The annexin superfamily consists of 12 proteins with a highly structural homology that binds to phospholipids depending on the availability of Ca2+-dependent. Different studies of overexpression, inhibition, or using recombinant proteins have linked the main function of these proteins to their dynamic and reversible binding to membranes. Annexins are found in multiple cellular compartments, regulating different functions, such as membrane trafficking, anchoring to the cell cytoskeleton, ion channel regulation, as well as pro- or anti-inflammatory and anticoagulant activities. The use of animals deficient in any of these annexins has established their possible functions in vivo, demonstrating that annexins can participate in relevant functions independent of Ca2+ signalling. This review will focus mainly on the role of different annexins in the pathological vascular remodelling that underlies the formation of the atherosclerotic lesion, as well as in the control of cholesterol homeostasis.
La superfamilia de anexinas esta constituida por 12 proteínas con alta homología estructural que se unen a fosfoslípidos de membrana de una manera dependiente de Ca2+. Diferentes estudios de sobreexpresión, inhibición o usando proteínas recombinantes han identificado que la función principal de estas proteínas esta relacionada con su unión dinámica y reversible a membranas. Estas proteínas se encuentran en múltiples compartimentos celulares participando y regulando diferentes funciones como el tráfico de membranas, el anclaje al citoesqueleto celular, la regulación de canales iónicos, así como actividad pro- o anti-inflamatoria y anticoagulante. El uso de animales deficientes en alguna de estas anexinas ha permitido establecer sus posibles funciones in vivo, demostrando que las anexinas pueden participar en funciones relevantes independientes de la señalización por Ca2+. En esta revisión nos centraremos principalmente en el papel que juegan las diferentes anexinas en el remodelado vascular patológico que subyace a la formación de la lesión aterosclerótica así como en el control de la homeostasis del colesterol.
Cardiovascular disease (CVD) is the leading cause of mortality in developed countries. Despite the considerable decrease in mortality rates due to cardiovascular causes over the last two decades, 17.3 million people die every year because of CVD and it is estimated that up to 23.6 million people will die by the year 2030.1 The term ‘CVD’ is used to describe different pathologies that affect the heart and the circulatory system, including cardiac failure, diseases of the coronary arteries, stroke, arterial hypertension, and atherosclerosis, amongst others. Atherosclerosis, in particular, refers to the development of atheromatous plaques in the inner lining of the arteries. The endothelial cells of the vessel, which are usually resistant to the binding of leukocytes, express adhesion molecules that enable white blood cells to anchor to the cell surface when they are in the presence of stimuli that activate them, such as dyslipidaemia, arterial hypertension, or proinflammatory molecules. The changes in endothelial permeability that occur in parallel, as well as in the composition of the extracellular matrix promote the entrance and retention of lipids, especially of low density lipoproteins (LDL), in the arterial wall.2 The retention of LDL in the interior of the wall will foster their oxidation, provoking an increase in the expression of adhesion molecules and proinflammatory proteins that stimulates the migration of the leukocytes toward the inner layer of the artery, called the ‘tunica intimae’. Once inside the wall arterial, the monocytes differentiate into macrophages, where they will capture the LDL-ox, and will differentiate into lipid-laden foam cells. The formation of the atheratomous plaque also involves the action of the vascular smooth muscle cells (VSMC) of the tunica media, which will migrate toward the tunica intimae. In the case of human arteries, the tunica intimae also contains resident VSMC that will proliferate and, together with the VSMC that have migrated from the tunica media, will produce extracellular matrix proteins, such as interstitial collagen and elastin, which foster the formation of the fibrous layer surrounding the plaque. This layer normally overlays foam cells, which can die, and release lipids that accumulate in the extracellular space. The inefficient elimination of the dead cells, a process known as efferocytosis, can give rise to the accumulation of cellular waste and extracellular lipids, forming the necrotic nucleus.3
The atherosclerotic plaque typically causes clinical manifestations derived from the stenosis it produces in the lumen of the vessel, limiting flow and causing tissue ischaemia, or by causing thrombi that can interrupt the local blood flow. The thrombi often arise following the physical rupture of the fibrous layer that exposes the procoagulant material of the nucleus of the plaque to the coagulation proteins of the blood, which triggers the thrombi. The plaques that are most vulnerable to rupture tend to be thinner, poor in collagen, and with few VSMC but abundant in macrophages. The inflammatory cells can accelerate the rupture, in that they synthesize collagenolytic enzymes that result in the degradation of the collagen. Furthermore, these cells are capable of synthesizing mediators that induce the cell death of VSMC, the largest source of arterial collagen.4 The macrophages of the plaque also produce the procoagulant tissue factor that makes the necrotic nucleus thrombogenic. When the atheratomous plaque fractures and the thrombus is formed, it can cause acute myocardial infarction, stroke, unstable angina, or sudden death.5
In this review, we will discuss the role played by a family of proteins, the annexins, in cholesterol homeostasis, as well as in the pathological vascular remodelling associated with the development and in the progression of the atherosclerotic lesion.
