The presence of lymph nodes metastasis in papillary thyroid cancer (PTC) modifies the type of surgical resection as well as the indication of the treatment with I131 in the postoperative period. This therapeutic approach is based on the results of the diagnostic tests, like the cervical ultrasonography. Currently other methods of diagnostic are tested as selective sentinel lymph node biopsy (SLNB). It can complement to the ultrasound results. The aim was to validate the SLNB for use in the diagnosis of lymph node metastasis by papillary thyroid cancer.
MethodsObservational prospective cohort study of 55 patients who underwent PTC without suspicion of lymph node involvement clinical or radiological, since February 2012 through February 2015, with a follow-up between 6 and 8 years. It was used 99Tc with intratumoral nanocoloid and a portable tube of the gamma camera for the detection of the sentinel node (SN). Variables: age, gender, histological, analytical and preoperative and postoperative staging. The sensitivity, specificity and predictive values of technique was calculated. The validation was determined by calculating the detectability and the false negative results of the test.
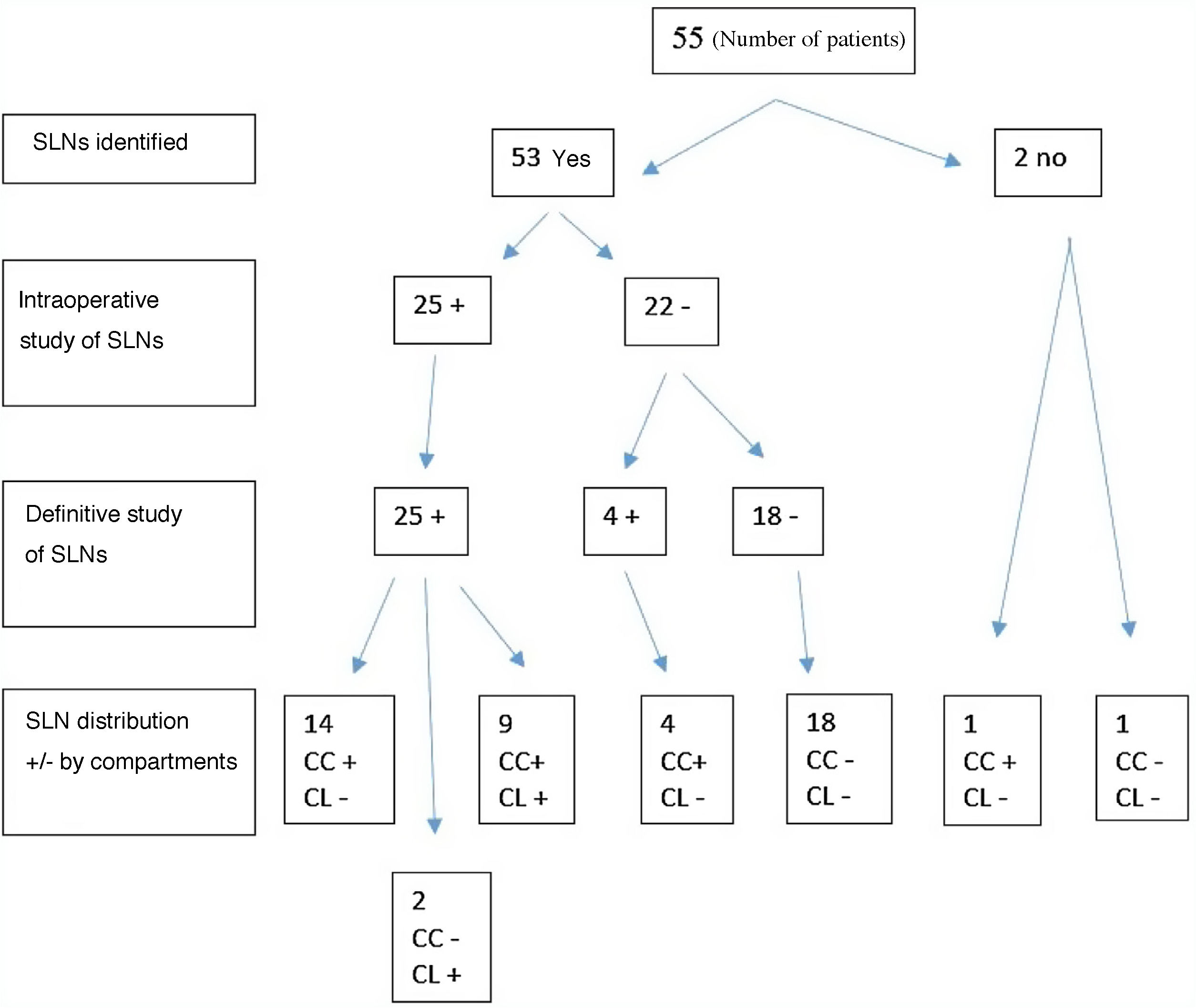
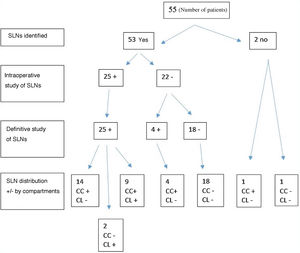
Results53 of the 55 patients (96,36%) there was the SN detection. The FN were 4 patients (7,5%). Of the rest, after applying the SLNB, 24 (48,9%) were kept as N0, 14 (28,5%) became N1a and 11 (22,4%) were classified as N1b. The differences observed in the study were significant (P < ,05). The sensitivity was 86,21%, the specificity of 100%, the PPV was 100% and the NPV of 85.71%. The diagnostic accuracy of 92,45%.
