The main objective of this study was to analyse the results of the surgical treatment of ampullary neuroendocrine tumours (NET) based on transduodenal ampullectomy and pancreatoduodenectomy, in a reference centre in hepatobiliopancreatic pathology.
MethodRetrospective, observational study, including all patients operated on for pancreatic and/or duodenal NET in a reference unit of hepatobiliopancreatic pathology and prospectively registered between January 1st, 1993 and September 30th, 2021. For those parameters not present, retrospective research was performed. Demographic, clinical, analytical and pathological data were analysed. A descriptive study was carried out. Overall and disease-free survival was calculated using Kaplan-Meier curves and the Log-Rank test.
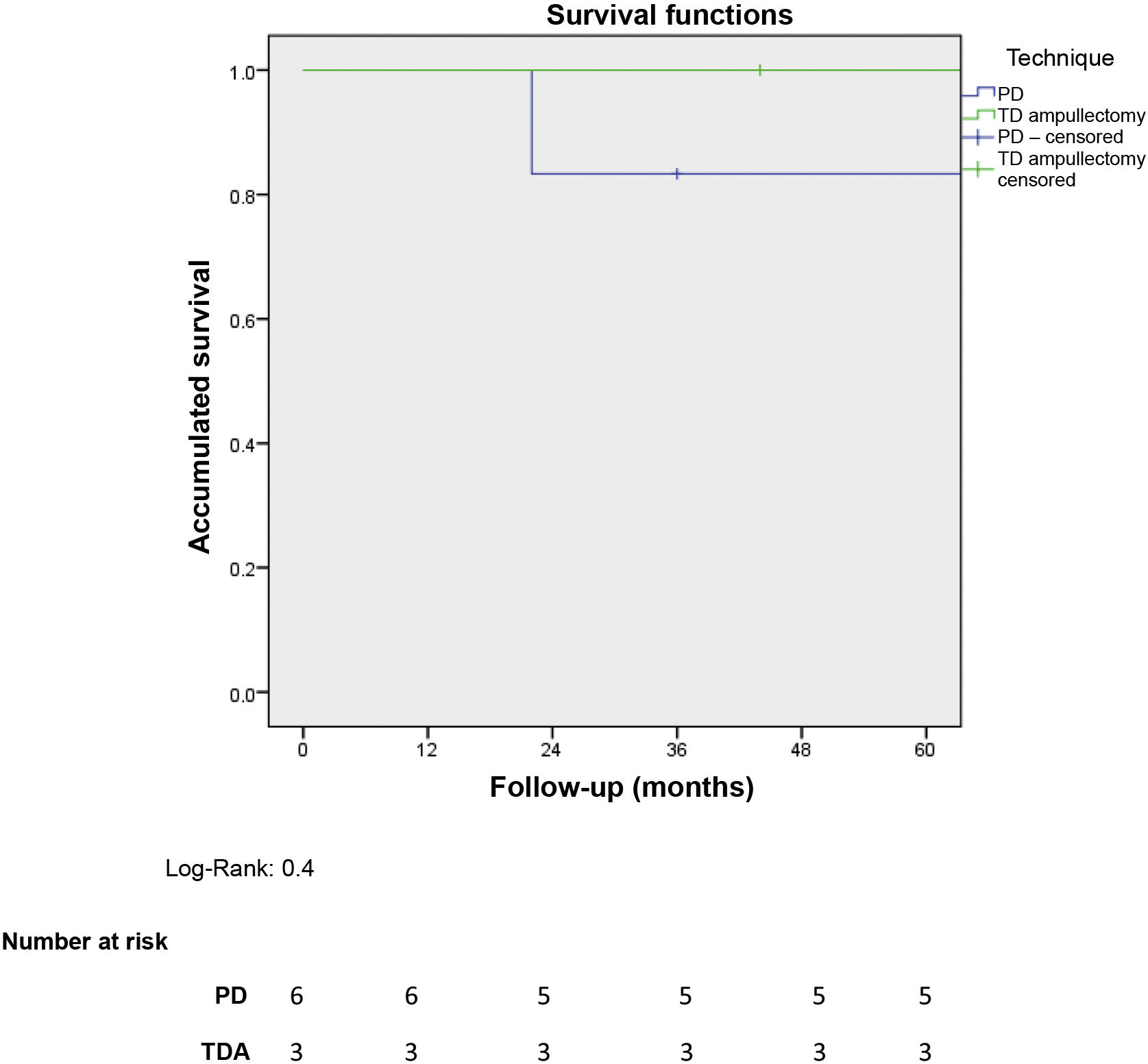
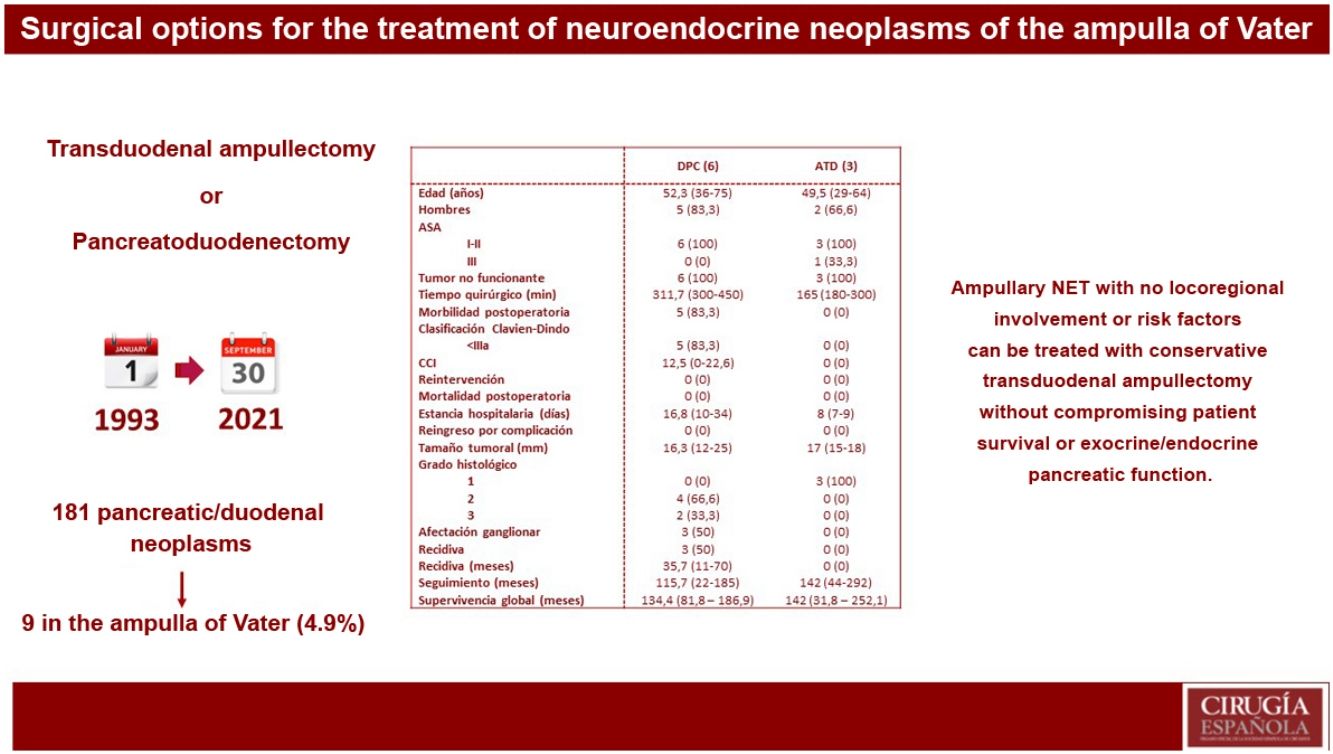
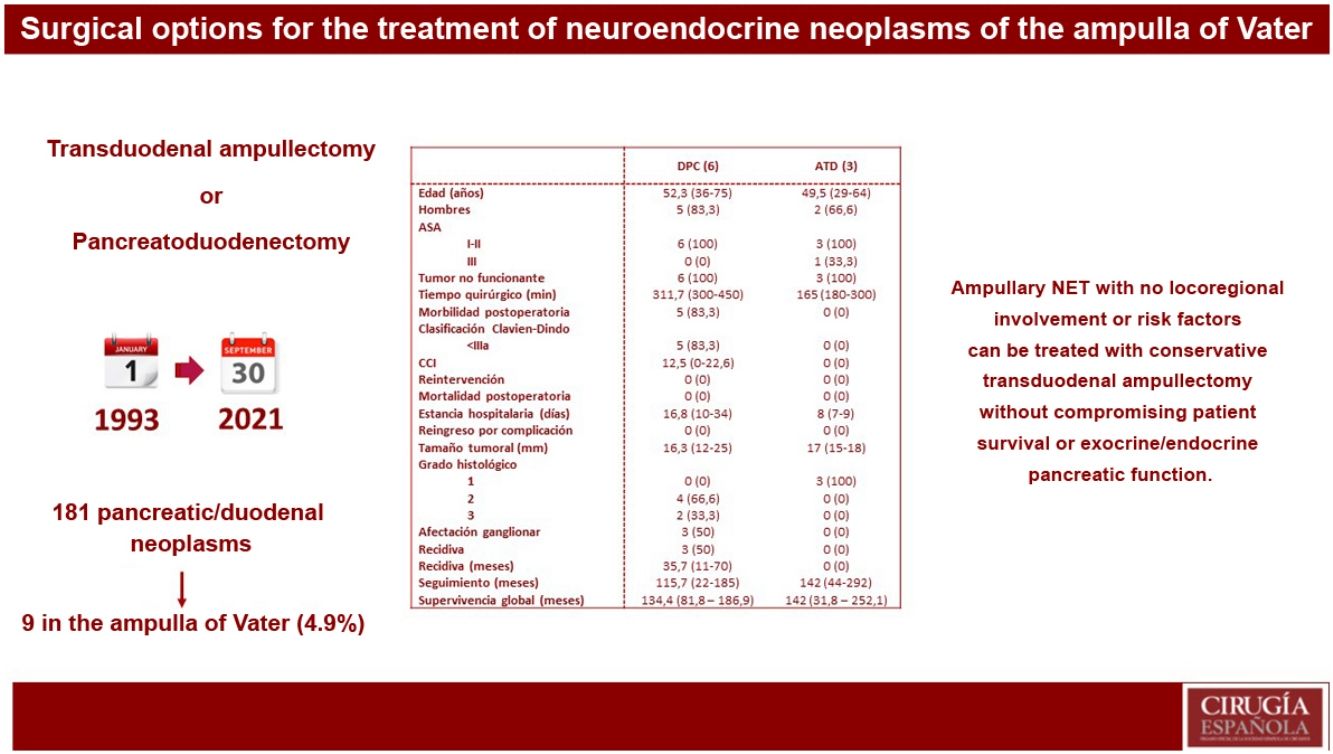
ResultsOf 181 patients operated on for pancreatic and/or duodenal NET, only 9 were located in the ampulla of Vater, which represents 4.9% of all pancreatic and/or duodenal NET. Pancreatoduodenectomy (PD) was performed in 6 patients, while 3 patients underwent transduodenal ampullectomy (TDA). Longer surgical time and more postoperative complications were observed in the PD group. There were no differences in hospital stay. Overall and disease-free survival at 5 years in the PD group compared to ATD was 83.3% vs. 100% and 50% vs. 100%, respectively.
ConclusionsAmpullary NET without locoregional involvement or risk factors, can be treated by conservative surgeries such as transduodenal ampullectomy.
