The aim of this paper is to propose our technique, namely three-port laparoscopic sleeve gastrectomy (TPLSG), to define the feasibility and expose the short-outcomes, as an alternative between the standard laparoscopic approach and the single incision (SILSG) for such patients.
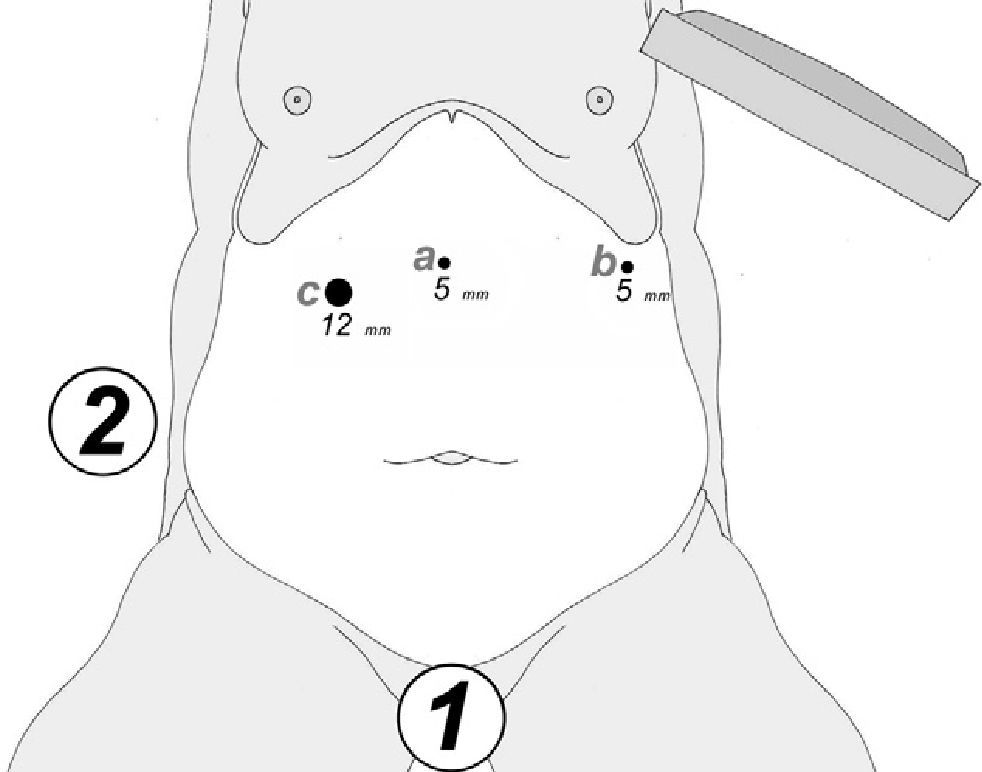
Material and methodsWe conducted a prospective study of 25 patients: 12 male and 13 female, reporting a mean BMI of 53kg/m2 (range: 50–72) and a mean age of 38 years (range: 29–55). To evaluate the feasibility of our technique we have always been respecting 3 pre-operatives conditions: BMI≥50kg/m2; preoperative abdominal US or CT to measure the liver and determine the hepato-splenic characteristics; and “intent to treat by 3 ports” (2 of 5mm and one 12mm in diameter). The short outcomes follow-up include: operative time, conversion, transfusions, fistula, reinterventions and parietal herniation at one and three months after surgery.
ResultsHepatomegaly was present in 19 (76%) patients, and it is greater on the left hepatic lobe in 9 (36%) patients. The mean operation time was 72min (range: 50–110). No per-operative complications were observed. Conversion to four ports procedure was necessary in one patient. The mean hospital stay was 3 days (range: 2–5). No mortality and 30th POD morbidity rate were reported. No patient developed an incisional hernia to date.
ConclusionThe TPLSG reduces the ports in number and in size and subsequently the parietal trauma; it also an instrumental triangulation, making surgery safe and reproducible.
El objetivo de este trabajo es presentar nuestra técnica de gastrectomía vertical laparoscópica a través de 3 puertos (GVLTP) como alternativa a la técnica laparoscópica convencional, por un lado, y a la de incisión única por otro; también describimos su viabilidad y presentamos los resultados a corto plazo.
Material y métodosSe realizó un estudio prospectivo con 25 pacientes: 12 hombres y 13 mujeres, con un IMC medio de 53kg/m2 (intervalo: 50–72) y una edad media de 38 años (intervalo: 29–55). Para evaluar la viabilidad de nuestra técnica, hemos respetado siempre 3 condiciones preoperatorias: IMC≥50kg/m2. Tomografía computarizada o ecografía abdominal para medir el hígado y determinar las características hepatoesplénicas. «Intención de tratar» con 3 puertos (2 de 5mm y uno de 12mm de diámetro). Los criterios de valoración del seguimiento a corto plazo incluyen: tiempo perioperatorio, cambio a otra técnica, transfusiones, fístulas, reintervenciones y hernia parietal al mes o a los 3 meses después de la cirugía.