ConclusionThe SLNB is a valid technique for use in patients suffering from papillary thyroid cancer with a high diagnostic accuracy.
La biopsia selectiva del ganglio centinela (BSGC), puede completar el estudio preoperatorio detectando adenopatías no visibles ecográficamente. De este modo, se puede estadificar a los pacientes y estratificar el riesgo de recidiva de forma más precisa y por tanto, ayudar a definir el tipo de tratamiento tanto quirúrgico como con I131 que debemos realizar. El objetivo fue validar la BSGC para su utilización en el diagnóstico de la metástasis ganglionar por cáncer papilar de tiroides.
MétodosEstudio observacional prospectivo de cohortes que incluye a 55 pacientes intervenidos por CPT sin sospecha de afectación ganglionar clínica o radiológica, desde febrero de 2012 hasta febrero de 2015, con un seguimiento entre 6 y 8 años. Se utilizó 99Tc con nanocoloide intratumoral y una sonda portátil de la gammacámara para la detección de los ganglios centinelas (GC). Variables: edad, género, histológicas, analíticas y estadificación preoperatoria y postoperatoria. Se calculó la sensibilidad, especificidad y valores predictivos de la técnica. La validación se determinó calculando la detectabilidad y los falsos negativos (FN) de la prueba.
ResultadosEn 53 de los 55 (96,36%) pacientes hubo detección del GC. Los FN fueros 4 (7,5%) pacientes. Del resto, tras aplicar la BSGC, 24 (48,9%) se mantuvieron como N0, 14 (28,5%) pasaron a ser N1a y 11(22,4%) se clasificaron como N1b. Las diferencias observadas en el estudio fueron significativas (P < ,05).
La sensibilidad fue del 86,21%, la especificidad del 100%, el VPP fue del 100% y el VPN del 85,71%. La precisión diagnóstica del 92,45%.
ConclusionLa BSGC es una técnica válida para su utilización en los pacientes afectos de cáncer papilar de tiroides, con una alta precisión diagnóstica.
Surgical treatment of papillary thyroid cancer (PTC) should include, in addition to thyroidectomy, lymphadenectomy of the affected lymph node groups.
Preoperative lymph node assessment is basically performed with cervical ultrasound. However, due to the sensitivity and specificity of this test, especially for the central neck compartment, a group of patients may be understaged.
Selective sentinel lymph node biopsy (SLNB) can complete the preoperative study by detecting lymph nodes that are not visible on ultrasound. In this way, patients can be staged and the risk of recurrence can be stratified more precisely, thus helping to define the type of treatment, both surgical and with 131I to be performed.
The aim of this study is to validate the sentinel lymph node (SLN) technique for use in PTC.
MethodsA prospective, observational study was conducted which included 55 patients operated on by PTC with clinical and radiological absence of lymph node involvement, from February 2012 to February 2015 and with a follow-up of between 6 and 8 years.