El objetivo del estudio fue analizar los resultados del tratamiento quirúrgico de las Neoplasias Neuroendocrina (NNE) ampulares mediante Ampulectomía transduodenal (ATD) y duodenopancreatectomía cefálica (DPC), en un centro de referencia en patología hepatobiliopancreática.
MétodoEstudio retrospectivo, observacional, incluyendo los pacientes intervenidos de NNE de páncreas y/o duodenales en una unidad de referencia en patología hepatobilipancreática y registrados prospectivamente entre el 1 de enero de 1993 y el 30 de septiembre de 2021. Para aquellos parámetros no presentes, se realizó una búsqueda retrospectiva. Se analizaron datos demográficos, clínicos, analíticos y anatomopatológicos. Se realizó un análisis descriptivo. La supervivencia global y libre de enfermedad se calculó mediante curvas de Kaplan–Meier y el test de Log-Rank.
ResultadosDe 181 pacientes intervenidos de NNE de páncreas y/o duodenales, solo 9 se localizaban en la ampolla de Váter, lo que representa un 4,9% de todos los NNE pancreáticos y/o duodenales. Se realizó DPC en 6 pacientes, mientras que a 3 pacientes se les practicó ATD. Se observó mayor tiempo quirúrgico y más complicaciones en el grupo DPC. No hubo diferencias en la estancia hospitalaria. La supervivencia global y libre de enfermedad a 5 años del grupo DPC respecto a la ATD fue del 83,3% vs. 100% y del 50% vs. 100%, respectivamente.
ConclusionesLas NNE ampulares sin afectación locoregional ni factores de riesgo, pueden ser tratadas mediante cirugías preservadoras como la ampulectomía transduodenal.