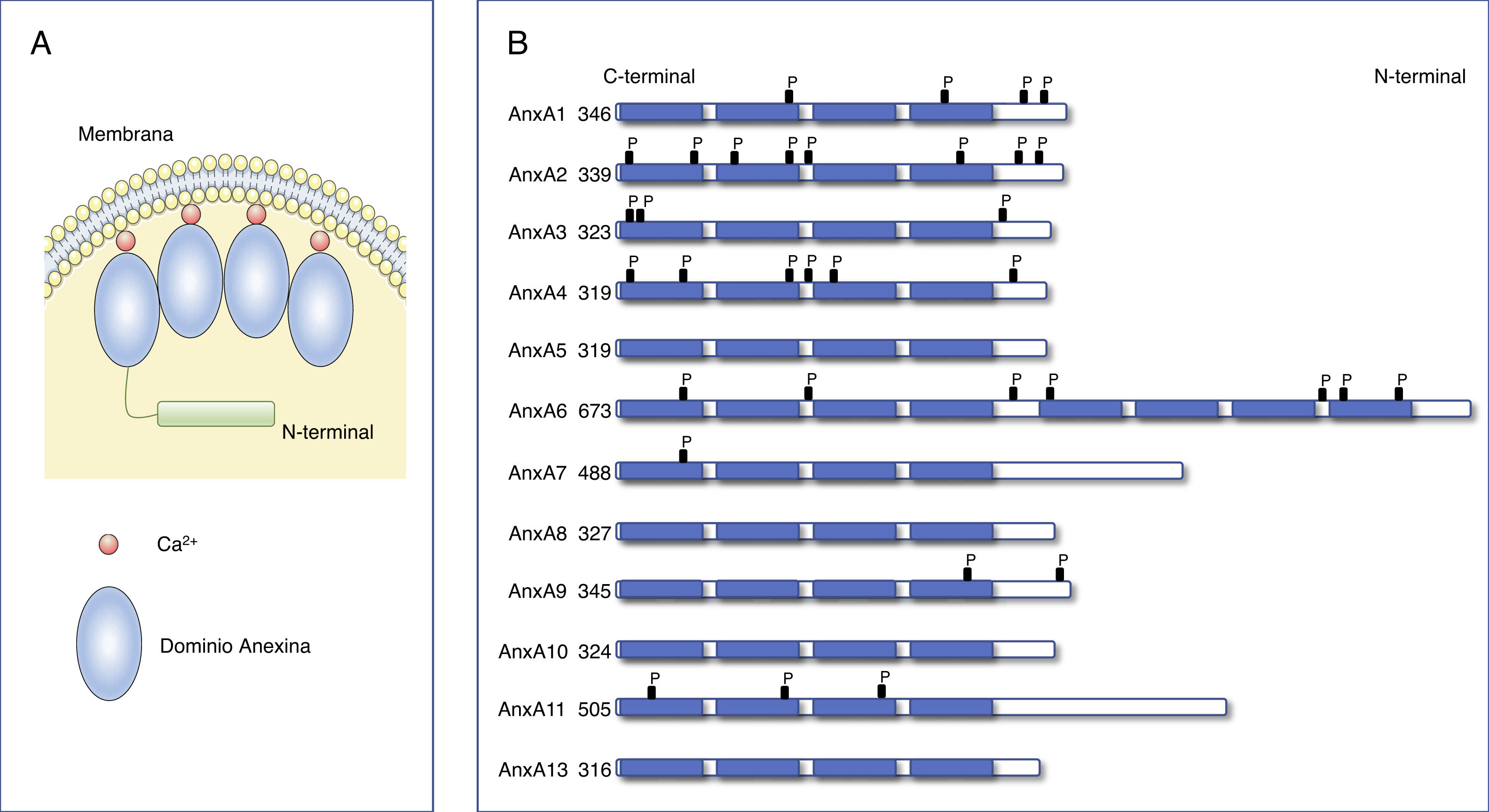
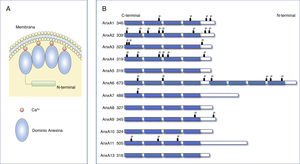
Annexins. Structure and functionThe family of the annexins represents a multigenic family of Ca2+-dependent proteins that bind to phospholipids. Approximately 100 different annexins have been described in several animal species. Of them, the annexins family comprises 12 members in humans and vertebrates denominated AnxA1-AnxA13 (AnxA12 has not been currently assigned).6,7 The crystallographic analysis of these proteins identified that all the annexins have with two structural domains: a fairly well-conserved C-terminal domain with four repetitions of 70-80 amino acids (eight in the case of AnxA6), and a variable N-terminal domain (Fig. 1). Each of the repetitions of the C-terminal domain codes for a Ca2+ binding site, enabling the annexins to translocate quickly to the plasma membrane or to intracellular membranes, binding to negatively charged phospholipids.6,8,9 The N-terminus varies in length and sequence among the different annexins and is in charge of regulating the interaction with different ligands and of the association of the annexins to the membranes.8
Structure of annexins.
A) Diagram of the structure of annexins. Annexins are anchored to membranes through Ca2+ binding. All annexins contain a variable N-terminal end and a conserved C-terminal end composed of 4 (8 for AnxA6) homologous annexin repeat domains that pack into a compact, slightly curved disk. (Modified from Gerke et al.6).
B) Primary structure of human annexins. Annexins consist of 4 annexin repeat domains and a variable N-terminal domain. The number on the left indicates the number of amino acids in each annexin.
