It has been suggested that endoprostheses are an effective treatment for fistulae after sleeve gastrectomy, but the results published are very variable. To analyze the effectiveness of stents as treatment of leakage after sleeve gastrectomy, the Spanish Society of Obesity Surgery (SECO) and the Obesity Division of the Spanish Association of Surgeons (AEC) set up a National Registry to record treatments of leaks after sleeve gastrectomy. We have analyzed patients with leaks after sleeve gastrectomy and treated with endoprostheses: 19 medical centers reported the use of endoprostheses, where 51 endoprostheses were used in 42 patients (34 women/8 men, mean age: 43.8 years, BMI: 47.6). Global effectiveness was 45%, with a complication rate of 35%. Uni- and multivariate analyses detected no factors influencing the efficacy of treatment. A larger diameter bouggie used to calibrate the stomach was related to a higher incidence of complications. No factors were found related with better stent efficacy. The effectiveness of a second stent was very low when the previous one had not been effective.
Se ha propuesto la endoprótesis como tratamiento eficaz de la fístula tras gastrectomía vertical, pero existe variabilidad en los resultados publicados. Para evaluar la efectividad de la endoprótesis como tratamiento de la fuga posgastrectomía vertical, La Sociedad Española de Cirugía de la Obesidad (SECO) y la Sección de Obesidad de la Asociación Española de Cirujanos (AEC) propusieron a sus miembros participar en un registro nacional donde incluir a pacientes con fístula posgastrectomía vertical. Analizamos los tratados con endoprótesis. Diecinueve centros han utilizado endoprótesis. Se colocaron 51 endoprótesis en 42 pacientes, 34 M/8 H, edad media: 43,8 años, IMC: 47,6. Efectividad global: 45%, con 35% de complicaciones. El estudio uni- y multivariado no objetivó factores determinantes de la eficacia del tratamiento. Un mayor diámetro del tubo gástrico se relacionó con una mayor incidencia de complicaciones. No hemos encontrado factores implicados en la efectividad de la endoprótesis. Apenas es efectiva una segunda endoprótesis si la primera no lo fue.
The excellent results obtained by sleeve gastrectomy (SG), not only in terms of weight loss but also related to the control of associated comorbidities, have made it one of the most popular bariatric techniques.1–3
However, it is not a procedure without complications. Despite its low incidence, suture line fistula is the most feared of these complications because of the morbidity and mortality involved4,5 and, in many cases, the difficulty to effectively treat fistulae.
Different options exist to treat post-SG fistulae, from antibiotic therapy to surgery. In recent years, endoluminal procedures have proven to be a good option in the treatment of these complications. These procedures include the use of stents, sealants, or endoclips. The growing experience gained with these techniques has provided increasingly satisfactory clinical results.6–9
Stenting is the most frequently used endoluminal procedure. Initially, its use was intended for the treatment of malignant intestinal stenosis, and subsequently its application has been extended to benign stenosis and suture dehiscence. Its objective is to isolate the fistula from the gastrointestinal tract during the healing process.
In this study, we have analyzed a cohort of patients diagnosed with post-SG fistula, who also had a stent placed at some point in the treatment process. Our objective was to evaluate the effectiveness of stents and the possible associated complications, as well as to analyze whether there are factors associated with greater effectiveness of these devices.
MethodsThe Spanish Society for Obesity Surgery (Sociedad Española de Cirugía de la Obesidad, SECO) and the Obesity Division of the Spanish Association of Surgeons (AEC) have proposed to their members to participate in a national registry that would retrospectively include patients diagnosed with post-SG fistula. The information was collected in an online database (www.survio.com/survey/d/registro-fistula-seco) between January 2016 and December 2017, preserving the confidentiality of patients in compliance with data anonymity requirements, as established by the Spanish Agency for Data Protection (AEPD).