ResultadosExistía hepatomegalia en 19 (76%) pacientes, y en 9 (36%) era mayor en el lóbulo hepático izquierdo. El tiempo medio de intervención fue de 72min (intervalo: 50–110). No se observaron complicaciones perioperatorias. En un paciente fue necesario cambiar a un procedimiento de 4 puertos. La estancia hospitalaria media fue de 3 días (intervalo: 2–5). La tasa de morbimortalidad a los 30 días de la operación fue cero. Ningún paciente ha desarrollado hernia incisional hasta la fecha.
ConclusiónLa GVLTP reduce el número y tamaño de puertos y, posteriormente, el trauma parietal; además, como utiliza la triangulación instrumental, la cirugía es segura y reproducible.
The first laparoscopic sleeve gastrectomy (LSG) was done in 1999 by Gagner. Initially, it was used as a restrictive component of a more complex intervention. It later became an independent procedure when it was demonstrated that it could reduce morbidity and mortality in cases of super morbid obesity (defined by a body mass index [BMI] ranging between 50 and 60kg/m2) when compared with other procedures, such as biliopancreatic diversion with duodenal switch (BPD-DS) and Roux-en-Y gastric bypass (RYGB).1–3
Lately, LSG is being used more frequently as a definitive procedure for the treatment of morbid obesity, and acceptable short-term results have been achieved.4,5
The current accepted indications for LSG include: primary weight loss procedure in super obese patients who often present hepatomegaly, initial stage of two-staged approach for weight loss (RYGB or BPD-DS in 2 stages) and patients with BMI≥40kg/m2, in addition to a serious medical condition or other important comorbidities.6–10 LSG could be useful in cases of adolescents with morbid obesity, in patients who present distorted anatomy (multiple abdominal adherences, situs inversus), a history of inflammatory bowel disease or intestinal lymphoma.11–14
LSG reduces the volume of the stomach and the production of ghrelin. This mechanism seems to explain the physiopathology of LSG in terms of weight loss and the sensation of hunger.15–19
An extensive multicenter meta-analysis recently established that the morbidity and effectiveness of LSG is between that of the laparoscopic adjustable gastric band (LAGB) and the RYGB.5
The rate of conversion to open surgery which has been reported in extensive reviews is less than 0.9%. The 30-day postoperative morbidity (POM 30) and the 30-day rate of reintervention are 5% and 3%, respectively. The short-term surgical complications are mainly due to leaks and hemorrhages (2.2% and 6%, respectively). Stenosis (4%) and delayed gastric emptying are the most frequent late complications. The POM 30 and 1-year mortality rate associated with LSG are 0.1% and 0.2%, respectively.5,20,21
The standard approach in LSG requires between 4 and 7 trocars.9,13,22 Recently, single-incision laparoscopic sleeve gastrectomy (SILSG) has been used successfully, with positive postoperative results and fewer wound complications.8,9,23–27
The objective of this paper is to present the new three-port laparoscopic sleeve gastrectomy (TPLSG) (12-5-5) technique as a feasible, reproducible, effective and economic alternative to the standard laparoscopic approach on one hand, and SILSG on the other, taking into consideration the short-term favorable results obtained in the ongoing prospective study with 25 super obese patients with hepatomegaly.
In order to evaluate the viability of our technique, we have respected one preoperative condition: “intention to treat with 3 ports: two 5mm and one 12mm in diameter”.
Material and MethodsThe patients received prophylactic treatment for deep vein thrombosis (DVT) and compression stockings against thromboembolism, in association with preoperative wide-spectrum antibiotics.
The patients were placed in the 20° reverse Trendelenbourg position and the operating table was tilted toward the right side of the patient at 15°. The surgeon stood between the patients’ bent legs and the assistant on the right side of the patients.
Pneumoperitoneum was created with 14mmHg using a Verress Needle® (Ethicon Endo-Surgery Inc., Johnson & Johnson, Cincinnati, OH, USA) in the left upper quadrant.