Several in vitro studies with purified annexins have been able to analyse their biochemical properties, folding, and their three-dimensional structure, as well as their membrane binding characteristics. In this way, they have also been able to determine their affinities for the membrane of different phospholipids and with other proteins, especially of those belonging to the S100 family of proteins.10 Annexins carry out diverse biological functions. Most are performed in the cell cytoplasm and their function is closely related to their capacity to bind to the membrane. These proteins are responsible for endocytic and exocytic events that are regulated by Ca2+ together with their capacity to facilitate the stabilization of organelle membranes and the plasma membrane.11 One of the main functions annexins have is to act as scaffolding proteins by means of calcium-regulated binding to the phospholipids in the membranes. This enables the cytoplasm and the cytoplasmic side of the cell membrane to interact in an orderly fashion.12 The intracellular mobilization of calcium triggers the recruitment of annexins by the cell membranes. However, some annexins can also bind to the membranes in the absence of calcium, such as annexins A9 and A10.8 Some members of the annexin family can interact specifically with certain actin assembly sites in the cell membranes. For example, the organization of microdomains of VSMC cell membranes is regulated by annexins A2 and A6 through interactions with the cytoskeleton.13
Although most of the actions of the annexins take place in the cell cytoplasm, under certain circumstances, some annexins (AnxA2 and AnxA11) can also interact with the cell nucleus.14 In particular, AnxA11 plays a key role in the final phase of cytokinesis, the process through which the cytoplasm is physically separated into two daughter cells during cell division. Without this process, the cells cannot form the midbody and enter into apoptosis.15 On the other hand, some annexins have demonstrated that they can be present anywhere on the cell surface. Thus, in cells that have been incubated in the presence of glucocorticoids, AnxA1 translocates from the cytoplasm to the cell surface.16 Likewise, as we will see later on, AnxA2 is a co-receptor for plasminogen in different cell strains, including macrophages, and endothelial cells.17 Some annexins also [substances] secrete to the outside of the cell. In this. line, AnxA5 has been proven to have anticoagulant capacity and AnxA1 has anti-inflammatory properties on leukocytes.18 The latter, AnxA1, is found in human serum and its concentration increases primarily in inflammatory processes, such as colitis.19 Different studies have demonstrated that AnxA1 inhibits the transendothelial migration of leukocytes, reducing the inflammatory response.20
The importance and participation of the different annexins in different diseases has been evident since the beginning of the 2000s, when animals have been created that are deficient in different annexins, such as AnxA1, A2, A4, A5, A6, A7, and A8.21 All animals deficient in the different annexins are viable, with the exception of AnxA7 for which two different phenotypes have been reported: one that is lethal because of brain hemorrhage22 and, later, another normal, viable one.23 The fact that most annexin-deficient mice are normal and viable points toward a redundance in the functions of annexins. Nevertheless, upon examination of the different strains of knockout animals and under disease conditions, have revealed different results, identifying specific mechanisms for each one of the annexins. Thus, subtle differences observed in their spatial-temporal location, differences in the kinetics of their binding to negatively-charged phospholipids, the diversity in their interactions through the N-terminal domain, their affinity for other lipids, including phosphatidilinositol-4,5-bisphosphate, cholesterol, and ceramides, post-translational modifications, and, even more relevant, their differential patterns of expression, mean that there are more chances of discovering functional diversity within the family of the annexins.6
Annexins and cholesterol homeostasisOne of the main functions of the different annexins is to control intracellular cholesterol homeostasis. Cholesterol is a sterol that is indispensable for life and that carries out structural and metabolic functions. It is mainly found anchored to the membranes, where it modulates their function.24 Cholesterol is essential for tissue growth, as well as for the synthesis of steroid hormones, biliary acids, and vitamin D.25 Consequently, the regulation of cholesterol homeostasis is crucial for cell function. As is well known, although cholesterol can be synthesized by the cells, most cellular cholesterol is acquired through LDL endocytosis. After internalization, the esterified LDL cholesterol reaches the late endosomes (MVB), where it is hydrolysed to be distributed inside the cell by means of the Niemann-Pick C1 and C2 (NPC1/2) proteins.26,27 Up to 30% of LDL cholesterol moves on to the endoplasmic reticulum (ER) to regulate the control of cholesterol biosynthesis by the cell itself in the form of negative feedback.27,28 From the ER, the cholesterol can be sent to other organelles, such as the plasma membrane or the mitochondria. Alternatively, the excess cholesterol can be esterified by means of acyl-coenzyme A:cholesterol acyltransferase (ACAT) to be stored in lipidic droplets.29 Cholesterol processing takes place in different subcellular locations, being moved among the various subcellular membranes by vesicular transport and by means of vesicular mechanisms.27 Therefore, cholesterol homeostasis must be strictly regulated in order to be maintained within appropriate ranges and so that it can carry out its biological functions properly.