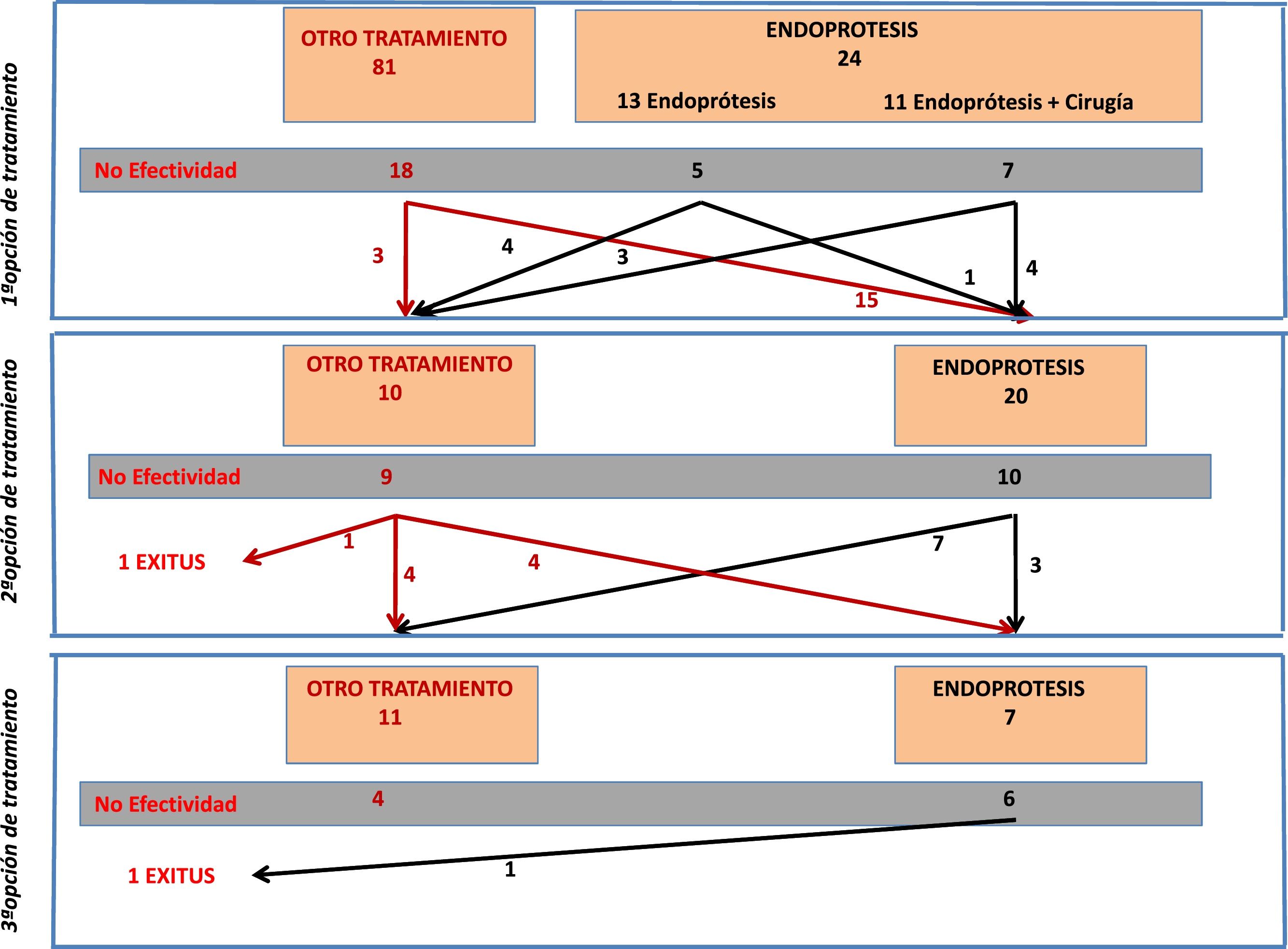
A significant percentage of patients received more than one treatment. Therefore, the results were collected based on the order of the treatment attempts made: first, second or third treatment option.
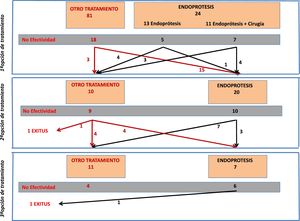
From the total number of patients included in the registry, in this study we analyzed those patients who had a stent placed as a therapeutic option during any of the treatment attempts. Fig. 1 is a flowchart of the treatments performed in the patients included in this study.
Epidemiological variables were recorded, as well as: time elapsed until the diagnosis of the fistula, main form of clinical presentation, Foucher calibration tube used, fistula location, number of treatments received, characteristics of the prosthesis, treatment effectiveness, and the complications derived from stent placement.
Statistical AnalysisData are expressed as mean ± standard deviation. For statistical evaluation, the Chi-square test or Fisher’s test was used for qualitative variables, and ANOVA or the Mann-Whitney test for quantitative variables. Likewise, in the subgroup of 24 patients who underwent stent placement as the first treatment option, the Cox regression model was used to analyze possible risk factors for stent treatment failure, and resolution of the fistula was established as a dependent variable. We also used the same model in the same group of patients to analyze the possible influence of factors on the incidence of complications, which would also be dependent variables. The SPSS Statistics 25.0 statistical package (IBM, Chicago, IL., USA) was used.
ResultsA total of 27 hospitals have participated in the registry, and 105 cases of post-SG fistula have been registered. During the different treatment options, 51 stents were placed in 42 patients. This is the group we have analyzed. A total of 19 hospitals have reported the use of stents for post-SG fistula treatment.