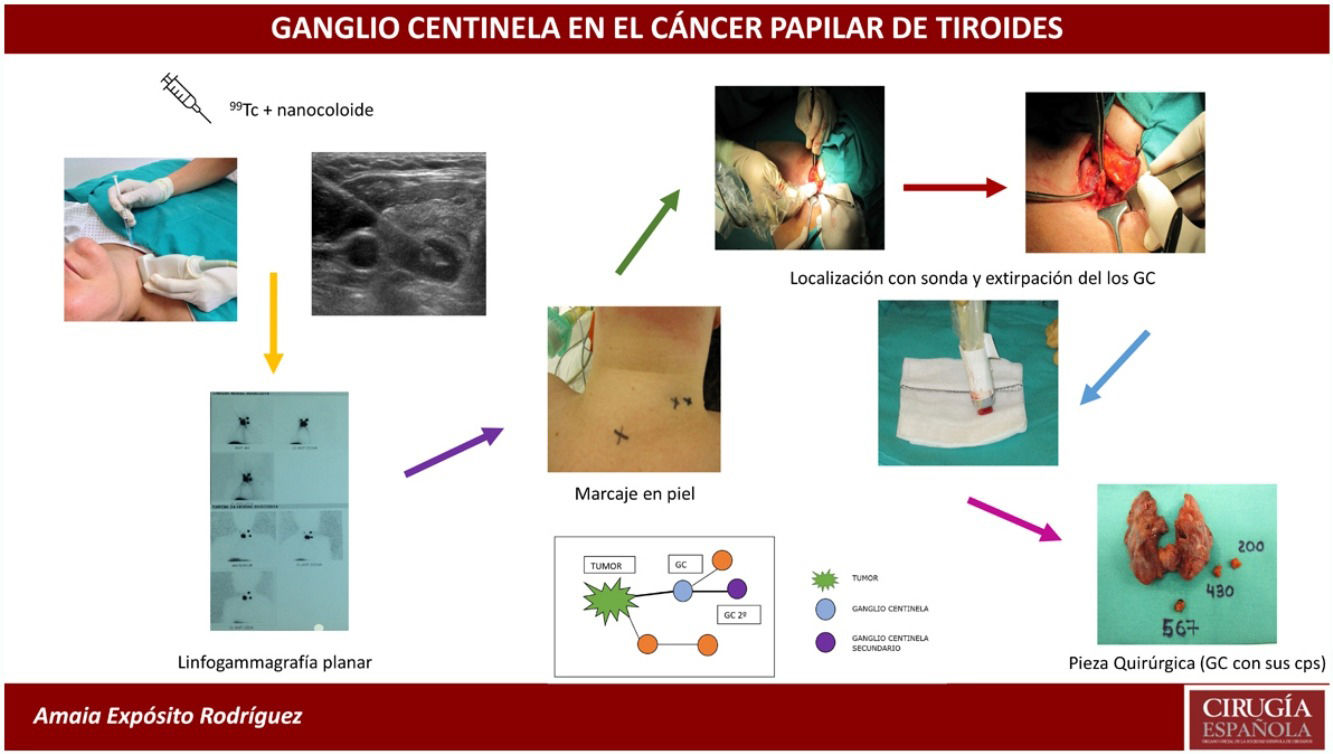
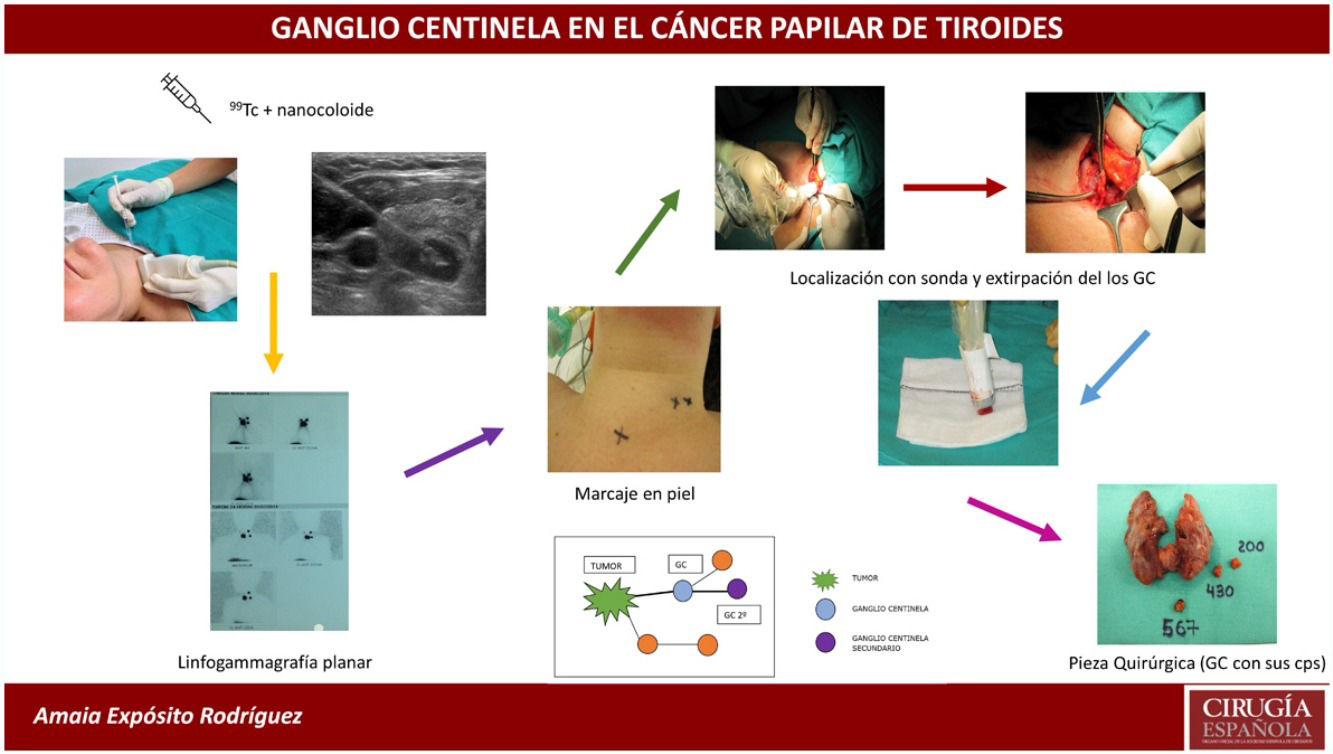
SLNB was performed with intratumoral injection of the radiopharmaceutical (0.2 mL of 4 mCi of 99mTc) with nanocolloid (Nanocoll®), with ultrasound monitoring 24 h before surgery.
Prior to surgery, the sentinel lymph nodes (SLNs) were located by planar lymphoscintigraphy and marked on the skin with indelible ink. Subsequently, total thyroidectomy was first performed in all cases (to avoid interference from radioactivity emitted by the thyroid gland), followed by all cervical lymph node compartments being systematically traced with the portable gamma camera probe (neo2000® Gamma Detection System). The lymph nodes were located and sent fresh for intraoperative freezing. The surgery was then completed by central compartment dissection (CCD) and in cases where the lymph node dissection of the lateral neck compartment was positive, the lateral lymph node dissection of the affected side was completed (levels II, III and IV). The procedure of removing the SLNs took an average of 20 min.
The main variables analysed were: age, gender, number of SLNs, location, percentage of positivity, relationship with the findings of the final anatomopathological study, follow-up results, sensitivity and specificity, predictive values and detectability of the test. Survival and recurrence rates were determined over a follow-up period of 6–8 years.
Statistical analysisAnalyses were performed using SAS® for Windows statistical software, version 9.2 (SAS Institute, Inc., Carey, NC, USA).