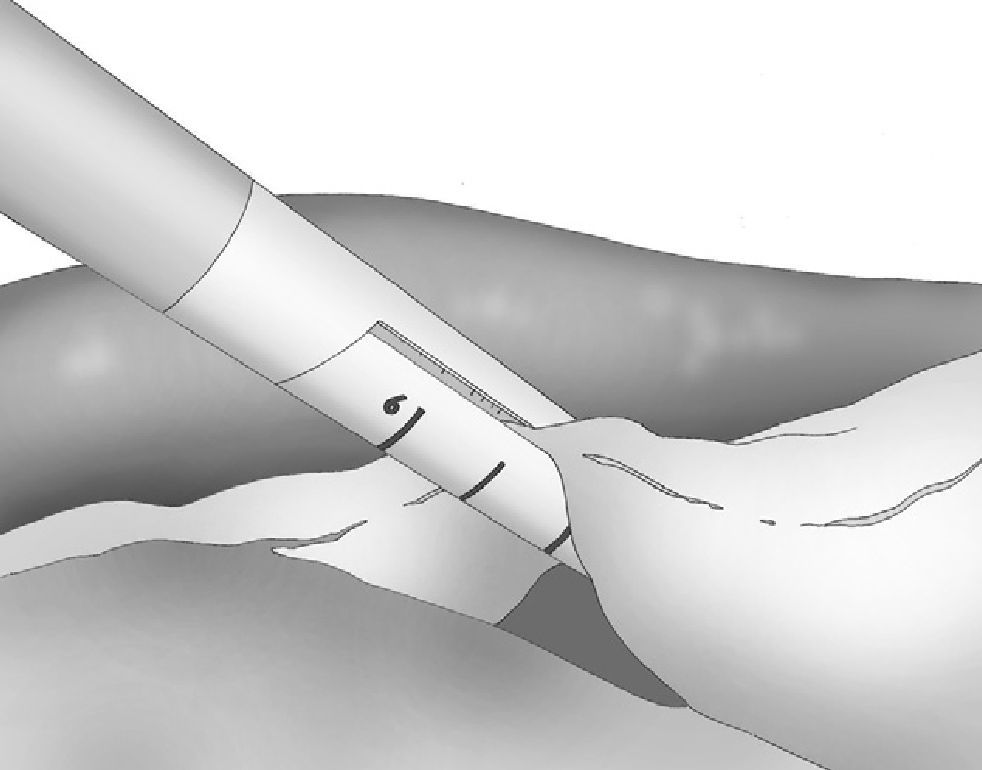
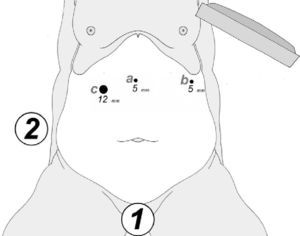
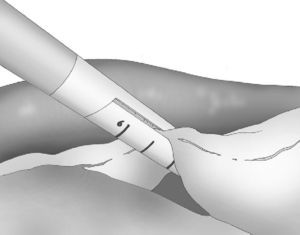
At either 0° or 30°, a 5mm camera was inserted through an atraumatic working port (ENDOPATH® Xcel™, Ethicon Endo-Surgery Inc., Johnson & Johnson, Cincinnati, OH, USA) and another 2 surgical ports (12 and 5mm) were inserted with visual control, as shown in Fig. 1.
The gastroepiploic division was initiated in the thinnest and most accessible part of the greater curvature and performed with an ultrasonic scalpel (Harmonic Scalpel®, Ethicon Endo-Surgery Inc., Johnson & Johnson, Cincinnati, OH, USA) that was introduced through the 5-mm left lateral port. The division of the vessels of the greater curvature was performed near the gastric wall in order to respect the gastroepiploic vessels.
