39.3%). However, these figures have been progressively decreasing with the improvement of surgical techniques9,31,32, reaching current figures of 7% and 5.6% in SPK transplantations performed in the periods 2006–2009 and 2011–2016, respectively22. Despite this, they remain the main cause of comorbidity in all types of pancreas transplants22.
The medical and surgical complications that appeared at the beginning of the pancreas transplantation program in our hospital have been described previously9. Currently, in our series, surgical complications continue to be the main cause of pancreas graft loss.
Graft thrombosis is the most frequent and serious complication33,34; it is also the main cause of reoperation and removal of a pancreatic graft32,35. In our series, the incidence of pancreatic graft thrombosis was 7.4% (6 cases). This incidence is similar to the report by the Spanish Pancreas Transplantation Group36, but it is somewhat lower than descriptions of other international series, between 8% and 13%33,37,38. Its etiology is multifactorial and related to the hypercoagulability state39, problems in the anastomosis, stasis of the splenic vessels, donor-related factors (greater if age >50 years, cardiovascular death, etc)37,38,40, procurement (excessive supply of preservation solution, inadequate blood drainage), and recipient factors (previous peritoneal dialysis, portal venous drainage, graft pancreatitis, thrombophilia syndromes)36–38. In this regard, the use of one preservation solution or another (Wisconsin or Celsior) has been reported to have no influence on the results of venous thrombosis25,41. In our series, the Celsior solution was used more than Wisconsin, which was only used until 2009, as previously described9. Once the graft thrombosis occurs, the treatment generally entails graft removal (‘transplantectomy’). Therefore, prevention is essential. Improvements aimed at reducing the incidence of graft thrombosis include antithrombotic prophylaxis, postoperative monitoring with Doppler ultrasound, improvement in donor and recipient selection, and improvement in surgical technique, avoiding portal venous drainage31,42.
Graft pancreatitis after surgery occurs as a consequence of ischemia-reperfusion injury or reflux of exocrine bladder diversions. Furthermore, pancreatitis is usually associated with the appearance of serious complications such as fistulae, thrombosis, infectious collections or necrosis31. Our series had an incidence of 4.9%, a figure somewhat lower than the 6% obtained by the Spanish Pancreas Transplantation Group36, probably because we did not perform exocrine bladder diversion, thus minimizing the risk of pancreatitis.
T1DM recurred in 2 patients (2.5%) who received SPK transplants in our series. This prevalence is also somewhat lower than other series (7%–9%), probably due to the fact that our cohort of patients is more recent and, consequently, the immunosuppression regimen had been improved compared to previous series10.
This implementation of the immunosuppression regimen, with tacrolimus and mycophenolate mofetil (greater immunosuppressive potency than azathioprine)43, has also contributed towards a reduction in rejection-mediated pancreatic graft loss. Thus, the incidence of immunological rejection of the pancreatic graft was also lower in our series (8.6%) compared to reports in the literature of around 9%–37.2%24,25,44, with a one-year pancreas graft loss rate of 20%. The incidence of rejection has also been shown to increase when the donor is older than 30 years of age and the recipient is black or younger than 30 years of age. In contrast, this incidence is lower in SPK transplantations compared to PAK or PTA and improves over time. Transplantations performed most recently have a lower incidence22, as has happened in our cohort, and we observed statistically significant differences between both study periods.
As a consequence of the intensified immunosuppression, infections have become the main cause of mortality during the first year after transplantation45–48, especially postoperative abdominal infections25,49. In our case, they are responsible for half of the deaths, despite the application of antibacterial, antifungal and anti-cytomegalovirus prophylaxis. These include anastomotic leaks, abscesses or surgical collections, infection of the surgical wound, etc. Some factors that may favor them are: advanced donor and recipient ages, longer cold ischemia time, the use of peritoneal dialysis versus hemodialysis, and more time spent on the waiting list for transplantation34,37,38,50.
Fistulae, however, are rare complications35, with an incidence of around 5%–8% in duodenum-enteric diversions51, and somewhat higher in duodenum-bladder diversions. In our series, they appeared in 4.9% of SPK transplantations.
When we compare the overall complications that arose in both time periods, 2002−2008 and 2009−2015, the first period had more fistulae, more pancreatic graft rejections, a greater need for ‘transplantectomy’ (removal of the pancreatic graft), and a higher mortality rate compared to the subsequent period. In this second period, the total number of postoperative venous thromboses and intra-abdominal infections was higher but less serious as there were fewer pancreas graft removals and deaths. However, with regard to the appearance of complications between both time periods, statistically significant differences were only observed for pancreatic graft rejections, which could be related to the improvement of the immunosuppressive regimen. Statistically significant differences were not reached for the remaining complications, probably due to the small sample size.
This study has several limitations. The first is the smaller sample size, especially compared to other series published internationally, but it is justified as it is a regional series with a population of 5 million inhabitants and a T1DM prevalence of 0.3%15. The second is that it is a retrospective study, so some data are incomplete or not available.
In conclusion, during our 13-year experience with SPK transplantation, patient and pancreatic graft survival rates and the rate of complications after pancreas transplantation achieved in the second time period are similar to those of other larger series. The experience of the medical-surgical team improves the survival of the pancreatic graft, without influencing patient survival.
NoteThe data utilized to support the findings of this study are available upon request to the author.
FundingAs this study was conducted as part of the daily clinical practice of the authors with the Consellería de Sanitat de la Comunidad Valenciana (Regional Healthcare Administration of Valencia, Spain), no funding was required.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Argente-Pla M, Martínez-Millana A, Espí-Reig J, Maupoey-Ibáñez J, Moya-Herráiz Á, Beneyto-Castello I, et al. Resultados tras 13 años de inicio del trasplante simultáneo de páncreas-riñón en pacientes con diabetes mellitus tipo 1 en la Comunidad Valenciana. Cir Esp. 2021;99:666–677.