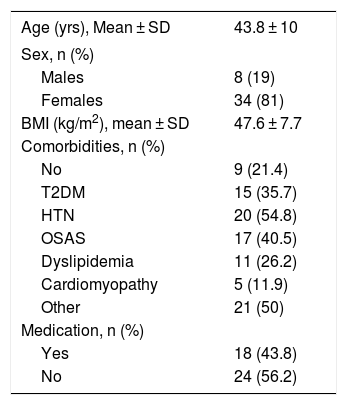
Out of these 42 patients, 34 were women (81%) and 8 were men (19%). The mean age was 43.8 ± 10 years, with a preoperative BMI of 47.6 ± 7.7 kg/m2. Table 1 presents the epidemiological data of the series.
Epidemiological Data.
| Age (yrs), Mean ± SD | 43.8 ± 10 |
|---|---|
| Sex, n (%) | |
| Males | 8 (19) |
| Females | 34 (81) |
| BMI (kg/m2), mean ± SD | 47.6 ± 7.7 |
| Comorbidities, n (%) | |
| No | 9 (21.4) |
| T2DM | 15 (35.7) |
| HTN | 20 (54.8) |
| OSAS | 17 (40.5) |
| Dyslipidemia | 11 (26.2) |
| Cardiomyopathy | 5 (11.9) |
| Other | 21 (50) |
| Medication, n (%) | |
| Yes | 18 (43.8) |
| No | 24 (56.2) |
SD: standard deviation; T2DM: type 2 diabetes mellitus; HTN: hypertension; BMI: body mass index; OSAS: obstructive sleep apnea syndrome.
The average period of time elapsed between the SG surgery and the appearance of the fistula was 11 ± 13 days.
Characteristics were recorded for 39 out of the 51 stents placed. Although a wide variability of models was used, most surgeons opted for fully or partially coated prostheses, with lengths between 10 and 20 cm and an approximate diameter of 25 mm. The composition of the stent was only indicated for 19 cases: 8 silicone, 8 metal, and 3 nitinol (nickel-titanium).
Stent Placement as the First Treatment OptionFrom the overall post-SG fistula series (n = 105), stent placement was the first treatment option in 24 patients (23% of the series). Placement was not possible in only one patient, and the technical effectiveness was 96%.
Out of these 24 patients, the stent was the only treatment in 13, while in 11 cases surgery was associated with flushing of the abdominal cavity and placement of a drain tube. Fistula resolution was achieved in 12 out of the 24 cases (50%), with no significant difference in effectiveness depending on whether or not it was associated with surgery (36% vs. 61%, respectively). It should be noted that the surgeries associated with stent placement consisted of flushing and drain placement, and that 9 out of the 13 patients in whom stent insertion was not associated with surgery either already had drain catheters from the first intervention, or they were inserted percutaneously.
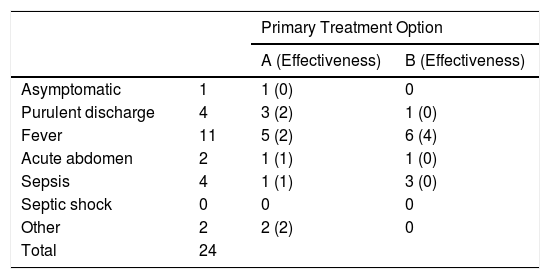
The form of presentation of the post-SG fistula in 16 out of the 24 patients was a febrile syndrome or discharge of purulent content through the drain, while the remaining 8 patients presented with sepsis or acute abdomen. The form of presentation did not determine a significantly greater performance of surgeries associated with stent placement (Table 2).
Main Form of Presentation in Patients Treated With Stents as the First Treatment Option.
| Primary Treatment Option | |||
|---|---|---|---|
| A (Effectiveness) | B (Effectiveness) | ||
| Asymptomatic | 1 | 1 (0) | 0 |
| Purulent discharge | 4 | 3 (2) | 1 (0) |
| Fever | 11 | 5 (2) | 6 (4) |
| Acute abdomen | 2 | 1 (1) | 1 (0) |
| Sepsis | 4 | 1 (1) | 3 (0) |
| Septic shock | 0 | 0 | 0 |
| Other | 2 | 2 (2) | 0 |
| Total | 24 | ||
A: stent; B: stent + surgery.
The average healing time in patients in whom the prosthesis was effective as the first treatment was 36 ± 20 days.
Seven 7 patients (29%) presented stent-related complications: one case of stent kinking, 2 displacements (which required relocation in one case and removal in the other), 2 cases of intolerance with vomiting, one esophageal erosion, and one gastric ulcer. In 3 of these cases, the fistula was resolved despite the complication (the patient with esophageal erosion, the case of gastric ulcer, and one of the patients who presented intolerance with vomiting).