With atraumatic forceps that were introduced through the 12-mm port, the posterior surface of the stomach was raised anteriorly and, afterwards, the liver was retracted without direct contact. The endoscope in the 5-mm port, situated in the epigastrium, aids in this maneuver by creating a tent effect that is particularly useful in the presence of hepatomegaly of the left lobe.
Once the greater curvature was liberated, a slight elevation of the stomach allowed for comfortable division of posterior gastric adherences with the anterior pancreatic surface, while always maintaining contact with the gastric wall.
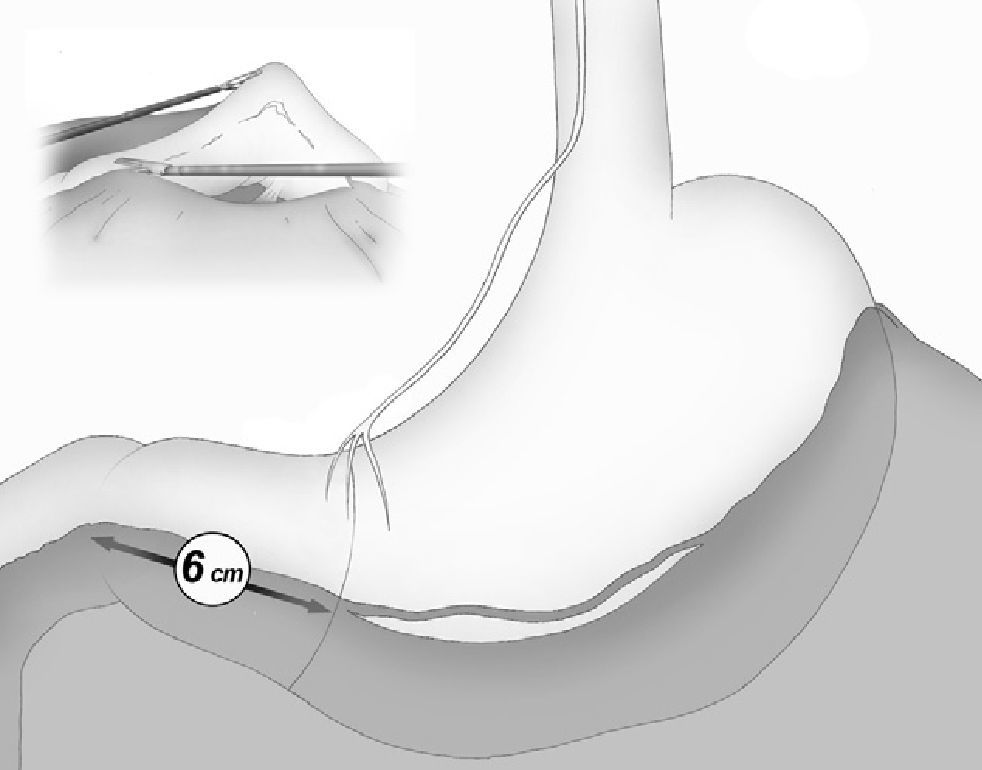
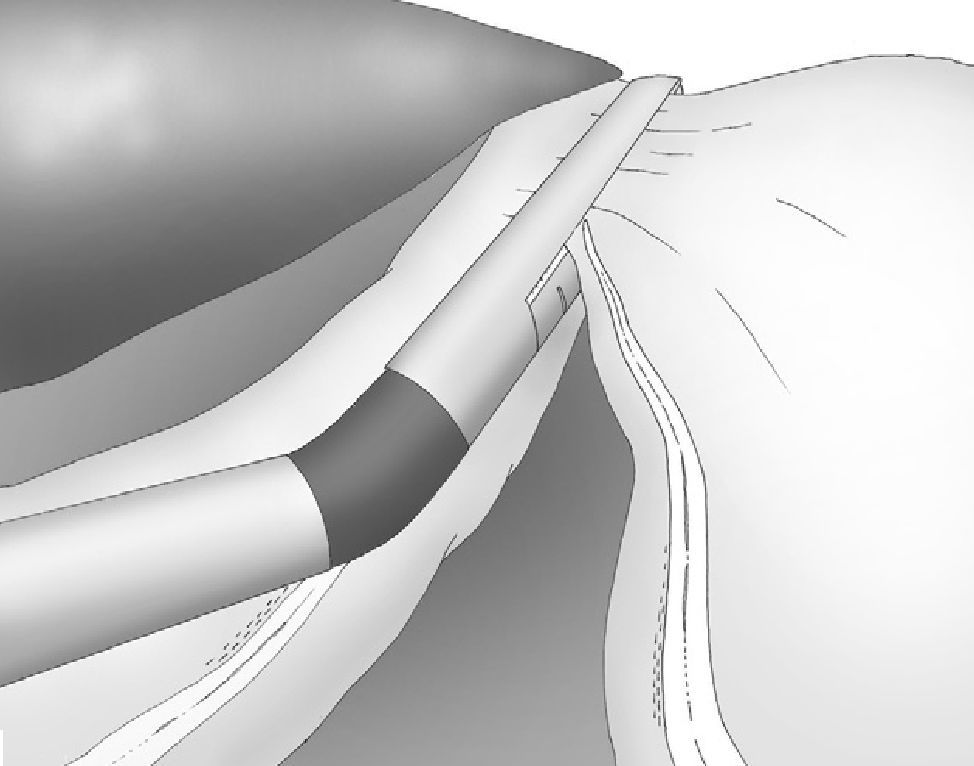
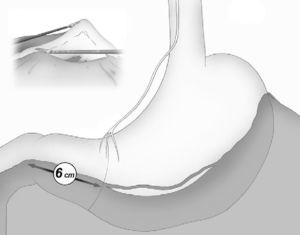
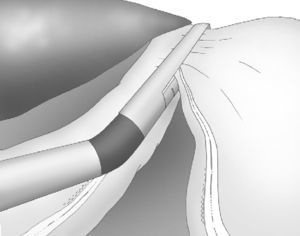
The gastroepiploic division was continued distally until a distance of 6cm proximal to the pylorus, just at the level of the second terminal branch of the nerve of Latarjet (Fig. 2).