For all analyses a result was considered statistically significant at P < .05. The result of the SLN technique was determined by estimating the sensitivity, specificity, positive predictive value and negative predictive value.
ResultsForty-seven (85.4%) of patients were women and 8 (14.6%) were men.
Mean age was 49.3 years, with a standard deviation (SD) of 15.
A total of 237 SLNs were isolated in 53 patients in whom drainage was present. The SLN mean was 4.5.
Mean SLN size was 7.1 mm (SD 3.3).
In 51 (96.23%) patients SLN was located in the central compartment compared to 2 (3.77%) patients with no drainage in the central compartment.
Thirty-two (60.38%) patients had drainage in the lateral compartment, while 21 (39.62%) had no drainage in the lateral compartment. Thirty (50.60%) patients had drainage in both the central and lateral compartments, while only 2 (3.7%) had drainage in the lateral compartment with no SLNs located in the central compartment. The rate of SLN “skip” or metastatic leap phenomenon in our study was 3.7%.
All SLNs positive in the intraoperative biopsy were positive in the definitive study (there were no false positives). In 52.38% of patients with positive SLNs located in the central compartment, these were the only positive lymph nodes in the central dissection, and in 75% of positive SLNs in the lateral compartment, these were the only positive lymph nodes in the lateral compartment dissection. The node positivity was due to the presence of macrometastases, while the false negatives (FN) were due to micrometastases that could not be detected in the intraoperative freeze-dissection. The total number of FN was 7.5% (4 patients). No drainage was obtained in 2 (3.6%) cases, so that the detectability of the test was 96.36%.