It has recently been reported that annexins can interact directly with cholesterol, regulating its homeostasis.30 For instance, AnxA1 has the capacity to establish contacts between the ER and the multivesicular bodies (MVB) leading to a decrease in the expression of the epidermal factor receptor and facilitating the transfer of cholesterol from the ER to the MVB. This is an unusual route cholesterol transport, inasmuch as the cells primarily obtain cholesterol by means of LDL endocytosis and, has previously commented, the cholesterol derived from LDL is transferred to the ER, downregulating the de novo synthesis of cholesterol.26,27 However, when cholesterol levels are low in the MVB, the route reverses and the cholesterol moves from the ER to the MVB to guarantee the presence of cholesterol for the formation of vesicles inside the cell.31
In the case of AnxA2, an association has been observed of this annexin with cholesterol-rich MVB32 that might be related to the transport of molecules from early endosomes to MVB. When there is a reduced expression of AnxA2, the transport of molecules toward MVB increases, suggesting a role of the AnxA2 that is dependent on cholesterol levels.32
One of the most important annexins in controlling the distribution of cholesterol in the various intracellular compartments appears to be AnxA6.28 The overexpression of AnxA6 has been correlated with an increment of this annexin in MVB that is associated with an increase in cholesterol inside of the endosomes and a decrease in cholesterol levels in the plasma membrane, in the Golgi apparatus, and in the recycling endosomes.26,33 The changes caused by AnxA6 in the cellular distribution of cholesterol interferes with the traffic of cholesterol-dependent SNARE proteins and integrins, compromising critical cell functions, such as cell adhesion and migration.34 SNARE proteins are proteins that are sensitive to the levels of cholesterol present in fusion membranes and transport vesicles. Cholesterol levels inside the endocytic compartments regulate the function and location of these proteins.26,35 Furthermore, cholesterol interacts directly with some SNARE proteins.36,37 As a result, annexins, specifically AnxA2 and AnxA6, in addition to regulating the fusion of vesicular membranes mediated by SNARE proteins and the specific destination of the load of these vesicles, also regulate cholesterol transport between different cell compartments.
The molecular mechanisms by which the binding of AnxA6 to the MVB alters cholesterol transportation are not fully analysed. While NPC1/2 proteins are key to cholesterol exiting the MVB, other proteins can be involved in the passage of cholesterol from MVB to ER. In the MVB, membrane proteins, such as NCP1, ORPL1, StARD3, and StARD3NL appear to be fundamental for the movement of cholesterol to the RE.38 Moreover, the activation of Rab7, a small G protein, promotes the mobility and repositioning of the MVB to properly transfer cholesterol.39 In this mobility, AnxA6 binding to the membranes must also be taken into account, as it activates the reordering of the cholesterol-rich microdomains, as occurs in the plasma membrane.40 These structural changes induced by AnxA6 can also take place in the MVB, modulating the distribution of cholesterol and, consequently, other membrane lipids.41 These domains that are both highly-ordered and rich in cholesterol affect the active transport of cholesterol toward the ER.
Finally, in addition to AnxA6, AnxA8 also controls cholesterol homeostasis in association with the MVB.42 Contrary to the role played by the increase in AnxA6 expression in the accumulation of cholesterol in the MVB, decreased expression of AnxA8 blocks the cholesterol from leaving the MVB, indicating a balance in the expression of these two annexins in the control of the cholesterol load inside the ET.42 Therefore, it appears that different annexins play an important role in the transport of cholesterol along the endocytic pathway and changes in their expression can give rise to changes in the amount of intracellular cholesterol. In addition, the increase in circulating cholesterol concentrations —and, as a result, the greater entrance and accumulation of cholesterol inside the cell— might be regulated, at least in part, by means of the regulation of the expression of different annexins.