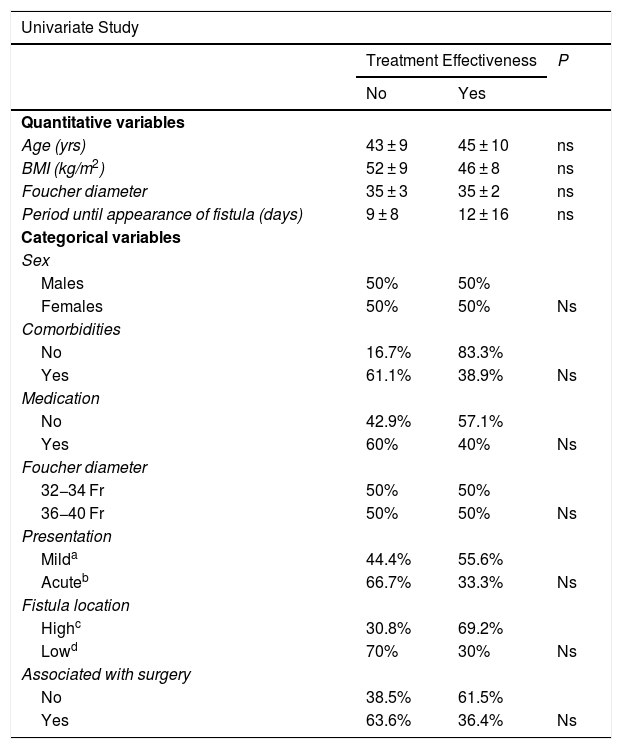
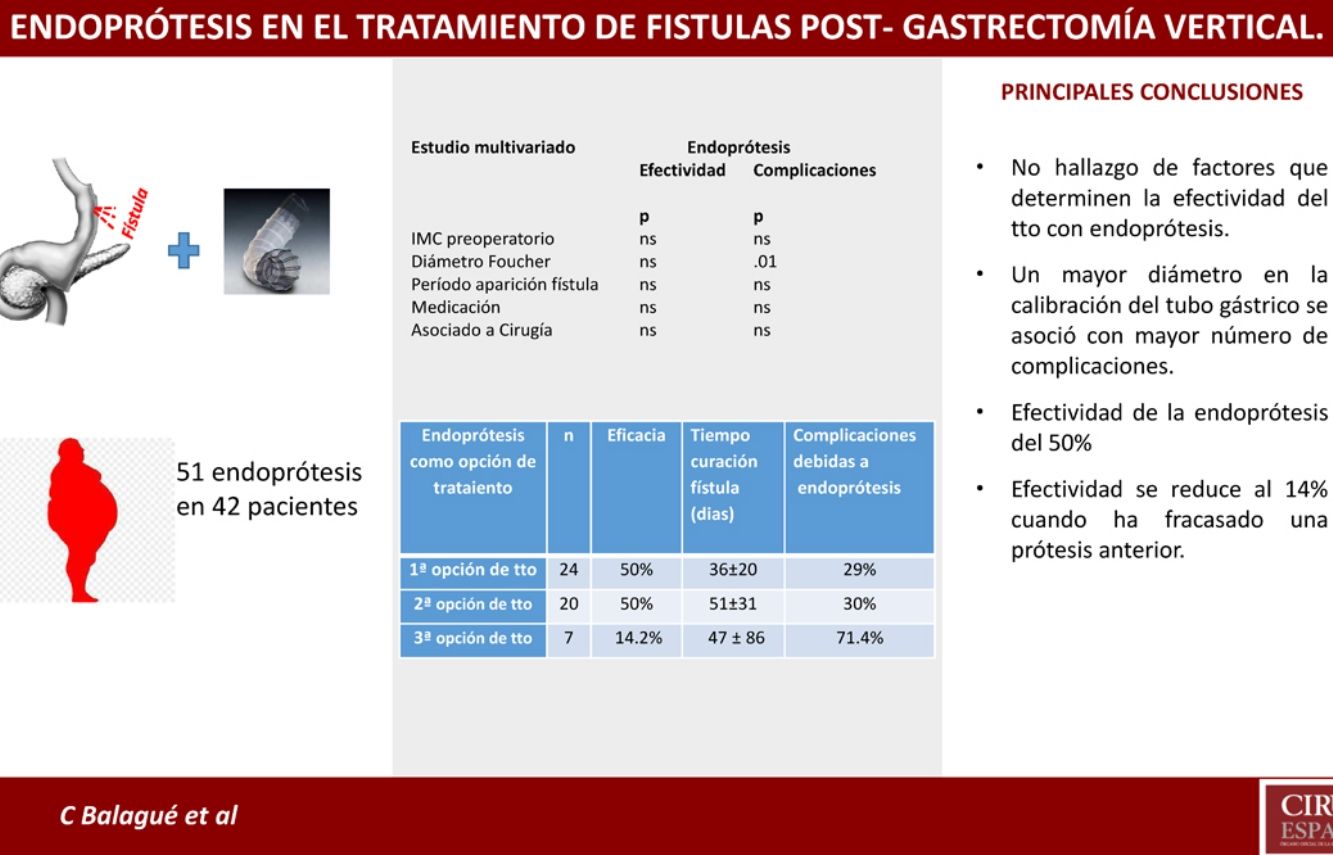
Univariate and multivariate studies were carried out with the objective of analyzing whether there were any factors that influenced the appearance of complications and also the effectiveness of the treatment. This analysis was performed with the group of patients receiving stents as the first treatment option (n = 24) because part of the factors to be analyzed were data from the initial fistula diagnosis, such as the time of the appearance of the fistula after the SG, the main form of presentation, or the association or not of surgery with the placement of the stent. This group of patients is not influenced by changes introduced by other previous fistula treatments that could cause bias. We also analyzed associated comorbidities, medication, the location of the fistula as a quantitative variable and as a categorical variable (esophagogastric junction and upper third vs. middle and lower thirds) and the diameter of the calibration bougie used to perform the SG (as a quantitative variable and as a categorical variable, greater or smaller than 36 Fr). In addition to the mentioned variables, demographic variables (age, sex, preoperative BMI) were also included. None of the variables analyzed demonstrated significant influence on the efficacy of the treatment, and only a larger size of the calibration tube showed a greater number of complications after the first treatment option (see univariate and multivariate analysis in Table 3 for complications and Table 4 for treatment effectiveness).
Univariate and Multivariate Studies for Complications.
| Univariate Study | |||
|---|---|---|---|
| Complications Due to Treatment | P | ||
| No | Yes | ns | |
| Quantitative variables | |||
| Age (yrs) | 45 ± 10 | 42 ± 9 | ns |
| BMI (kg/m2) | 47 ± 7 | 52 ± 12 | ns |
| Foucher diameter | 34 ± 2 | 37 ± 2 | .03 |
| Period of appearance of fistula (days) | 8 ± 8 | 16 ± 20 | ns |