Neuroendocrine tumours (NET) originate in the cells of the neuroendocrine system distributed throughout the body, although they most commonly affect the gastrointestinal tract.1 The involvement of the ampulla of Vater by this type of tumour is extremely rare and, although it is difficult to estimate since most of the published evidence is case series2–5 and retrospective reviews,6 about 6% of pancreaticoduodenal NET affect the ampulla of Vater.5
The prognosis of ampullary NET tends to be worse than duodenal neuroendocrine tumours since they tend to have greater lymph node dissemination, which means that disease-free survival is lower.7 In terms of treatment, according to the recommendations of the ENETS,8 tumours <1 cm can benefit from endoscopic treatments, but there is no consensus on the ideal treatment for tumours >1 cm. Surgical options include pancreaticoduodenectomy (PD) and transduodenal ampullectomy (TDA).
Given the lack of clear evidence about the best surgical approach for ampullary NET, the objective of this study is to analyse the results of conservative surgery using TDA versus PD to treat ampullary NET at a reference hospital for hepato-biliary-pancreatic pathology.
MethodWe have conducted a retrospective observational study at the Bellvitge University Hospital (Hospitalet de Llobregat, Barcelona) including all patients treated surgically for pancreatic and/or duodenal NET that had been and registered in a prospective database of the Pancreatic Surgery Unit between 1 January, 1993 and 30 September, 2021. For parameters that were not present, a retrospective search was performed. As this is a retrospective study, informed consent was not required. The study was approved by the Ethics Committee of our institution (Manuscript reference number PR281/22 and Minutes 20/22).
Data collectionThe following data were analysed: sex, age, diagnostic method, ASA classification (American Society of Anesthesiology), preoperative tumour markers (Chromogranin A), surgical treatment (PD or TDA), 90-day postoperative complications as well as their severity according to the Clavien–Dindo classification,9 postoperative complications specific to pancreatic surgery (pancreatic fistula, delayed gastric emptying and postoperative bleeding) according to the definition of the International Study Group of Pancreatic Surgery (ISGPS),10–12 reoperation, definitive pathological anatomy, tumour size, histological grade according to the WHO Classification of Tumours of the Digestive System,13 hospital stay, 90-day hospital readmission, adjuvant treatment, recurrence, and mortality. Overall survival and disease-free survival were calculated from the date of surgery.
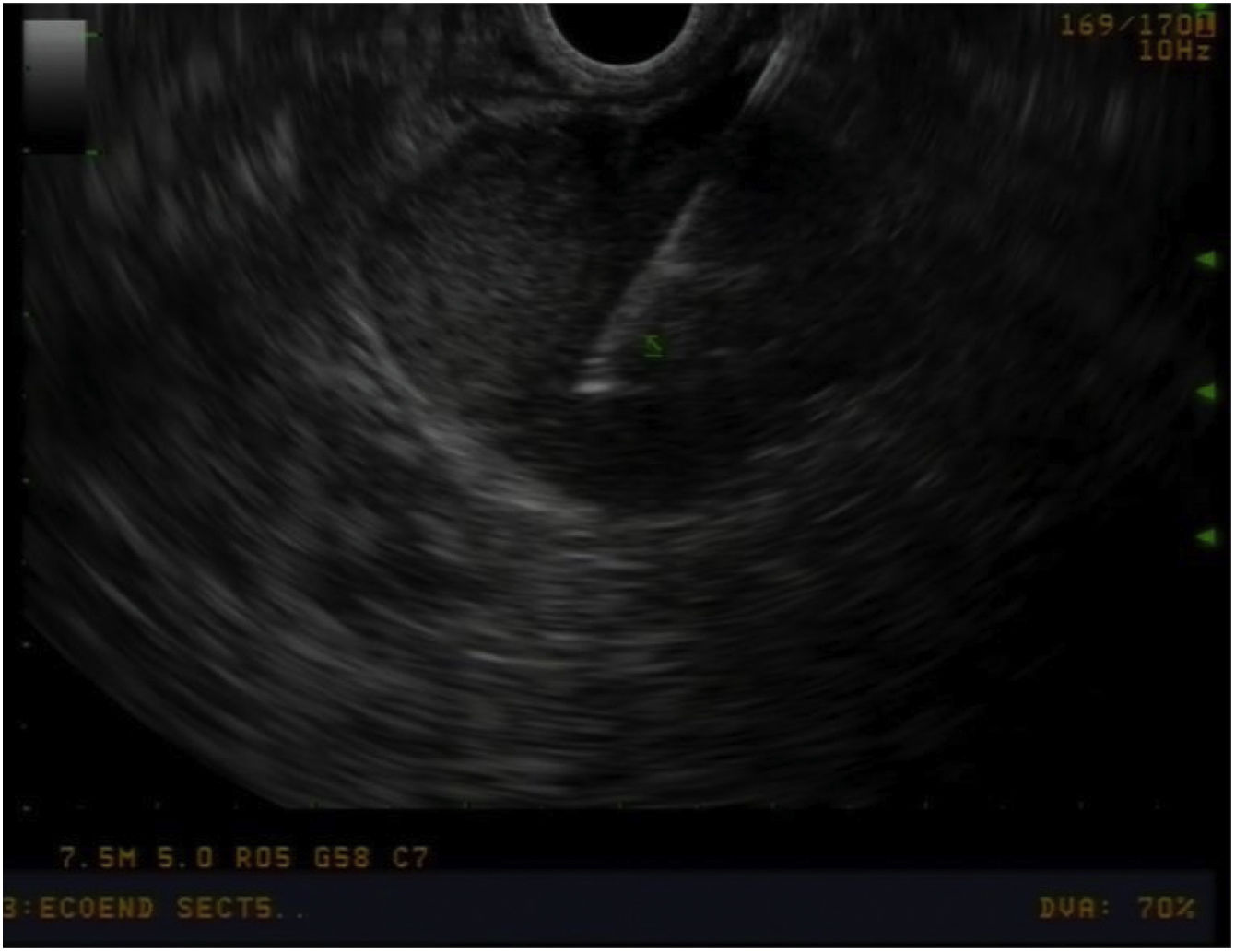
Preoperative study and follow-upAll patients underwent a thorough preoperative study, which included: thoracoabdominal computed tomography (CT) scan, tumour markers (chromogranin A, CA 19.9), complete liver profile, laparoscopy and endoscopic ultrasound to assess the characteristics of the ampulla of Vater and locoregional lymphadenopathies (Image 1), and biopsies. For patients whose diagnosis was uncertain (ampullary neoplasm vs. choledocholithiasis vs. tumour of the head of the pancreas), the study was completed with magnetic resonance cholangiopancreatography and octreotide scan. In patients with jaundice or cholangitis, we tried to avoid preoperative drainage of the bile duct to prevent pre- and postoperative complications14,15 as well as the risk of recurrence.16
All patients were assessed and discussed individually in a clinical-radiological meeting, where the treatment approach was decided by the multidisciplinary committee.