Annexins and vascular remodellingIn recent years, the role of different annexins has been revealed in the inflammatory response and in the underlying vascular remodelling that takes place during the development and progression of the atherosclerotic lesion. The most salient of these are annexins A1, A2, A3, A5, and A7. The role that other annexins may play has yet to be dilucidated.
Annexin A1AnxA1, also known as lipocortin 1, was first reported when the factors that mediate the anti-inflammatory actions of glucocorticoids were being identified.43,44 It is expressed in most cell and tissue types, although it is especially abundant in macrophages, neutrophils, and the nervous and endocrine systems.6,21,45 Just like other annexins, AnxA1 is found in different cell locations, including the plasma membrane, endosomal apparatus, secretory vesicles, cytoskeleton, and nucleus. In these locations, AnxA1 participates in cell processes, such as membrane transport (endo/exocytosis), signal transduction, actin dynamics, and the regulation of metabolic enzymes related to proliferation, differentiation, migration, and apoptosis.6,8,9,45–47 AnxA1 also plays an important extracellular function, acting as an anti-inflammatory molecule by means of its regulation by glucocorticoids and by means of its binding to the membrane receptor coupled to G proteins, known as receptor 2 of the N-formyl peptide (formyl peptide receptor 2 [FPR2]).48
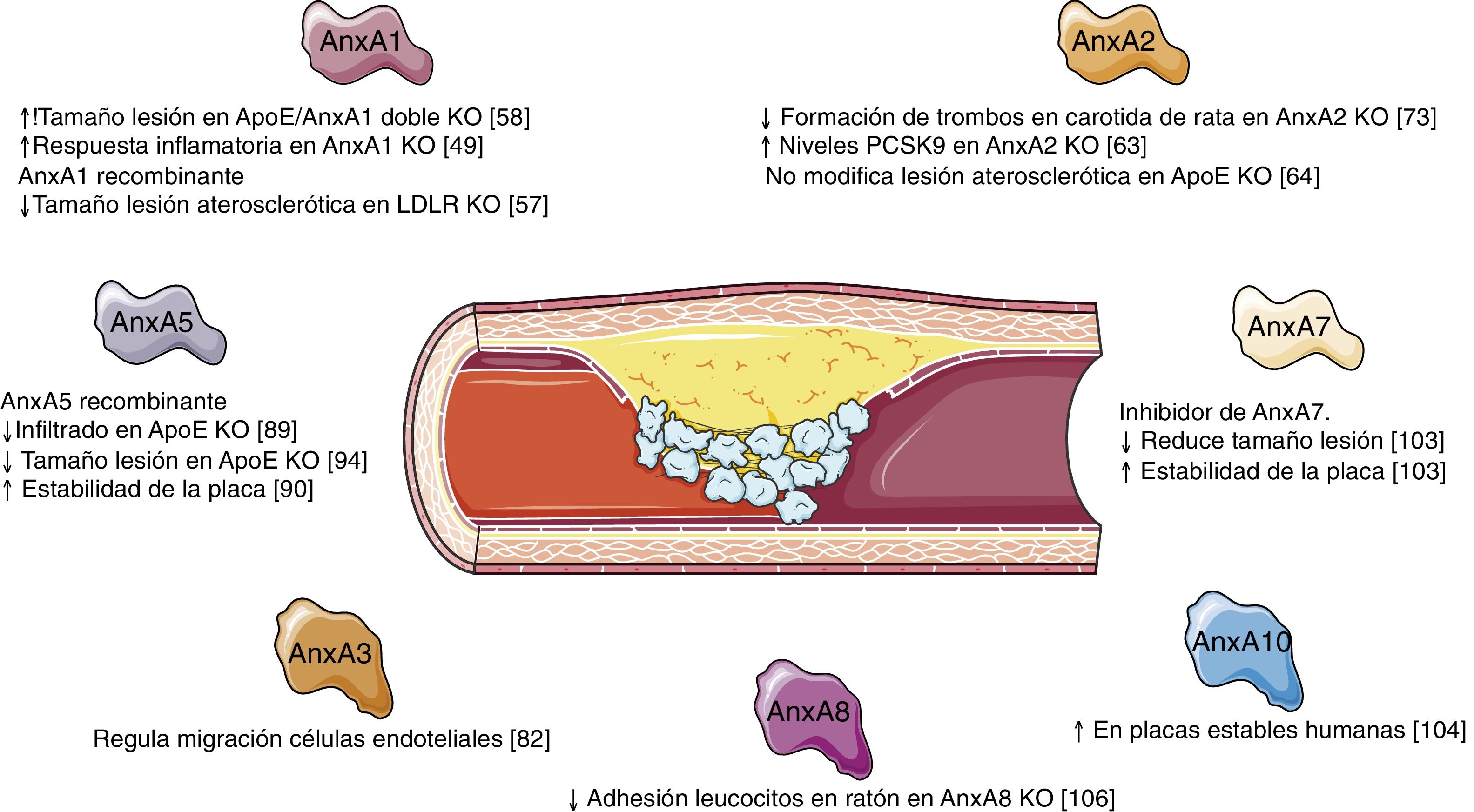
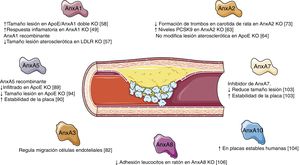
The development of the AnxA1 knockout mouse revealed the anti-inflammatory role of this protein and how its absence caused an exacerbated and prolonged inflammatory response with an increase in leukocyte migration and resistance to the anti-inflammatory effect of glucocorticoids.49 In fact, an increase in the levels of proinflammatory proteins, such as cyclooxygenase 2 or phospholipase A2 has been seen in these mice.49 In addition, mice deficient in its FPR2 receptor revealed that AnxA1 exerts its function by activating the innate immune response.50 The molecular mechanisms that underly the activation of FPR2 by AnxA1 have been linked to the activation of mitogen-activated kinases (MAPK), such as Erk1/2, p38MAPK, Akt, or c-Jun.51,52 The activation of these signalling cascades translates into a decrease in the adhesion of neutrophils to the vascular endothelium,49 increased apoptosis of neutrophils por though intrinsic apoptotic pathways53 or by decreased survival signals induced by other inflammatory mediators,54 and the induction of monocyte recruitment, as well as their subsequent polarization toward an anti-inflammatory phenotype.55 All of these functions that have been described for AnxA1 are fundamental in the development of the atherosclerotic lesion. Hence, different studies have revealed its therapeutic potential and its capacity to limit the formation of the atherosclerotic lesion and the cardiovascular complications derived from it.56 In this regard, in mice deficient in LDL receptor (LDLR) who were fed a fatty diet, the intraperitoneal administration of human recombinant AnxA1 was seen to significantly reduce the adhesion and the rolling of FPR2-dependent neutrophils on endothelial cells, thereby attenuating the progression of the atherosclerotic plaque.57 Likewise, in animals deficient in AnxA1 or FPR2 in ApoE―/― resources an increase has been observed of atherosclerotic lesions associated with an increase in the adhesion of myeloid cells in the damaged arterial walls, as well as an increase in the proliferation of macrophages within the lesion.56,58
Therapeutically, the in vivo administration of the Ac2-26 peptide (derived from the N-terminal domain of the AnxA1 protein) reduced the recruitment of FPR2-dependent myeloid cells, thereby decreasing the size of the lesion and the accumulation of macrophages within the atherosclerotic lesions.51 In this regard, the AnxA1/FPR2 axis inhibits the expression of the integrin-dependent chemokines that are necessary for leukocyte recruitment.51 Furthermore, in an advanced model of atherosclerosis in ApoE knockout mice treated with nanoparticles specifically targeting collagen IV (Col IV) that contained Ac2-26 (Col IV-Ac2-26 NP), increased thickness of the fibrous capsule, decreased collagenase production, and, in consequence, greater stability of the atherosclerotic lesions was observed.59 These data indicate that the administration of AnxA1-derived peptides might provide alternative and efficacious drug forms for the treatment of chronic inflammatory diseases, countering the continuous recruitment of leukocytes and macrophage activity during atherosclerotic progression.