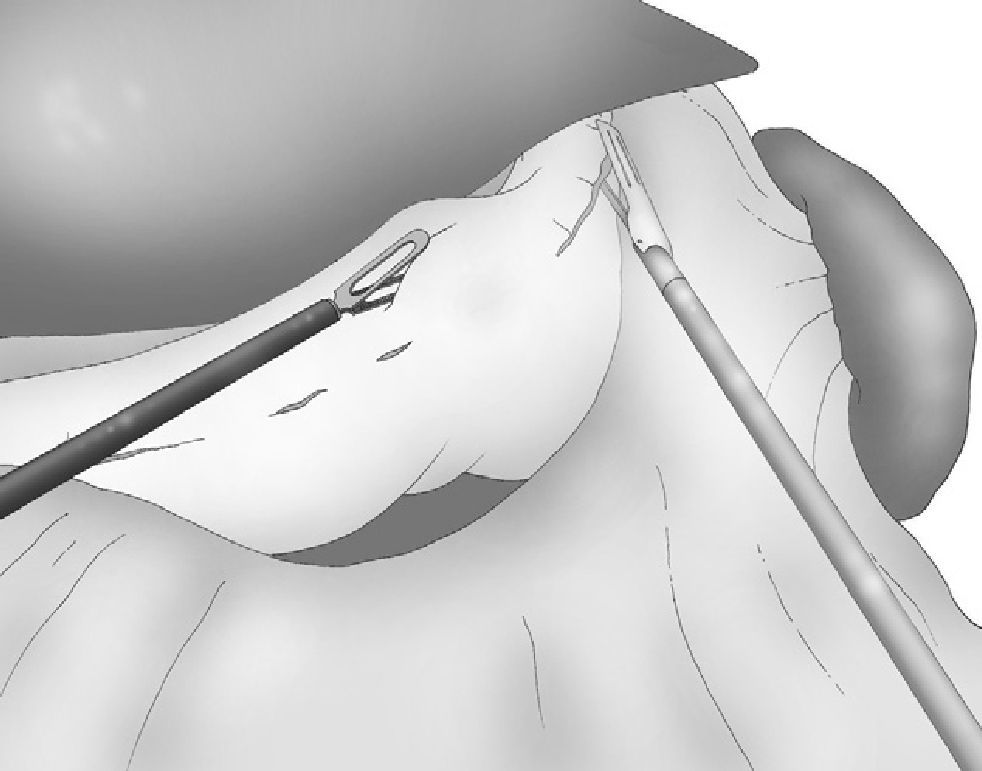
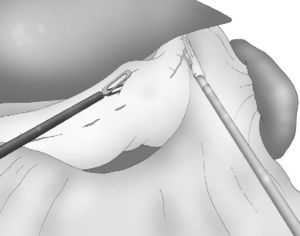
Correct, light medial and anterior retraction of the free portion of the greater curvature allowed for adequate exposure of the short gastric vessels without using a hepatic separator (Fig. 3).
It may be useful to place a sponge under the gastrosplenic ligament while cutting the short gastric vessels, which is also done with the harmonic scalpel.
The angle of His was then accessible and, once retracted, the left crural pillar and the gastroesophageal junction were clearly exposed, where the dissection of fat was done without any risk of injury to the spleen, in strict and careful contact with the stomach wall. The greater curvature was now completely dissected from the antrum to the gastroesophageal junction, and the posterior side of the stomach was freed from other structures of the lesser sac.
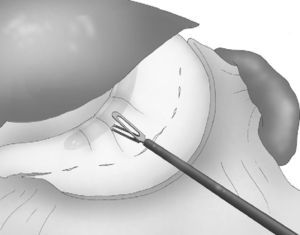
A 33 F oral gastric tube was introduced in order to obtain correct calibration of the LSG (Fig. 4).
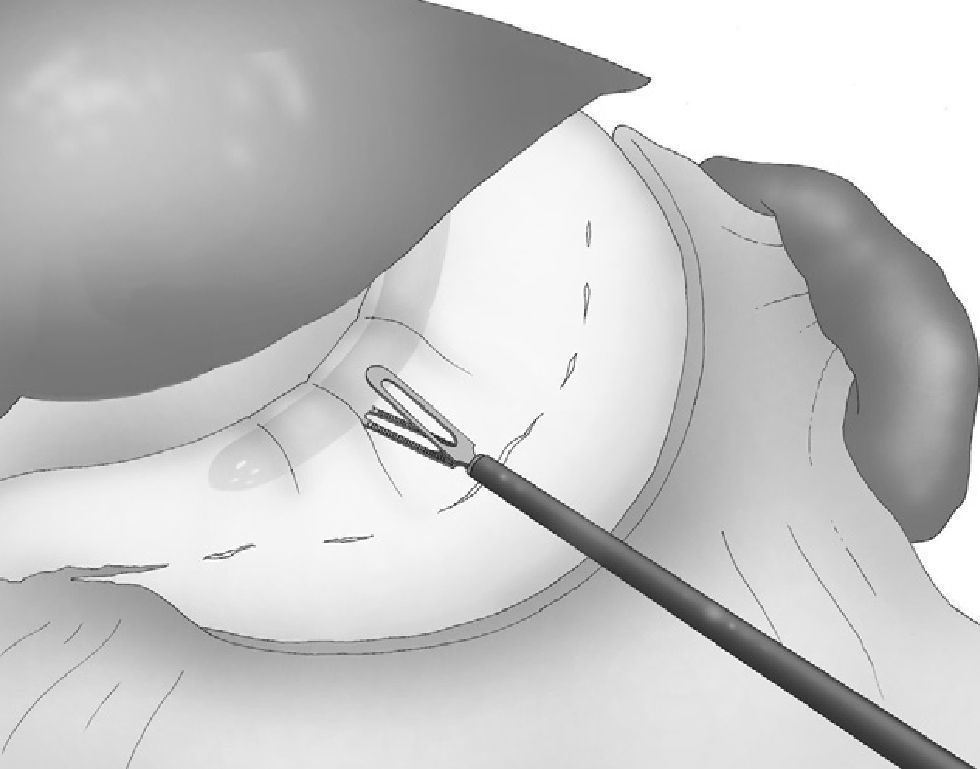
An articulating linear 6-mm stapler (Echelon Flex™ 60 Endopath®, Ethicon Endo-Surgery Inc., Johnson & Johnson, Cincinnati, USA) was inserted through the 12-mm right trocar, and the laparoscopic sleeve gastrectomy was done with sequential staples along the gastric tube, starting at the distal end of the gastrocolic division. Gold staple cartridges were selected for the antrum, and blue staples were later used for the rest of the gastric transection (Fig. 5).