| Categorical variables | |||
| Sex | |||
| Males | 50% | 50% | |
| Females | 75% | 25% | ns |
| Comorbidities | |||
| No | 83.3% | 16.7% | |
| Yes | 66.7% | 33.3% | ns |
| Medication | |||
| No | 78.6% | 21.4% | |
| Yes | 60% | 40% | ns |
| Foucher diameter | |||
| 32−34 Fr | 91.7% | 8.3% | |
| 36−40 Fr | 50% | 50% | .03 |
| Presentation | |||
| Milda | 72.2% | 27.8% | |
| Acuteb | 66.7% | 33.3% | ns |
| Fistula location | |||
| Highc | 69.2% | 30.8% | |
| Lowd | 70% | 30% | ns |
| Associated with surgery | |||
| No | 61.5% | 38.5% | |
| Yes | 81.2% | 18.2% | ns |
| Multivariate study | |
|---|---|
| Preoperative BMI (kg/m2) | ns |
| Foucher diameter | .01 |
| Period of fistula appearance (days) | ns |
| Medication | ns |
| Associated with surgery | ns |
BMI: body mass index.
Univariate and Multivariate Studies for Treatment Effectiveness.
| Univariate Study | |||
|---|---|---|---|
| Treatment Effectiveness | P | ||
| No | Yes | ||
| Quantitative variables | |||
| Age (yrs) | 43 ± 9 | 45 ± 10 | ns |
| BMI (kg/m2) | 52 ± 9 | 46 ± 8 | ns |
| Foucher diameter | 35 ± 3 | 35 ± 2 | ns |
| Period until appearance of fistula (days) | 9 ± 8 | 12 ± 16 | ns |
| Categorical variables | |||
| Sex | |||
| Males | 50% | 50% | |
| Females | 50% | 50% | Ns |
| Comorbidities | |||
| No | 16.7% | 83.3% | |
| Yes | 61.1% | 38.9% | Ns |
| Medication | |||
| No | 42.9% | 57.1% | |
| Yes | 60% | 40% | Ns |
| Foucher diameter | |||
| 32−34 Fr | 50% | 50% | |
| 36−40 Fr | 50% | 50% | Ns |
| Presentation | |||
| Milda | 44.4% | 55.6% | |
| Acuteb | 66.7% | 33.3% | Ns |
| Fistula location | |||
| Highc | 30.8% | 69.2% | |
| Lowd | 70% | 30% | Ns |
| Associated with surgery | |||
| No | 38.5% | 61.5% | |
| Yes | 63.6% | 36.4% | Ns |
| Multivariate Study | |
|---|---|
| Preoperative BMI (kg/m2) | ns |
| Foucher diameter | Ns |
| Period fistula appearance (days) | Ns |
| Medication | Ns |
| Associated with surgery | Ns |
BMI: body mass index.