The importance of the regulation of AnxA1 expression is dictated by the different studies carried out in human samples. Thus, AnxA1 is expressed in atherosclerotic plaques of human coronary arteries, collocating with macrophages and endothelial cells, and in the plaques of patients with carotid stenosis undergoing carotid endarterectomy.60–62 The expression of AnxA1 was greater in the carotid plaques in asymptomatic patients compared to patients with neurological symptomatology, which indicates a protective role of AnxA1 in atherosclerosis.60 Moreover, AnxA1 is mainly expressed in areas with a high content of apoptotic cells, which speaks to its importance in eliminating this kind of cell.61
Annexin A2AnxA2 is expressed in a wide variety of cell types and varies depending on the type of tissue analysed. Thus, it is highly expressed in the lung and kidney, and, in contrast, its expression is lower in the liver.63 AnxA2 is predominantly found in the plasma membrane, the endosomal apparatus, and in secretory vesicles, and only in the odd case, is it found in the cell nucleus.64,65 Just like other annexins, AnxA2 regulates the traffic and organization of membranes by means of endo/exocytosis processes, the formation of microdomains, or repair of the plasma membrane. Similarly, its interaction with the actin filaments of the cellular cytoskeleton has involved AnxA2 in different cell functions, such as cell growth, differentiation, apoptosis, and migration.6,9,46,47,65
Most AnxA2 is found creating heterotetramers with the p11 protein (S100A10), a member of the S100 family of proteins. The interaction of AnxA2/p11 acts as a plasminogen receptor and plasminogen tissue activator (tPA) in endothelial cells, both molecules in charge of promoting vascular fibrinolysis.64,66,67 In addition, plasmin/plasminogen signalling in human monocytes has been shown to use the AnxA2 heterotetramer as a receptor and triggers signalling by means of JAK/STAT, activation Akt-dependent NF-κB, as well as ERK1/2 and p38 MAPK, which leads to the induction of proinflammatory genes and to the recruitment of inflammatory cells in the context of atherosclerosis.68
The development of the AnxA2 knockout mouse demonstrated that these animals are viable and fertile, but that they exhibit significant deposition of fibrin in some tissues.69 In a model of acute thrombi in the carotid artery in animals deficient in AnxA2 or in p11, these animals were seen to display an increase in the thrombi, which proved that the binding of AnxA2/p11 is involved in vascular fibrinolysis.70
The role played by AnxA2/p11 and its consequences in vascular homeostasis has been highlighted in different experimental models. On the one hand, the induction of hyperhomocysteinemia by means of administering a diet has been seen to provoke an accumulation of fibrin and alterations in neoangiogenesis in AnxA2 knockout mice.71 In addition, in ischaemic cerebral disease, the administration of recombinant AnxA2 should serves as an adjuvant to amplify tPA-mediated thrombolysis and to prevent stroke.72 However, the deficit of AnxA2 had no effect on the size of the atherosclerotic lesion in ApoE knockout mice, despite the fact that the therapeutic inhibition of plasminogen generation had been proven to be beneficial for atherosclerosis.64 In this regard, the administration of recombinant AnxA2 decreased the formation of thrombi in a rat model of thrombi in the carotid artery.73 These results suggest that the improvement in fibrinolytic activity induced by AnxA2 might modulate the hypercoagulability state in atherosclerosis.