During this latter stapling, it is necessary to have a satisfactory posterior visual control of the staple line in order to avoid injury to the spleen. This is easily achieved with the cooperation of the stapler traction and the inclination of the camera (Fig. 6).
Hemostasis of the staple line was achieved by means of localized monopolar coagulation and, when necessary, with interrupted sutures. Normally, we do not do continuous sutures on the staple line or suture reinforcement of the gastric staples as they do not reduce the rate of leaks.21,28,29
Finally, the gastric specimen was withdrawn through the 12-mm port and the fascia of the 12-mm port was closed with Vicryl 2/0 sutures.
Liquid diet was initiated on the first postoperative day and also implemented on the second day. The patients received orientation about nutrition during their hospital stay and were generally released on the third day post-op after a light lunch, with oral analgesia, gastric protection with proton-pump inhibitors and prophylaxis for DVT including a standard dose of low molecular weight heparin. Three weeks later, all patients were examined and 3-, 6- and 12-month follow-up appointments were scheduled. All patients underwent CT or parietal ultrasound 3 months after surgery to examine the scars.
ResultsAfter having clearly defined the technique, from 2010 to 2011, 25 patients were included in a prospective, continuous series according to 3 preoperative conditions:
(i) BMI≥50kg/m2;
(ii) abdominal CT or ultrasound during the preoperative period in order to measure the liver and determine hepatosplenic characteristics;
(iii) “intention to treat using 3-trocar LSG” (two 5-mm and one 12-mm trocars).
The patients of our series included 12 men and 13 women with a mean BMI of 53kg/m2 (interval: 50–72) and a mean age of 38 (interval: 29–55). Signed informed consent was obtained from all the patients who participated in this study. All patients underwent preoperative ultrasound in order to determine the degree of hepatomegaly.
Surgery was performed by 4 different surgeons, two of whom were in training. Mean surgical time was 72min (range: 50–110). No perioperative complications were observed.
A 4-trocar procedure was required in one male patient with a BMI of 69kg/m2. In this case, it was necessary to insert an additional 5-mm port for adequate exposure of the esophagogastric junction and the left pillar of the diaphragm, hidden by the fatty tissue of the phrenogastric ligament. This was the only patient in the entire series that required drainage. None of the patients required transfusion.
Mean hospital stay was 3 days (interval: 2–5).
No morbidity or mortality was registered within the 30 days post-op.