Fig. 1 shows the correlation between the intraoperative findings and the final anatomopathological study of the SLNs after central and lateral dissection.
There were no complications related to the application of the SLN technique, nor was there any mortality in our series.
The sensitivity of the SLN technique in our study was 86.21%, specificity 100%, PPV 100% and PNV 85.71%. The diagnostic accuracy was 92.45% (Table 1).
After performing the SLN technique, the N (according to TNM 7th edition classification) was modified. Preoperatively all patients were N0, whereas after locating and analysing SLNs, 48.98% remained N0, 28.57% became N1a and 22.45% became N1b. The differences observed in the study were significant (P < .05). Excluding FNs, 48.98% of patients had concordance between preoperative and postoperative staging, while 51.02% did not (Table 2).
Tumour staging (TNM) was modified after SLNB. Preoperatively, 46 (86.8%) patients were classified as Stage I and 7 (13.2%) as Stage II, while postoperatively, 32 (60.4%) were included in Stage I, 5 (9.4%) in Stage II and 16 (30.2%) in Stage III (Table 3).
The mean tumour size was 16.7 mm (SD 12.2). Microcarcinomas accounted for 31.6%.
Tumours were multicentric in 50.9% of cases and bilateral in 43.7%.
Regarding follow-up, 88.6% of patients had an excellent response. Incomplete biochemical response without structural findings presented in 5.7% of patients and recurrence in the remaining 5.7%. Recurrences occurred in 3 patients: a pulmonary metastasis in one case; a diffuse sclerosing variant that recurred in both lateral compartments and after dissection of these was found to be disease-free in another patient; the third case of recurrence was in the right lateral compartment, a classic variant with 3 right tumour foci and another in the isthmus in which the isotope was only placed in the tumour described in the ultrasound scan. After dissection of the affected compartment, the patient is also free of disease. The 3 patients who recurred had positive SLNS only in the central compartment.
Survival in our series was 100%.
DiscussionWe consider it of interest to address whether the surgical treatment we perform in PTC is appropriate for each patient. We may be applying an overtreatment when we perform prophylactic CCD, with the consequent increase in morbidity that this type of dissection implies. Conversely, failure to perform such a dissection may lead to failure to remove all tumour tissue, which may affect recurrence, disease persistence, prognosis and/or survival.
SLNB can determine the type of lymph node dissection to be performed, orienting such dissections only to those with a therapeutic purpose.
The SLN is defined as the first lympthatic drainage station of a tumour1. Its interest lies in the fact that its detection and intraoperative study will reveal the existence or not of lymphatic metastases. However, for reasons that will be discussed below, not all authors2,3 agree on its usefulness in PTC.
Some of the risk factors in the PTC will be described in the study of the surgical specimen and therefore may help in the indication of the ablative treatment with131I. However, others, such as the presence of locoregional adenopathis are based on preoperative studies, where the most widely used diagnostic test is cervical ultrasound. Notwithstanding, the sensitivity and specificity of this test in the diagnosis of lymph node metastases in the central compartment is 30% and 86.8%, respectively, and in the lateral compartment it is 93.8% and 80%4.
It is accepted that around 50% (25%–90% depending on the series) of patients with PTC may present with lymphatic metastases5.
Authors such as Hughes et al.6 describe an increase in recurrence in patients over 45 years of age with lymph node involvement in a total of 931 patients, although without significant differences in survival.
In contrast, Lundgren et al.7 published a case-control study of 5123 patients in 2006 in which they found a higher mortality rate in those with metastatic lymph node involvement. In our experience, SLNB detected in 51.02% of patients the presence of metastatic adenopathies that had not been located in the preoperative ultrasound.
Regarding lymph node dissection, international guidelines agree on the need to perform it when it is therapeutic, and do not consider prophylactic dissection of the lateral compartment to be indicated8–10. Regarding prophylactic CCD (levels VI-VII), there is no consensus.
The European Thyroid Association10 and the National Comprehensive Cancer Network11 do not recommend prophylactic CCD, although they recognise that it contributes to better staging and facilitates correct follow-up.