Postoperative follow-up was performed in accordance with ENETS recommendations.17
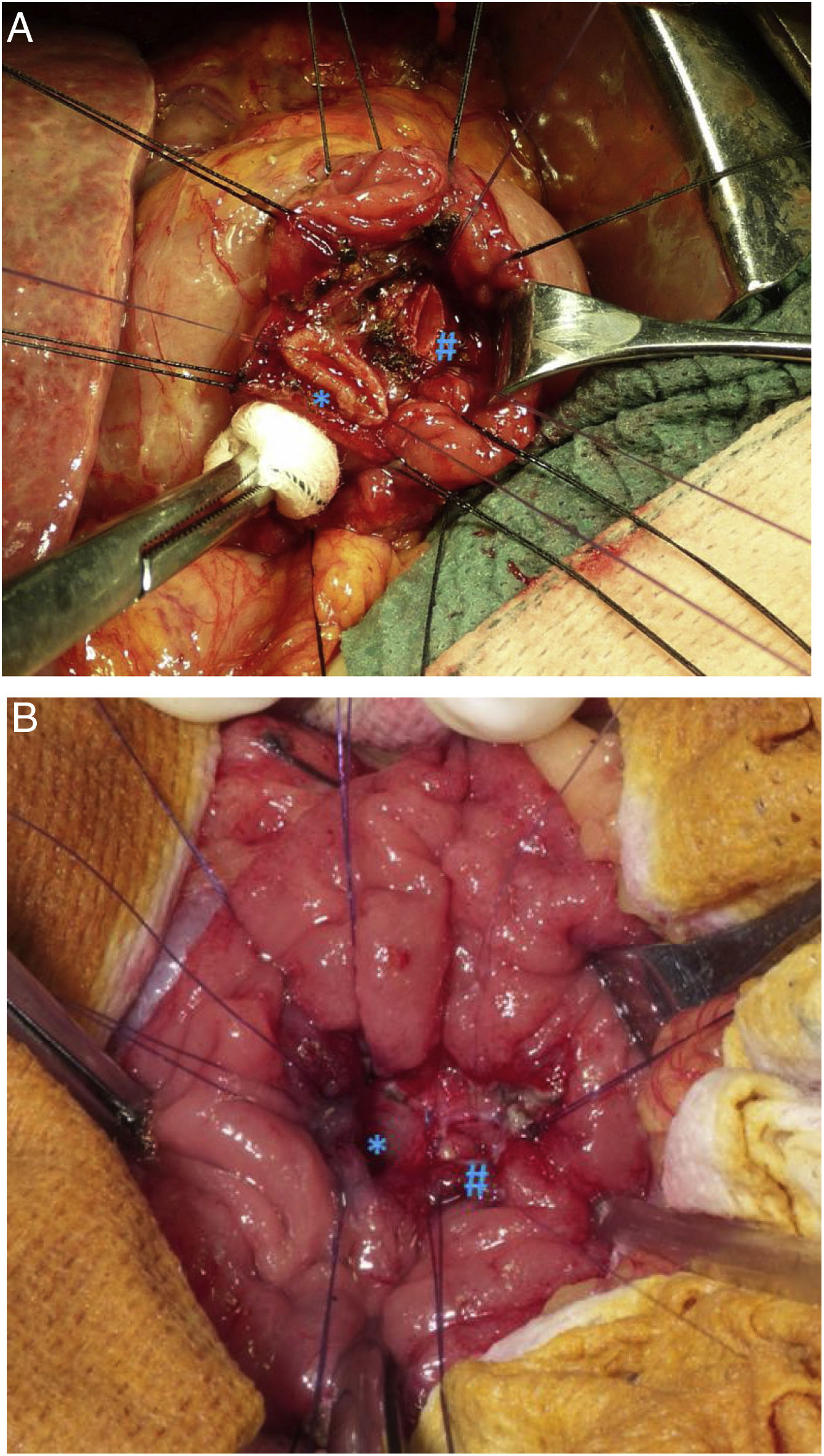
Surgical techniqueIn patients with tumours and no preoperative suspicion of locoregional invasion, no lymph node involvement, nor poor differentiation in the preoperative biopsy, we performed conservative treatment, which included DTA and reimplantation of the bile duct and the main pancreatic duct (Image 2). In these cases, an intraoperative lymph node biopsy and intraoperative pathological study of the surgical resection piece were performed to assess the involvement of the surgical margins and verify the good differentiation of the neoplasm. When margin involvement was observed in the intraoperative study, we performed PD.
In patients with tumours and suspected locoregional or lymph node involvement, a more aggressive treatment was applied, using PD according to the Whipple technique and Child’s loop reconstruction.
Main objectiveThe main objective of this paper is to descriptively analyse the results of surgical treatment of ampullary NET using TDA and PD in a high-volume referral hospital for hepato-biliary-pancreatic pathology.
Secondary objectiveThe secondary objective is to determine whether treatment by TDA in patients with ampullary NET is a safe technique that provides acceptable oncological results.
Statistical analysisData are expressed as frequencies and percentages for categorical variables and as mean (minimum/maximum) for continuous variables. We created a descriptive study of the patients who were treated with PD and TDA, and Table 1 summarizes the main characteristics of each of the patients. Overall survival and disease-free survival were calculated using Kaplan–Meier curves, and the SPSS statistical program, version 25, (SPSS Inc., Chicago, IL, USA) was used.