In 20 patients, a stent was placed as a second treatment option after the failure of a different initial treatment. The effectiveness of the stent in these 20 cases was 50% (n = 10). In 4 cases, the treatment that had previously failed was the placement of a stent. Out of these 4 cases, the placement of a second device was only effective in one patient (25%). In contrast, the effectiveness in the other 16 patients who had a stent placed for the first time (after another previous treatment) was 56% (9/16).
In patients in whom stent placement was effective as the second treatment option, an average of 51 ± 31 days had elapsed since stent placement.
The incidence of stent-related complications was 30% (6 cases), and the causes were: 3 cases of migration; one case of a lodged stent that required repositioning; one distal stenosis due to hyperplastic tissue; and one antral ulcer, which was observed upon removal of the stent. Out of these 6 cases, the stent was not effective in 2 of them (one case of a displaced prosthesis and the patient with distal stenosis).
Stent Placement as a Third Treatment OptionA stent was placed as the third treatment option in only 7 patients. In this small group of patients, the effectiveness was 14% (only in one patient), with a complication rate of up to 71.4% (5 patients): 4 of these cases were resolved with the removal of the device (2 cases of stent migration, one case of severe bleeding, and one case of distal stenosis due to tissue hyperplasia). In the fifth case, there was a displacement of the stent but no repositioning was required and the fistula resolved.
In 3 out of the 7 cases in which a stent was placed as the third treatment option, it had been a second device after failure of the first, and in none of them was the condition resolved.
If we assess the entire series of 42 patients who had a stent placed at any stage of treatment, we see that in 7 patients a second stent was placed after failure of the first. The effectiveness in this group of patients was 14% (only resolved in one case).
The mortality rate of this group of 42 patients was 4.7% (n = 2). The stent had been placed as the first treatment option in one case and as the second option in the other. In both cases, however, the cause of death was due to evolution of the septic condition that did not respond to treatment, and in neither patient was it related to the device.
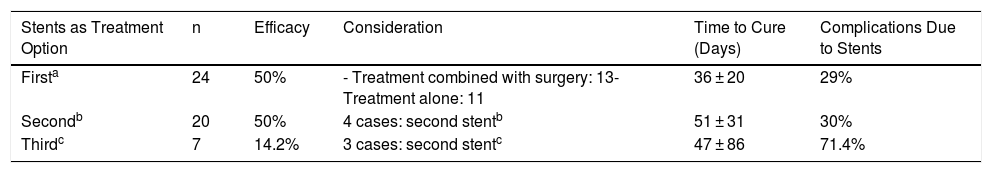