The American Thyroid Association8 recommends it in advanced tumours (T3-T4) and the British Thyroid Association9 in patients considered high risk, provided it is performed by expert surgeons to avoid increased morbidity.
In this respect, SLNB is a perioperative diagnostic test that can help define whether or not lymph node dissection is necessary. Furthermore, in the event that SLNS is negative, such dissection is avoided and, therefore, the morbidity that this type of surgery entails.
Consequently, cervical lymphadenectomy will always be indicated for therapeutic purposes, avoiding the controversy of whether or not to perform prophylactic lymph node dissection. Furthermore, we endorse the usefulness of this technique in that it defines for most patients (92.5%) the indication for the type of dissection to be performed.
In this sense, according to our study, SLNB would have allowed us to avoid overtreatment in 48.98% of patients, since the presence of negative SLNs would have led us not to perform CCD. This circumstance is also advocated by Garau et al.12, who promote the use of SLNB in view of the published evidence and as a system for avoiding prophylactic CCD.
However, in the remaining 51.02%, positive SLNs were found, so that in this group of patients, lymph node dissection was justified.
These facts led to significant changes in the TNM classification of the patients, so that the indication for ablative treatment with 131I, and the follow-up could be adjusted.
In 2009 Pelizzo et al.13 published a series of 99 patients who underwent the radioisotope technique (99Tc nanocolloid), with a detectability of 99%, localising metastatic SLNS in 49% of patients.
In 2011, Balasubramanian and Harrison14 published a meta-analysis in which 24 studies were evaluated, defining a detectability of 98.4%. They concluded that 57% of prophylactic lymphadenectomies could have been avoided with the use of SLNB.
In 2012 Larrad Jiménez et al.15 presented their preliminary results on 23 patients using methylene blue dye with a detectability of 91.3% and 7.1% FN. Sensitivity was 87.5% and specificity 100%.
In our study, the percentage of FN was 7.5% and the diagnostic accuracy was 92.5%. SLNs were detected in 53 out of 55 patients, so the detectability was 96.36%.
In the interpretation of these data, it should be borne in mind that the published studies are mostly small series.
To this fact must be added the methodological variability of the different existing techniques, which limits their interpretation. Even so, in most of them, the results of sensitivity and specificity are similar, as are the detectability and percentage of FN13,16–19.
In our experience, we can substantiate that SLNB is an easily reproducible and safe technique, as there were no procedure-related complications5.
However, not all authors3 defend the benefits of SLNB; for example, authors such as Huang et al.2 published a prospective study in 2007 with 90 women with low-risk PTC, with a high percentage of FN (14.3%). They defended the use of prophylactic CCD, since 38.1% of the patients had this compartment affected. Despite the high percentage of FN, they did conclude that the molecular study of the lymph nodes (OSNA) could reduce this percentage. In this regard, Iglesias Felip et al.20 published a study in which they concluded that OSNA helps to improve the diagnosis of SLNs and therefore to personalise the surgical treatment of patients with PTC. Likewise, in a meta-analysis published in 2019, Garau et al.21 support the use of SPECT/CT in the diagnosis of SLNs outside the central compartment.
With regard to the indeterminate response (3 patients) and tumour recurrence (3 patients) described in the study, we must consider that the validation of SLNB is determined with the parameters of FN and detectability22. The absence of recurrence is not an object for validating the technique, as any surgical procedure will inevitably involve a percentage of recurrence, i.e., we cannot achieve a 100% cure rate just by performing this technique, as this depends on many factors. SLNB aims to help detect as much tumour tissue as possible, which will help us to stage and stratify the risk of recurrence more accurately after its removal and, consequently, to adjust the indication for subsequent radioiodine treatment.
Like any diagnostic test, SLNB has its limitations and poses certain problems that must be considered, such as: the absence of drainage/blood passage of the radiotracer, the number of SLNs, FN in the intraoperative study, bilaterality and multicentricity, and the presence of micrometastases.
If we compare the benefits obtained (correct staging and personalised indication of surgical and ablative treatment) with respect to the problems or possible complications of SLNB, we consider that it is undoubtedly a technique that can be applied in PTC and complement the diagnostic tests usually employed.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Expósito Rodríguez A, Corta Gómez I, Domínguez Ayala M, García Carrillo M, González García AI, Gutiérrez Rodríguez MT, et al. Ganglio centinela en la metástasis ganglionar por cáncer papilar de tiroides: validez diagnóstica y aplicación en la práctica clínica. Cir Esp. 2022;100:416–421.