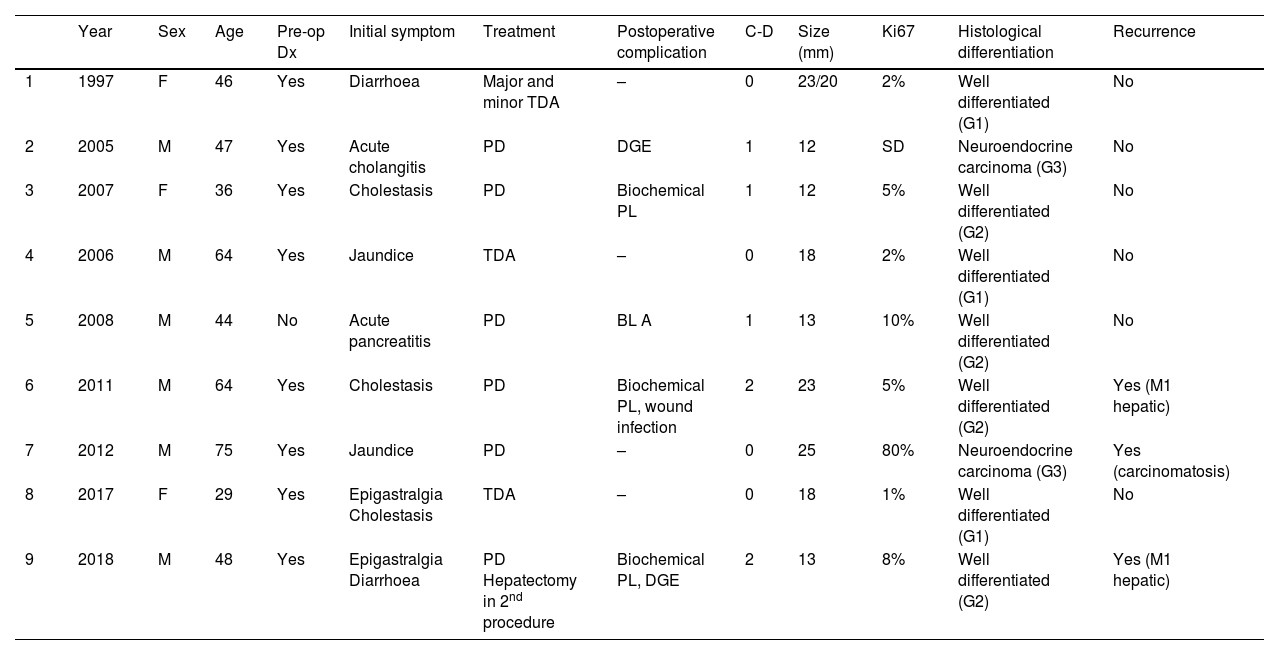
Summary of the main clinical variables of patients with ampullary NET.
| Year | Sex | Age | Pre-op Dx | Initial symptom | Treatment | Postoperative complication | C-D | Size (mm) | Ki67 | Histological differentiation | Recurrence | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1997 | F | 46 | Yes | Diarrhoea | Major and minor TDA | – | 0 | 23/20 | 2% | Well differentiated (G1) | No |
| 2 | 2005 | M | 47 | Yes | Acute cholangitis | PD | DGE | 1 | 12 | SD | Neuroendocrine carcinoma (G3) | No |
| 3 | 2007 | F | 36 | Yes | Cholestasis | PD | Biochemical PL | 1 | 12 | 5% | Well differentiated (G2) | No |
| 4 | 2006 | M | 64 | Yes | Jaundice | TDA | – | 0 | 18 | 2% | Well differentiated (G1) | No |
| 5 | 2008 | M | 44 | No | Acute pancreatitis | PD | BL A | 1 | 13 | 10% | Well differentiated (G2) | No |
| 6 | 2011 | M | 64 | Yes | Cholestasis | PD | Biochemical PL, wound infection | 2 | 23 | 5% | Well differentiated (G2) | Yes (M1 hepatic) |
| 7 | 2012 | M | 75 | Yes | Jaundice | PD | – | 0 | 25 | 80% | Neuroendocrine carcinoma (G3) | Yes (carcinomatosis) |
| 8 | 2017 | F | 29 | Yes | Epigastralgia Cholestasis | TDA | – | 0 | 18 | 1% | Well differentiated (G1) | No |
| 9 | 2018 | M | 48 | Yes | Epigastralgia Diarrhoea | PD Hepatectomy in 2nd procedure | Biochemical PL, DGE | 2 | 13 | 8% | Well differentiated (G2) | Yes (M1 hepatic) |
In our centre, 181 patients had been treated surgically for pancreatic and/or duodenal NET, 141 of which were non-functioning and 40 functioning. Among these patients, only 9 presented NET located in the ampulla of Vater, representing 4.9% of all pancreatic and/or duodenal NET.
Global descriptive studyTable 1 reports the main variables studied in each of the patients. Most were male (77.7%), and mean age was 51.2 years (range: 29–75). In terms of the ASA physical status classification, 88.8% of the sample were ASA Classes I–II. All included patients had non-functioning tumours. The most common initial symptoms were cholestasis (44%) and jaundice (22%). Preoperative pathological diagnosis was obtained in 8 patients by laparoscopy with biopsy. It should be noted that the presence of a 1% focus of adenocarcinoma was described in 1 patient.
Regarding surgical treatment, PD was performed in 6 patients: 5 due to suspected locoregional invasion or presence of lymph node involvement, and one due to the presence of intraoperative margin involvement during TDA. In contrast, 3 patients underwent TDA. Likewise, synchronous liver metastases were detected in one patient at the time of preoperative diagnosis; treatment of the primary tumour was first performed by PD, while the liver metastases were treated in a second procedure with right hepatectomy.
Mean surgical time was 253 min (180–450), specifically 311 min in the PD group and 165 min in the TDA group. Regarding postoperative morbidity, 5 patients (55.5%) presented some type of complication, all of which were Clavien–Dindo I–II. Biochemical pancreatic leak was present in 2 patients (22.2%) and type B in one (11.1%). Biliary fistula type A occurred in one patient (11.1%), and delayed gastric emptying type A was observed in 2 patients (22.2%). In the first 90 days after surgery, no complications due to bleeding, reoperations, or mortality were recorded.
Mean hospital stay was 16 days (range: 7–36), and no readmissions were recorded. At the pathological level, the mean tumour size was 16.5 mm (range: 12−25), while the histological type was well differentiated in 7 patients and neuroendocrine carcinoma in 2 patients. It should be noted that, at the immunohistochemical level, 3 patients presented hormonal expression of somatostatin and 1 patient gastrin, with normal preoperative analytical parameters for gastrin and somatostatin.
Descriptive study according to the results of each surgical techniqueTable 2 shows the results of the patients who underwent PD and those treated by TDA.