Table 5 indicates the different results obtained with the placement of stents in terms of first, second or third treatment option.
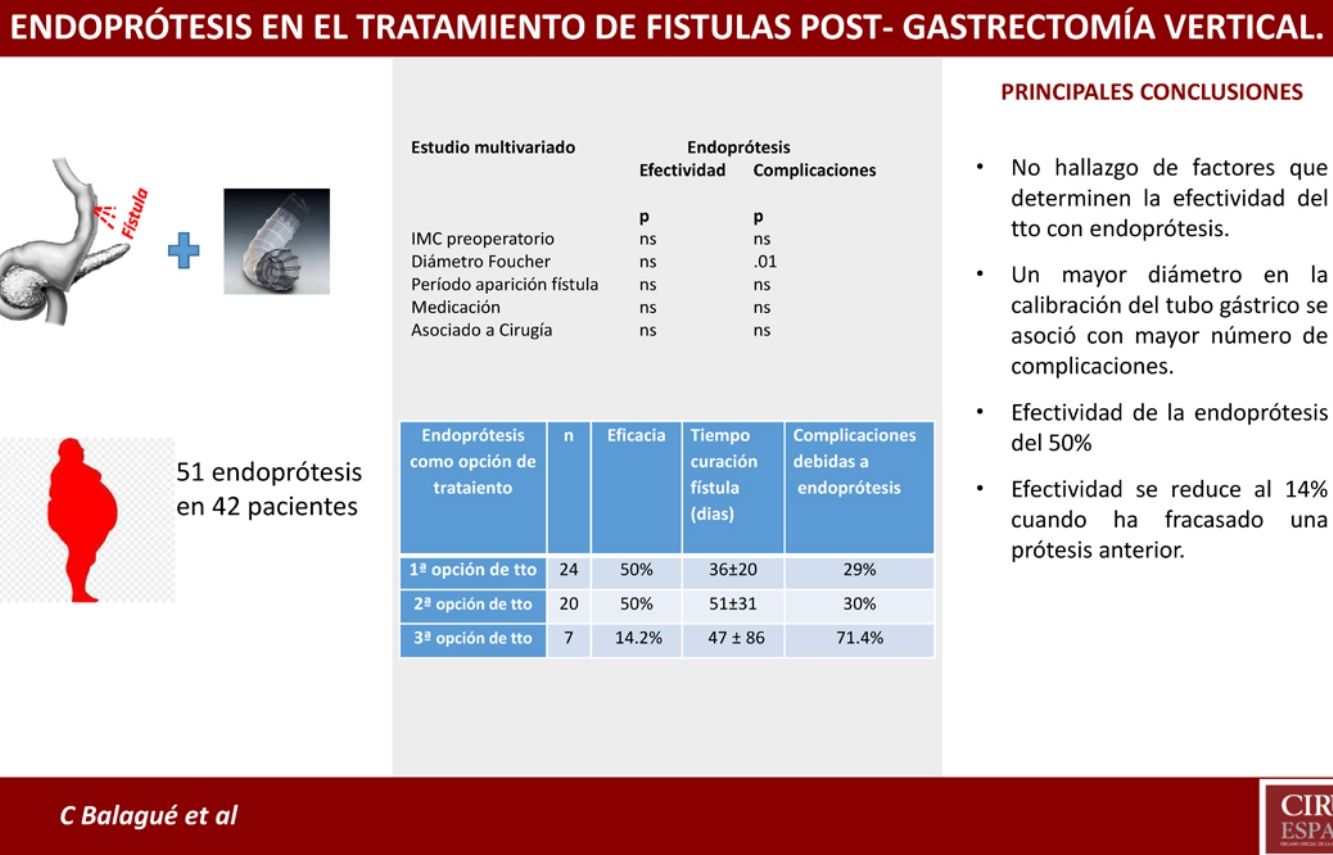
Results of Stents According to Time of Use.
| Stents as Treatment Option | n | Efficacy | Consideration | Time to Cure (Days) | Complications Due to Stents |
|---|---|---|---|---|---|
| Firsta | 24 | 50% | - Treatment combined with surgery: 13- Treatment alone: 11 | 36 ± 20 | 29% |
| Secondb | 20 | 50% | 4 cases: second stentb | 51 ± 31 | 30% |
| Thirdc | 7 | 14.2% | 3 cases: second stentc | 47 ± 86 | 71.4% |
The most important complications of SG are bleeding from the suture line (2%), stenosis of the gastric tube (1%) and the appearance of fistulae (<2%). Fistulae are the most feared complications due to their associated morbidity and mortality as well as the complexity of their treatment. Their prevalence varies among various series from 0.7% to 7%, and mortality can reach 35%.10
Once this complication appears, its treatment may include several therapeutic options,11 but the heterogeneity of the published studies and the absence of randomized studies make it difficult to standardize treatment.
While surgery had classically been the treatment for fistulae developed after bariatric surgery,12 several articles have shown the good results obtained with less invasive treatments. Endoluminal techniques, such as the placement of endoclips, stents or fibrin sealants, are being increasingly used.13–15
Currently, the use of stents has a prominent role. The guidelines of the European Society for Gastrointestinal Endoscopy approve their temporary use in the treatment of fistulae and benign esophageal perforations.16 Likewise, the American Society for Bariatric and Metabolic Surgery (ASMBS) recommends stent placement for this type of complications, compared to other endoluminal alternatives.17
It should be noted that, in our setting, the application of stents in this type of complications is progressively increasing. In the present registry, stents were the first treatment option in 24 patients, representing 23% of the global series of 105 patients treated for post-SG fistula. In the end, up to 40% (n = 42) of the cases were treated with prostheses at some point in the process. But we have not found evidence of any factor that would enable us to obtain better results with the placement of stents as initial treatment of post-SG fistula.