As previously commented on, one of the main functions of AnxA2 is in relation to thrombi. Nevertheless, this protein has been proven to participate in other cell responses. Thus, in ApoE knockout given a cholesterol-rich diet, AnxA2 expression was seen to be significantly increased in the macrophages present in the atherosclerotic lesions.74 This resulted in an increase in the migration of macrophages by means of AnxA2-dependent Akt/NF-κB and ERK signalling.74 Likewise, the interaction of AnxA2 with integrin α5 through the tyrosine phosphatase 1B protein participates in the inflammatory response associated with the development of atherosclerosis in ApoE-deficient mice.75 Additionally, AnxA2 has been implicated in VSMC migration processes. In this sense, in vitro rat VSMC migration trials have proven that the overexpression or inhibition of AnxA2 increases or decreases, respectively, the migration of these cells in the presence of cell growth factors.76 Accordingly, AnxA2 expression is increased during the formation of the neointima in the carotid following balloon injury.76
Another major characteristic of AnxA2 is its capacity to interact with the proprotein convertase subtilisin kexin type NF-κB9 (PCSK9), a potent inductor of hepatic LDL receptor degradation.63,77 AnxA2 or AnxA2/p11 inhibits the decrease of the PCSK9-mediated LDL receptor in HuH7 or HepG277 cells. In addition, AnxA2 knockout mice have elevated LDL and PCSK963 levels. As a result, the modulation of AnxA2 levels could be considered an endogenous PCSK9 inhibitor, a relevant fact inasmuch as the treatment of hypercholesterolemia has benefitted from the use of monoclonal antibodies against PCSK9. Recent years has also witnessed the identification of variants of the AnxA2 gene thanks to the analysis of polymorphisms of a single nucleotide or SNP that directly impacts the circulating levels of LDL, pointing once again, to AnxA2 as a potential therapeutic target to lower LDL concentrations.78
Likewise, AnxA2 is also related to glucose and fatty acid metabolism. In this regard, AnxA2 has been proven to be responsible for the translocation of insulin-inducible GLUT4, the primary glucose transporter from the intracellular compartments to the cell surface in adipocytes.79 Furthermore, in endothelial cells and adipocytes in white adipose tissue, AnxA2 is essential for the capture of fatty acids via its binding to the fatty acid transporter CD36.79 In in vivo studies, AnxA2 knockout animals exhibited a frank delay in the clearance of fatty acids, which illustrates that the lack of AnxA2 compromised CD36-mediated elimination of fatty acids from the bloodstream.80 However, this same study demonstrated that AnxA2 knockout mice displayed stable levels of glucose, as well as normal tolerance, indicating that AnxA2 is not involved in the translocation of GLUT4 in vivo.80
Annexin A3Like other members of the family, AnxA3 is expressed in a wide variety of tissues, such as heart, lung, kidney, brain, liver, and, to a larger extent, in adipose tissue.81 This protein is connected with various cell functions, such as cell differentiation and migration, immune regulation, and bone formation6. Experimentally, AnxA3 has been shown to be linked to endothelial cell migration and in tube formation.82 What’s more, VEGF has been shown to increase AnxA3 expression in HUVEC cells, increasing their migration trials of wound repair and angiogenesis.83
While the AnxA3-deficient mouse is viable, the lack of animal models using mice deficient for this protein has thus far made it impossible to analyse the role it plays in the development of the atherosclerotic lesion. However, the inhibition in vivo of its expression using a sh-RNA (short hairpin RNA) in a model of myocardial infarction in rats led to a decrease in the inflammatory response, the size of the infarct, the expression of collagen I and II, promoting myocardial tissue repair.84 Moreover, by conditionally inhibiting vascular endothelium in mice (AnxA3f/f;Tie2-Cre), the loss of AnxA3 has been shown to cause no defects in the development of vasculature, albeit it is necessary for the parallel alignment of arteries and veins, indispensable for blood flow and proper functioning of the vessels.85
Annexin A5AnxA5 is the most abundant and most widely studied member of the family of annexins. It is expressed in most cells and tissues with the exception of neurons.6,86 This annexin has been associated with the traffic of membranes, the regulation of Ca2+ ion channels and entrance, and, even more importantly, with the regulation of the cell cycle and apoptosis. That is why AnxA5 has, for a long time, become a diagnostic tool to detect cell death, in that it binds to phosphatidylserine in the external part of the plasma membrane in apoptotic cells. In addition, AnxA5 has other extracellular functions that have to do with coagulation, phagocytosis, viral infection, or the processes of cellular membrane repair.86–88
Insofar as its relation to vasculature is concerned, AnxA5 has antithrombotic, antiapoptotic, and anti-inflammatory properties by binding to the phosphatidylserine expressed on the cell surface.89,90 Moreover, it is associated to antiphospholipid syndrome during pregnancy.18,91 Studies in AnxA5 knockout mice show that pregnant females are more prone to embryo loss and, therefore, to smaller litters.92 The administration of anticoagulants, such as heparin, prevents the formation of thrombi and, consequently, the loss of embryos during gestation in these mice.
Likewise, there is evidence as to the role AnxA5 plays during the processes of vascular remodelling. Treatment with recombinant AnxA5 decreased adhesion and the infiltration of leukocytes into atherosclerotic plaques present in ApoE knockout mice.89 In addition, in a model of femoral damage in ApoE―/― mice, treatment with AnxA5 was seen to bring about a dose-dependent decrease the early adhesion of leukocytes and macrophages, as well a long-term decrease in the development of atherosclerosis.93 Later studies focusing on the effects of administering exogenous AnxA5 revealed that treatment with this protein reduces inflammation in advanced atherosclerosis, as well as contributing to the stabilization of atherosclerotic plaques in ApoE-deficient mice, without modifying the size of the lesions, collagen content, or VSMC90. However, pre-treatment with AnxA5 in a model of early atherosclerosis in ApoE―/― was capable of reducing the formation and size of the plaques, decreasing the rate of apoptosis, and regulating the infiltration and activation of macrophages.94 These results could point toward AnxA5 playing a relevant role in the initial stages of development of the atherosclerotic lesion, being less relevant its role in advanced stages. In vitro studies endorse the idea of the role of AnxA5 on inflammatory cell recruitment. Thus, AnxA5 displayed anti-inflammatory effects in macrophages, significantly decreasing the rolling, adhesion, and transmigration of mononuclear cells on endothelial cells activated by TNF-α.90 Similarly, AnxA5 inhibits the proatherogenic and proinflammatory effects of phosphatidylcholine (PTC) in macrophages in vitro, disrupting the binding and uptake of oxidated LDL, as well as in the very proinflammatory effects of LPC.95