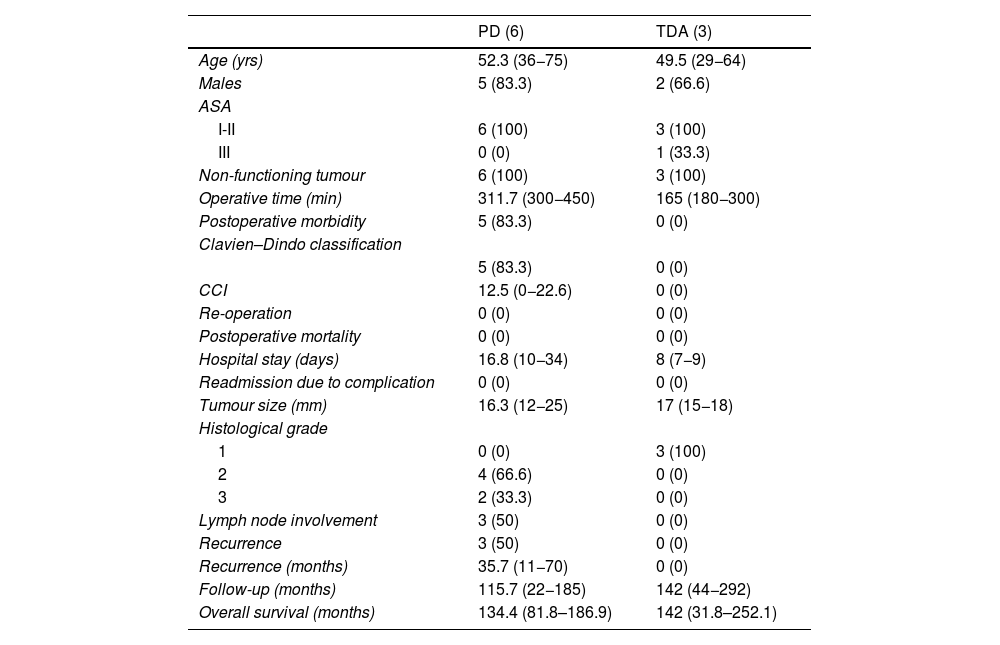
Descriptive analysis according to the type of surgical treatment conducted (PD and TDA).
| PD (6) | TDA (3) | |
|---|---|---|
| Age (yrs) | 52.3 (36−75) | 49.5 (29−64) |
| Males | 5 (83.3) | 2 (66.6) |
| ASA | ||
| I-II | 6 (100) | 3 (100) |
| III | 0 (0) | 1 (33.3) |
| Non-functioning tumour | 6 (100) | 3 (100) |
| Operative time (min) | 311.7 (300−450) | 165 (180−300) |
| Postoperative morbidity | 5 (83.3) | 0 (0) |
| Clavien–Dindo classification | ||
| 5 (83.3) | 0 (0) | |
| CCI | 12.5 (0−22.6) | 0 (0) |
| Re-operation | 0 (0) | 0 (0) |
| Postoperative mortality | 0 (0) | 0 (0) |
| Hospital stay (days) | 16.8 (10−34) | 8 (7−9) |
| Readmission due to complication | 0 (0) | 0 (0) |
| Tumour size (mm) | 16.3 (12−25) | 17 (15−18) |
| Histological grade | ||
| 1 | 0 (0) | 3 (100) |
| 2 | 4 (66.6) | 0 (0) |
| 3 | 2 (33.3) | 0 (0) |
| Lymph node involvement | 3 (50) | 0 (0) |
| Recurrence | 3 (50) | 0 (0) |
| Recurrence (months) | 35.7 (11−70) | 0 (0) |
| Follow-up (months) | 115.7 (22−185) | 142 (44−292) |
| Overall survival (months) | 134.4 (81.8–186.9) | 142 (31.8–252.1) |
A longer operative time was observed in patients who underwent PD. Postoperative complications occurred in the PD group, all of which were type I–II according to the Clavien–Dindo classification. The hospital stay tended to be shorter in the TDA group. In terms of definitive tumour size, it is worth noting that 33.3% of the patients who underwent PD presented WHO histological grade 3 (neuroendocrine carcinoma), as well as lymph node involvement in 3 patients (50% of the sample), with a mean of 2 affected lymph nodes. In a patient affected by neurofibromatosis, a somatostatinoma was detected in both the major and minor papillae (Von Recklinghausen Syndrome18), and major-minor ampullectomy was performed using a transduodenal approach.
Five-year disease-free survival was 50% in the PD group and 100% in the TDA group. Recurrence occurred in 2 patients in the form of liver metastases (one being the patient who had undergone hepatectomy due to the presence of synchronous liver metastases) and in one patient in the form of carcinomatosis. All of them were treated for recurrence, which included arterial embolization and radiofrequency ablation in patients with liver metastases, and systemic chemotherapy was administered to the patient with carcinomatosis.
Five-year overall survival of the entire sample was 90%. When we analysed the overall survival according to the type of treatment received, it tended to be lower in the PD group compared to the TDA (83.3% vs. 100% after 5 years) (Fig. 1). The only recorded death occurred in the PD group after 22 months of follow-up in the context of recurrence in the form of carcinomatosis and after starting systemic chemotherapy. The rest of the patients from both groups are still alive today.
Last of all, during follow-up, pancreatic endocrine and exocrine function was studied by means of plasma levels of glycemia and faecal elastase together with the clinical history (steatorrhea, abdominal pain after ingestion, poor digestion, etc.), respectively. Neither endocrine nor exocrine pancreatic insufficiency was observed in any of the patients undergoing TDA.
DiscussionThis paper presents a series of 9 cases of ampullary NET treated at a referral hospital for hepato-biliary-pancreatic pathology. Our study shows that patients with ampullary NET and no locoregional invasion or lymph node involvement can be treated with conservative surgery, which provides good local and systemic control of the disease as well as 5-year overall and disease-free survival rates of 100%. These results are similar to those published previously by other groups.5 In contrast, among the patients of our series treated by PD, some 50% of cases presented lymph node involvement, which resulted in recurrence of the disease in all of these patients. It should also be noted that the patients who presented lymph node involvement had the largest tumours (25, 23 and 13 mm).
The preoperative diagnosis of ampullary NET is usually difficult, since it requires high clinical suspicion due to its multiple clinical manifestations5 or because they are functioning neoplasms.17 The diagnostic tests that are considered the “gold standard” by the ENETS8 are upper gastrointestinal endoscopy and endoscopic ultrasound with biopsy, while in the extension study, CT and MRI play a relevant role. Other diagnostic tests include octreotide scan (111indium-prentreotide) and fluorodeoxyglucose positron emission tomography (18FDG-PET). Although its role is not defined in the ENETS8 recommendations, octreotide scan has shown a diagnostic sensitivity of 77% versus 72% for 18FDG-PET in comparative studies. This sensitivity increases up to 80% in well-differentiated NET (73% in pancreatic NET and 81% in gastrointestinal NET) compared to 60% for 18FDG-PET. In contrast, in poorly differentiated tumours, 18FDG-PET achieves a sensitivity close to 100% versus 57% for octreoscan.19 Likewise, other nuclear tracers are able to increase the detection of NET, such as gallium-PET (68Ga-DOTA-DPhe,1Tyr3-octreotide),19,20 which was performed in 3 patients in our series and provided the diagnosis in 2 of them. In this manner, we believe that, in cases where the extension study needs to be completed or when there is diagnostic doubt, it is preferable to use gallium-PET before octreotide scan.