On the other hand, the heterogeneity of data published in the current literature regarding the time of stent placement and removal, as well as the dimensions or materials, does not allow us to define an optimal treatment model. In our series, the majority of surgeons used fully or partially coated prostheses, with lengths longer than 10 cm and diameters smaller than 3 cm. The new prostheses available on the market, which are angled, flexible to adapt to the morphology of the gastric tube, longer in length (up to 240 mm) and coated with silicone, are likely to provide greater tightness and, consequently, better sealing of the fistula. At the moment, however, the experience with these new devices is scarce, and in the literature there are only isolated clinical cases.18
Undoubtedly, one of the advantages stents provide over other types of treatments is that they allow fistulae to stay separated from the transit of saliva and food. The isolation that stents provide enables the patient to benefit from oral intake, with reduced hospital stay and even costs, according to some authors.19 Meanwhile, the coverage of the prosthesis allows us to assume that there will be immediate control of the leak, contributing to less peritoneal contamination and reduced sepsis. Likewise, stent placement enables the endoluminal pressure of the gastric tube to be reduced, one of the possible factors favoring the appearance of this complication.20,21 In addition, stents can treat possible gastric tube stenosis, if observed.
However, the use of these devices is not without complications, as reflected in our series, where the complication rate reached 35% (18/51). The larger diameter of the calibration bougie used to prepare the gastric tube was the only factor associated with a higher incidence of complications after stent placement as the first treatment option, showing statistical significance in both the univariate and multivariate analyses. This may be due to poor adaptation of the stent to the gastric tube because the complications consisted of migration, kinking or incorrect coverage of the leak in 4 out of the 7 cases.
Stent migration is one of the major limitations, and it can cause serious problems, requiring removal of the device, even laparoscopically. In our series, migration represented 44% of complications (8/18), and its relationship could not be established with one type of stent or another. Other authors have published a higher migration rate in polyester stents (60% vs. 54%).19 Overall, polyester prostheses present a greater risk of migration than fully coated metallic devices, and the latter more than partially coated ones.22,23 In order to prevent this phenomenon, various strategies have been devised, including: fixation with sutures, overlapping of 2 stents to thereby increase the anchoring surface of the mucosa, the use of longer stents, or stents adjusted to the size and morphology of the gastric tube. But, although these mechanisms can reduce the incidence of stent migration, other adverse effects have also been described, such as the appearance of ulcers, bleeding or even duodenal perforation.18 All of these are mainly related to anchoring the distal end of the stent. Currently, prostheses specifically designed for bariatric surgery are available on the market with rounded ends and greater amplitude to facilitate their anchoring to the antrum or duodenal bulb. Hence, the percentage of migrations is reduced other possible adverse effects are minimized.18
The accumulated experience of our cohort is low, which makes it difficult to draw conclusions. In addition, there are aspects to take into consideration: on the one hand, we cannot analyze the 42 patients globally, since the results obtained with the stents as the first treatment option are not comparable with those patients who had received some other treatment before opting for the stent. This has limited the univariate and multivariate study to the 24 patients in whom the stent was the first treatment. Another factor to consider is that 21.5% (11/51) of the stents placed were part of a treatment combined with surgery for drainage tube placement. This fact further increases the difficulty of analyzing the results, although it has been one of the factors analyzed.
Out of the 27 hospitals that participated in the inclusion of patients with post-SG fistula, 19 have reported cases of stents used as treatment, with significant variability not only in terms of different types of devices, but also regarding the criteria for their use. This fact is a limitation for our study, making it difficult to obtain definitive conclusions. But we must accept the reality of each hospital, as the maneuvers performed to treat this complication will mainly depend on the surgeon and her/his degree of familiarity with different endoluminal treatments, as well as the experience of endoscopists and/or radiologists at each center.
Nevertheless, despite the limitations of the present study, it is worth noting the reduced effectiveness of stents after the failure of a previous stent. In our series, this effectiveness dropped to 14% (1/7) when a previous stent had failed, compared to an effectiveness of 50% (22/44) when it was placed for the first time.
In conclusion, fistula after SG is a rare complication but important enough to make surgeons concerned. Although the published data is heterogeneous, making it difficult to standardize treatment, the use of stents as treatment for post-SG fistula is an option to consider in patients with good general health and if experienced endoscopists are available. According to our results, the decision of its use would be independent of the time elapsed since the surgery. Factors to consider when placing the stent include the diameter of the gastric tube as well as the decision of whether to place a surgical drainage system. Regardless of the type of stent used, radiological and endoscopic follow-up studies are important during the period that the device remains implanted in order to minimize the impact of any complications that may occur, such as bleeding or migration.
FundingThis study has been financed by the Fundación de la Sociedad Española de Cirugía de Obesidad (Funseco) (Beca n.oSECO-2018-9).
Conflict of InterestsThe authors have no conflict of interests to declare, nor any economic, personal, political, financial or academic interests that could have influenced our work on this manuscript.
Please cite this article as: Balagué C, Fernandez-Ananín S, Ibarzabal A, París M, Vilallonga R, Julian Puche J, et al. Papel de las endoprótesis en el tratamiento de las fistulas posgastrectomía vertical laparoscópica. Análisis de un registro nacional. Cir Esp. 2020;98:373–380.