AnxA5 has also been proposed as a biomarker of atherosclerosis in different studies. It has been reported as a diagnostic biomarker of subclinical atherosclerosis in patients with systemic lupus eritematous.96 Circulating levels of AnxA5 have also been inversely linked to the severity of coronary stenosis and the oxLDL/AnxA5 ratio has even been seen to be a better marker of the severity coronary stenosis than the concentration of LDL in and of itself.97 In this same regard, several SNPs in the AnxA5 gene are associated with the risk of suffering re-stenosis in patients who undergo coronary angioplasty.93 Finally, AnxA5 has been used as a biomarker in imaging studies to visualize inflammation and cellular stress.98
Annexin A7Within the family of annexins, AnxA7 is the only one that que exhibits two isoforms: a 47-KDa isoform that is basically expressed in all tissues except for skeletal muscle and another, larger 51-KDa isoform that is found in cardiac, brain, and transverse tubule tissues.99 Its functions are related to Ca2+ homeostasis; it has GTPase activity; it intervenes in the production of prostaglandins in processes of cardiac remodelling and in inflammatory myopathies.100 Two different phenotypes have been described for AnxA7 knockout mice. First, a phenotype was described that is lethal due to cerebral hemorrhage,22 and it was later shown that the AnxA7-deficient mouse was normal and viable.23 The isolation and subsequent analysis of their adult cardiomyocytes identified that they exhibited diminished frequencies in cell contraction, which indicated that Ca2+ homeostasis or the functioning of the machinery in charge of contraction was altered. AnxA7 interacts with two proteins (sorcin and RyR), responsible for coupling Ca2+ channels to the contractile machinery of the cardiac muscle.23,101 Likewise, AnxA7 knockout mice have been shown to exhibit high susceptibility to atrial fibrillation, ventricular tachycardia, arrhythmia, and impairment of cardiac remodelling.100 In a model of transverse aortic constriction in these mice, a pronounced increase in heart size associated with increased expression of several hypertrophy genes was observed, possibly induced by the Ca2+-regulated cardiac nuclear factor of activated T cells (NFAT) in an AnxA7-dependent fashion. Furthermore, AnxA7 is an endogenous regulator of the Homeobox 1 gene (HMBOX1), critical for endothelial cell survival. Thus, decreased AnxA7 levels, due to treatment with its 6-amino-2,3-dihydro-3-hydroxymethyl-1,4-benzoxazine inhibitor raised HMBOX1102 concentrations. As for vascular remodelling associated with atherosclerotic lesion formation, treating ApoE-deficient mice with an AnxA7 inhibitor has been shown to reduce the area of atherosclerotic plaques, lipid deposition, and proinflammatory macrophages, in addition to increasing anti-inflammatory macrophages, collagen content, and SMCs in atherosclerotic lesions, thereby increasing plaque stability.103
Other annexinsThe role of other annexins in pathological vascular remodelling is currently known only slightly or remains totally unknown. In this sense, only AnxA10 has been correlated with atherosclerotic lesion formation, its expression being associated with the presence of more stable plaques,104 possibly related to the anticoagulant, anti-inflammatory, or antiapoptotic activity seen for other annexins. Moreover, AnxA8 regulates protein transport by means of the late endosome in endothelial cells that aids in the control of CD63 exposure to the exterior of the plasma membrane for proper leukocyte recruitment.105,106 In this regard, mice deficient in AnxA8 exhibit less leukocyte adhesion and rolling.106
Finally, AnxA4 has 3 different transcripts (AnxA4a, AnxA4b and AnxA4c) that presented unique expression patterns.107 Although its relationship with atherosclerotic lesion has not been demonstrated, its main function is associated with -adrenoreceptor (-AR)/cAMP signalling in cardiomyocytes.108 Given that cardiomyocytes from adult AnxA4-deficient mice showed increased cAMP levels, it was reported that it was, in all likelihood, due to the loss of the inhibitory action of AnxA4 on adenylyl cyclase5, which controls the conversion of ATP to cAMP. Furthermore, AnxA4-/- mice treated with -AR agonists were seen to exhibit elevated cAMP levels, associated with an improvement in cardiac contractile force.108
ConclusionAnnexins are a family of proteins that apparently play a noteworthy role in cholesterol homeostasis and in the vascular remodelling that underlies the formation and development of atherosclerotic lesions (Fig. 2). While progress has been made in recent years in what we know about the functions of some of the members of this family, we have yet to determine what role others may play; it therefore appears that we must broaden our knowledge about the actions of the different annexins and their possible overexpression/inhibition as a therapeutic option in the treatment of vascular remodelling.
FundingThis work was supported by a 2018 FEA/SEA basic research grant from the Spanish Society of Arteriosclerosis, Instituto de Salud Carlos III (ISCIII-FEDER)PI16/01419 and PI19/00128. N. M-B was awarded a Miguel Servet contract from the ISCIII (CP19/00151).
Conflict of interestsThe authors have no conflict of interests to declare.
The authors apologize to all those researchers whose work could not be cited due to space limitations.
Please cite this article as: Méndez-Barbero N, Gutiérrez-Muñoz C, Blázquez-Serra R, Martín-Ventura JL, Blanco-Colio LM. Anexinas: implicación en la homeostasis del colesterol, la respuesta inflamatoria y la aterosclerosis. Clin Investig Arterioscler. 2021;33:206–216.