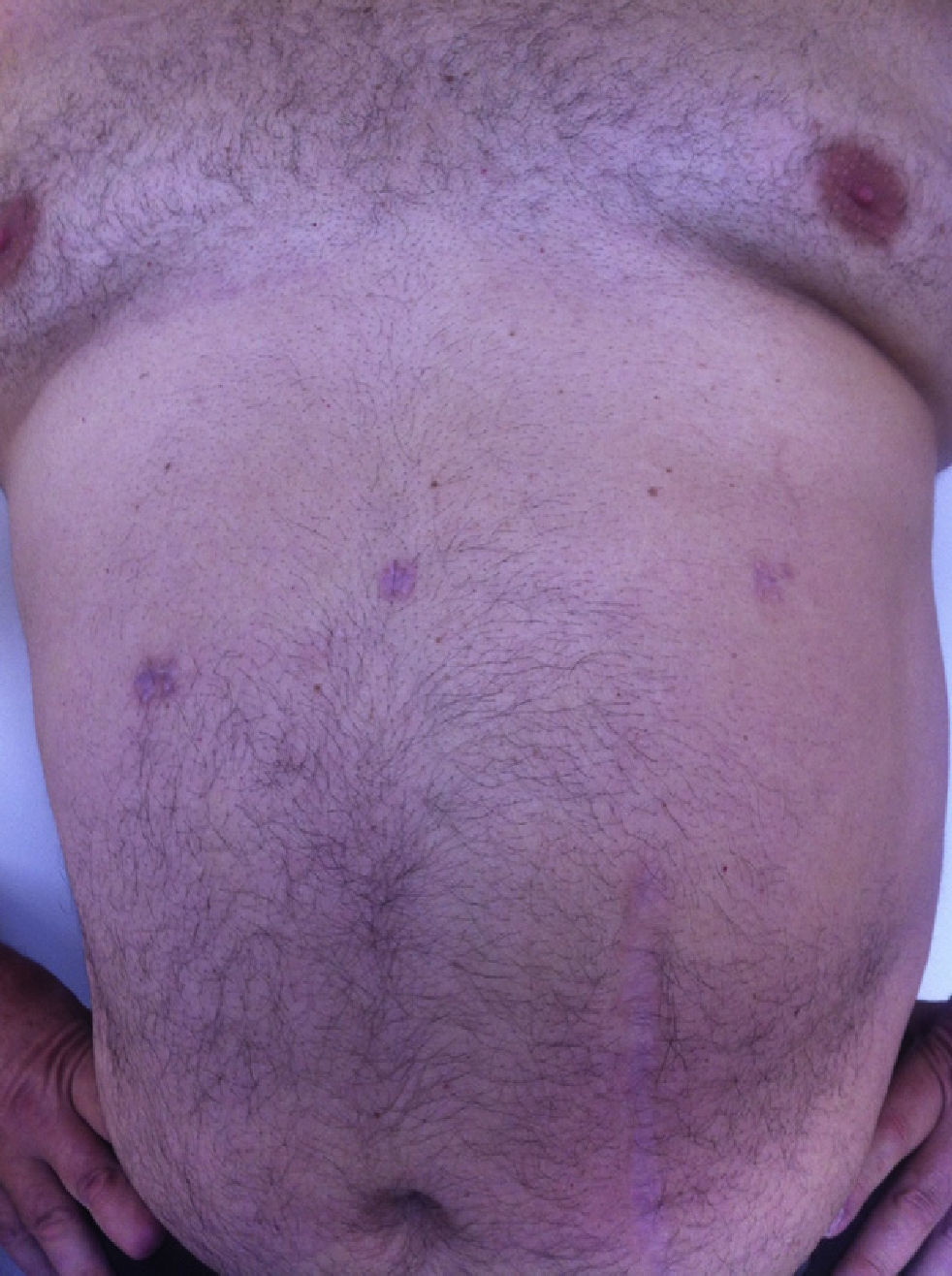
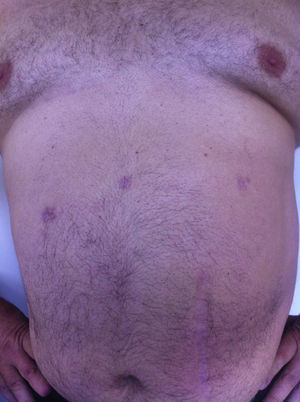
All the patients were examined one and three months after the procedure; mean follow-up was 8 months (range: 3–15). No pain or incisional hernias were observed in the examination of the parietal scars. The CT or parietal ultrasound ordered for all patients documented the absence of postoperative incisional hernia. The cosmetic effect was excellent since the 5-mm scars were almost invisible (Fig. 7).
With regard to the excess weight loss and its effect on comorbidities, no differences were observed when compared with the results obtained with standard LSG.
Discussion and conclusionsStandard LSG is a new bariatric procedure that is used with growing frequency in the treatment of obesity and obesity-related diseases. Recently, clinical practice guidelines based on international reports have been published.30 The procedure characteristically requires the placement of between 4 and 7 laparoscopic ports,9,13,22 even though some reports recommend reducing the number and size of the ports.31,32
It has recently been observed that fewer incisions (using a 12-mm access incision specifically) later translate into less postoperative discomfort and better esthetic results, as well as a reduction in parietal trauma, pain and risk of hernia.
According to specialized literature, the rates of infection and incisional hernia in ≥10mm ports range between 0.6% and 2.8%.32,33 Papers on bariatric surgery have not published a rate for this type of complications with 5-mm ports, which suggests that the rate of wound complications is low when small-diameter ports are used.
Recently, SILSG has been proposed and used successfully, with good immediate results and less wound complications. However, because the required learning curve is quite unique and specific, use of the technique has not been widespread. This is also due to the technical difficulties associated with instrument navigation within a limited area of movement. In fact, most authors coincide in the opinion that the loss of the classic laparoscopic triangulation makes extracorporeal dissection and suture more difficult than in conventional laparoscopy with several ports.23
To the best of our knowledge, there is only one published prospective randomized study that compares traditional LSG and SILSG.33 This study was done with selected patients and highly skilled surgeons. The study shows that SILSG is as safe and effective as standard LSG (five 12-mm ports), although the only statistical significance in its favor is less use of analgesia during the first postoperative day in the SILSG group.