The use of preoperative biopsy in our sample was performed in practically all cases (8/9) and was diagnostic in all patients. These results are similar to those published by other groups, where the preoperative biopsy ranges from 75%–100%.16,21,22 We believe that a thorough preoperative study with imaging techniques to rule out locoregional disease, a preoperative biopsy to identify well-differentiated tumours, as well as a meticulous intraoperative study to rule out lymph node metastases are all essential to indicate conservative surgery, as described in recently published international recommendations.23
For the treatment of ampullary NET, there are several options to consider. In terms of decision-making for endoscopic treatment versus surgery, some recommendations are based on tumour size,8 although there is no definitive consensus. Ampullary NET have been shown to be larger, have higher histological grades, and present more advanced stages than duodenal NET,6 which is associated with poorer survival. On the other hand, contrary to the concept based on tumour size, SM Ruff et al.24 and RK Schmocker6 have recently published that only the histological grade of the tumour correlates with overall survival. Thus, SM Ruff et al. found that 21% of patients with ampullary T1 tumours had lymph node involvement, while duodenal T1 tumours had only 6.7% lymph node involvement. Along these lines and with the aim to improve overall and disease-free survival, the ENETS recommends adjuvant therapy with regimens based on cisplatin and etoposide in G3 and metastatic NET, although therapy is not clearly defined in patients with lymph node involvement (regardless of histological grade). As H Zhai et al. conclude,25 the patients who best respond to adjuvant treatments are those with poorly differentiated pancreatic NET or a degree of poor differentiation, regardless of TNM staging.
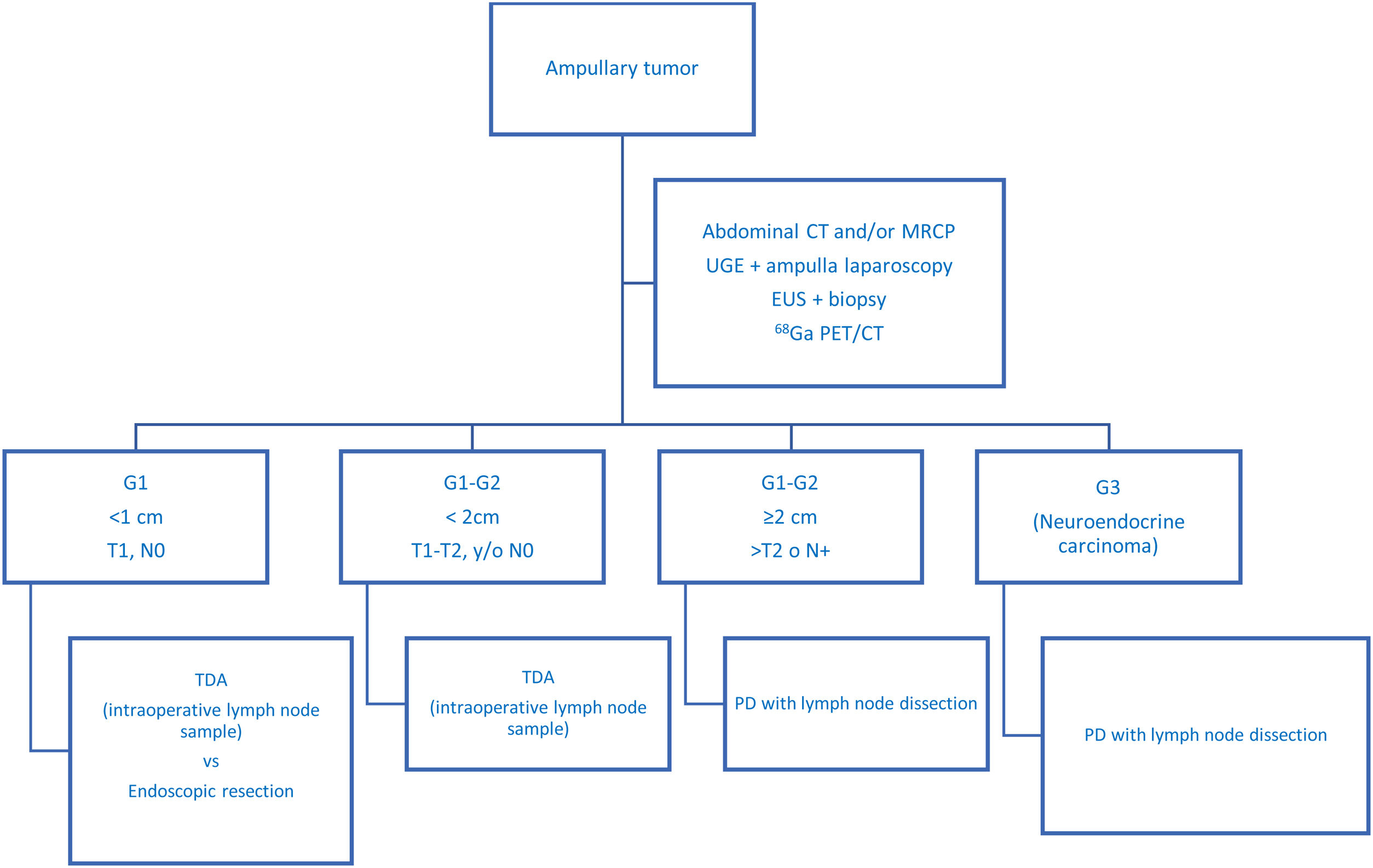
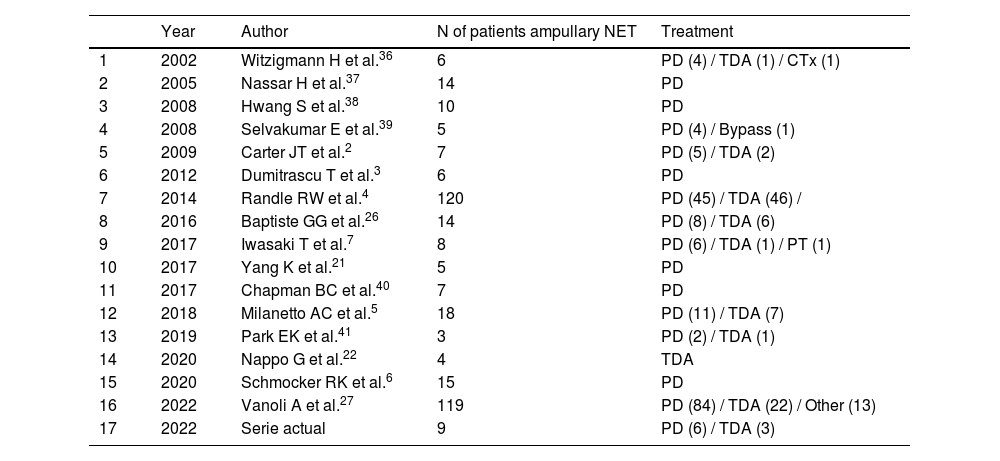
In recent years, historical series have been published comparing PD to TDA,4,26 with similar postoperative overall survival rates for both treatments. However, some authors concluded that TDA did not provide good oncological control of the disease26 since it tended to understage patients. In this regard, intermediate options have been published, such as intraoperative lymph node sampling at level 13,7 so that PD would be performed in cases with lymph node involvement, while local resection could be performed if the sample was negative (Table 3 presents a summary of the most relevant series published to date). In our experience, we have systematically performed intraoperative lymph node sampling, even though the patients had been extensively studied preoperatively. Thus, in no case of the TDA group has locoregional recurrence been recorded. This is probably due to the meticulous patient selection, which allows treatment to be individualized according to the characteristics of each. In Fig. 2, we present our diagnostic-therapeutic algorithm (which is similar to the Millaneto AC et al.5 proposal and in line with the largest published series27), where we define the criteria that we believe should be followed when proposing more aggressive treatment (PD) or parenchyma-sparing surgery (TDA).