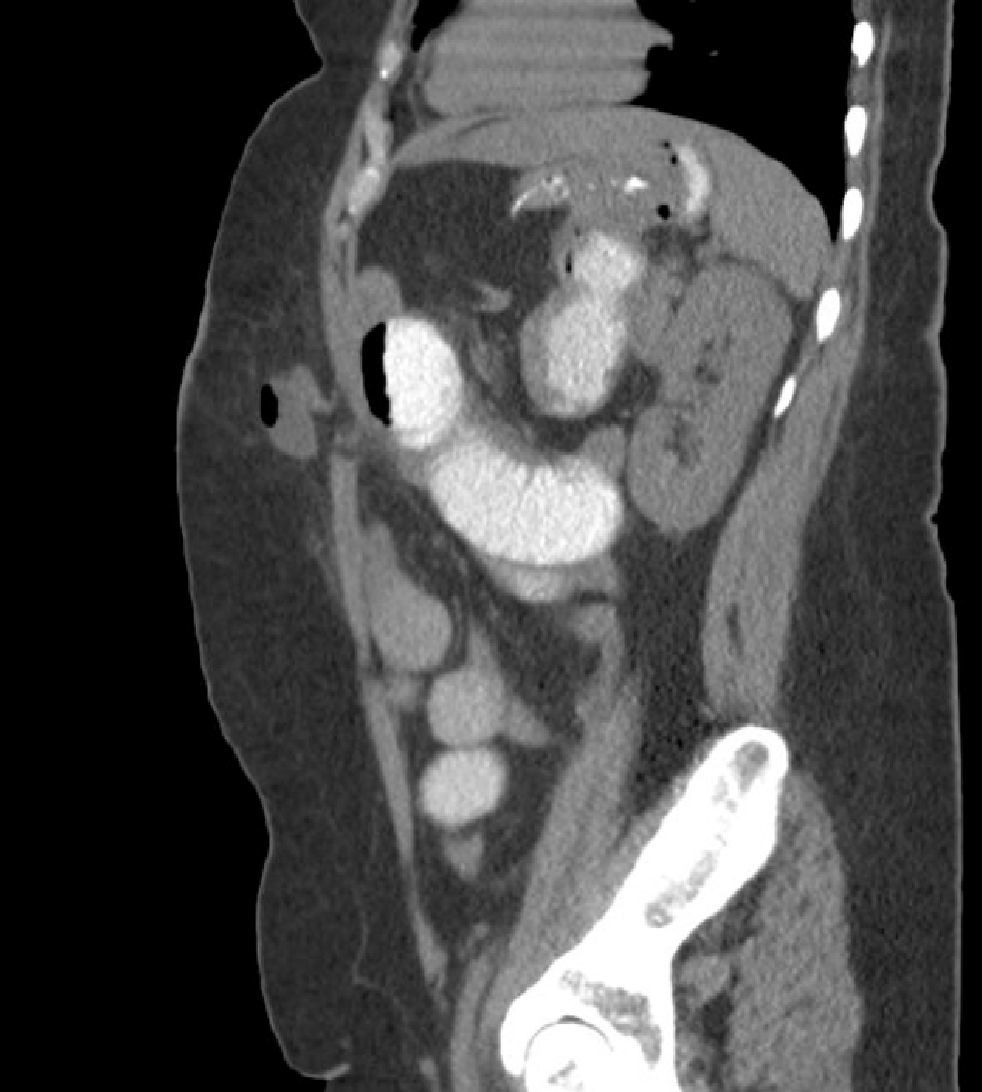
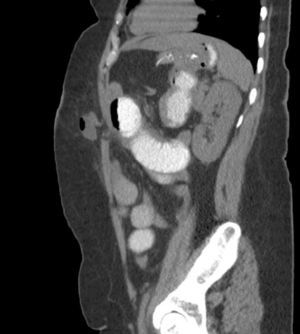
We also believe that the need to train surgeons for SILSG and the new instruments required for adequate anatomic exposure currently limit the propagation of the SILSG technique. Lastly, we consider that it is essential to properly follow up patients and to use appropriate diagnostic tools to determine the actual rate of postoperative incisional hernia (Fig. 8). In these patients, we believe that CT is fundamental in the context of evidence-based medicine.
Nonetheless, the three-port LSG procedure could be a comfortable and effective alternative to the standard laparoscopic approach in order to reduce parietal trauma, as well as another option to the new SILSG technique because no specific skills, training or equipment are required.
The TPLSG described herein for patients with morbid obesity or hepatomegaly is safe, technically feasible and reproducible, as shown by the low rate of conversion to a standard laparoscopic procedure (4%) and by the lack of conversion to laparotomy.
The interventions in our series were performed by different surgeons and included surgeons-in-training, which demonstrates that it is an easily reproducible technique. This is probably due to the possibility of using conventional instruments and to operate with comfortable instrument triangulation.
Our initial results do not reach statistically significant conclusions. Furthermore, randomized, prospective, controlled studies are needed to demonstrate the benefits of surgery with fewer ports.
By reducing the parietal risk, three-port LSG improves the results obtained with the standard laparoscopic technique and is similar to SILSG, with no increased risk for patients or social cost. Only prospective, randomized clinical trials could determine its effectiveness in terms of evidence-based medicine.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Arru L, Azagra JS, Goergen M, de Blasi V, de Magistris L, Facy O. Gastrectomía vertical laparoscópica a través de 3 puertos: viabilidad y resultados a corto plazo en en una serie de 25 pacientes con hiperobesidad. Cir Esp. 2013;91:294–300.