List of bibliographic references with the most relevant series published related to surgical treatment of ampullary NET.
| Year | Author | N of patients ampullary NET | Treatment | |
|---|---|---|---|---|
| 1 | 2002 | Witzigmann H et al.36 | 6 | PD (4) / TDA (1) / CTx (1) |
| 2 | 2005 | Nassar H et al.37 | 14 | PD |
| 3 | 2008 | Hwang S et al.38 | 10 | PD |
| 4 | 2008 | Selvakumar E et al.39 | 5 | PD (4) / Bypass (1) |
| 5 | 2009 | Carter JT et al.2 | 7 | PD (5) / TDA (2) |
| 6 | 2012 | Dumitrascu T et al.3 | 6 | PD |
| 7 | 2014 | Randle RW et al.4 | 120 | PD (45) / TDA (46) / |
| 8 | 2016 | Baptiste GG et al.26 | 14 | PD (8) / TDA (6) |
| 9 | 2017 | Iwasaki T et al.7 | 8 | PD (6) / TDA (1) / PT (1) |
| 10 | 2017 | Yang K et al.21 | 5 | PD |
| 11 | 2017 | Chapman BC et al.40 | 7 | PD |
| 12 | 2018 | Milanetto AC et al.5 | 18 | PD (11) / TDA (7) |
| 13 | 2019 | Park EK et al.41 | 3 | PD (2) / TDA (1) |
| 14 | 2020 | Nappo G et al.22 | 4 | TDA |
| 15 | 2020 | Schmocker RK et al.6 | 15 | PD |
| 16 | 2022 | Vanoli A et al.27 | 119 | PD (84) / TDA (22) / Other (13) |
| 17 | 2022 | Serie actual | 9 | PD (6) / TDA (3) |
*Including studies with n > 2, where the ampullary location of the NET and surgical treatment are clearly defined.
Proposed diagnostic-therapeutic algorithm for non-disseminated ampullary neuroendocrine tumours, adapted by Millaneto AC et al.5
The advantages of performing TDA are determined above all by the high morbidity of PD, which is present in around 50% of patients,26 and the complication is serious in 20%–30% of cases (Clavien–Dindo > IIIa). In contrast, TDA shows an overall complication rate of 44%, and 14% are serious complications (Clavien–Dindo > IIIa).22 In our series, no serious complications were recorded in either of the 2 groups; however, the complications that did occur all affected the PD group. Likewise, the exocrine and endocrine pancreatic insufficiency that appears as a consequence of extended pancreatic resections (PD) should be mentioned, as the rate of pancreatic insufficiency is from 40%28–45%29. These affect patient quality of life due to steatorrhea, malnutrition and the presence of de novo diabetes. In this context, parenchyma-sparing surgeries like TDA have emerged and evolved as alternatives to standard pancreatic resection techniques, since they allow the anatomy of the upper digestive tract to be maintained. The main objective of their use is the preservation of the duodenum and the main bile duct, thereby reducing long-term morbidity. Our study includes a follow-up of endocrine and exocrine pancreatic function in patients treated with TDA, and dysfunction was not detected in any of them.
In our opinion, the good results presented in this study are probably due to the high level of specialization of the surgical team, the large volume of highly complex procedures performed, and meticulous patient selection.
In our series, all surgeries were performed using an open approach, but there is increasing evidence about the benefits of the minimally invasive approach, both laparoscopic and robotic, in hepato-biliary-pancreatic surgery.30 National and international groups with extensive experience in hepato-biliary-pancreatic surgery have already presented cases of laparoscopic TDA31 or using a robotic platform,32 with very good results. We believe that this is the line to follow if the patient's condition allows.
Another therapeutic approach that is on the rise today is endoscopic resection. Despite advances made in endoscopic techniques, there are very few published series of endoscopic ampullectomy in ampullary NET, with small numbers of patients.33–35 Most are in patients with tumours <2 cm, but even so, 44% are R1 resections,35 and recurrence rates reach 16%.34 Despite being a less aggressive procedure, complications are not negligible and range between 38% and 66%,34,35 the most common being postoperative bleeding. Recently, the ESAP study (Endoscopic Papillectomy vs. Surgical Ampullectomy vs. Pancreaticoduodenectomy for Ampullary Neoplasm - A Pancreas20007EPC Study) has been initiated, which aims to compare endoscopic treatment versus ampullectomy versus PD in ampullary tumours of any histological type. The results will soon help us better define the criteria and indications for each of these treatments.
The limitations of our study are related to the small number of patients as well as its retrospective nature. Likewise, given the low number of patients and the meticulous preoperative selection, the 2 groups cannot be compared with each other, so the study can only provide descriptive data.
In conclusion, small ampullary NET without locoregional involvement or risk factors can be treated with parenchyma-sparing surgery, such as transduodenal ampullectomy, without compromising patient survival or pancreatic and endocrine function.
Contributions of the authors(1) Study concept and design: Juan Fabregat; (2) Data collection: Jaume Tur-Martínez, Lluis Secanella, Nuria Pelaez, Maria Sorribas; (3) Data analysis and interpretation: Jaume Tur-Martínez, Teresa Serrano, Juan Fabregat; (4) Article draft: Jaume Tur-Martínez; (4) Critical review of the intellectual content: Juan Fabregat, Lluis Secanella, Juli Busquets, Joan Gornals; and (4) Final approval of the version presented: Jaume Tur-Martínez, Juan Fabregat, Juli Busquets
FundingThe authors have received no funding for this article.
Conflict of interestThe results of this study have been presented as a Final Master-Level Project by Jaume Tur-Martínez as part of the Masters’ Course in Hepato-Biliary-Pancreatic Surgery and Transplantation at the Instituto de Formación de la Universitat of Barcelona.