Cardiovascular disease is the primary cause of death in obese and diabetic patients. In these groups of patients, the alterations of epicardial adipose tissue (EAT) contribute to both vascular and myocardial dysfunction. Therefore, it is of clinical interest to determine the mechanisms by which EAT influences cardiovascular disease. Two key factors contribute to the tight intercommunication among EAT, coronary arteries and myocardium. One is the close anatomical proximity between these tissues. The other is the capacity of EAT to secrete cytokines and other molecules with paracrine and vasocrine effects on the cardiovascular system. Epidemiological studies have demonstrated that EAT thickness is associated with not only metabolic syndrome but also atherosclerosis and heart failure. The evaluation of EAT using imaging modalities, although effective, presents several disadvantages including radiation exposure, limited availability and elevated costs. Therefore, there is a clinical interest in EAT as a source of new biomarkers of cardiovascular and endocrine alterations. In this review, we revise the mechanisms involved in the protective and pathological role of EAT and present the molecules released by EAT with greater potential to become biomarkers of cardiometabolic alterations.
Las enfermedades cardiovasculares son la primera causa de muerte en pacientes obesos y diabéticos. Las alteraciones del tejido adiposo epicárdico (TAE) contribuyen a la disfunción vascular y del miocardio en estos pacientes. Es por tanto de interés clínico determinar los mecanismos por los cuales el TAE influye en la enfermedad cardiovascular. Aquí resumimos los mecanismos que subyacen a la asociación entre TAE, síndrome metabólico y enfermedades cardiovasculares. Dos factores contribuyen a la estrecha intercomunicación entre el TAE, las arterias coronarias y el miocardio. Uno es la estrecha proximidad anatómica entre estos tejidos. El otro es la capacidad del TAE para secretar citocinas con efectos paracrinos y vasocrinos en el sistema cardiovascular. Estudios epidemiológicos han demostrado que el grosor del TAE está asociado no solo con el síndrome metabólico sino también con la aterosclerosis y la insuficiencia cardíaca. La evaluación del TAE utilizando técnicas de imagen, aunque eficaz presenta desventajas tales como la exposición a la radiación, la disponibilidad limitada y los costes elevados. Por lo tanto, existe un interés clínico en el TAE como fuente de nuevos biomarcadores de alteraciones cardiovasculares y endocrinas. En este artículo, revisamos los mecanismos implicados en el papel protector y patológico del TAE y presentamos las moléculas liberadas por el TAE con mayor potencial para convertirse en biomarcadores de alteraciones cardiometabólicas.
The inability of subcutaneous adipose tissue (SAT) to store the excess of fatty acids results in ectopic fat accumulation in tissues, such as the liver, skeletal muscle, heart, and pancreatic beta cells. Ectopic fat extension is currently considered clinically useful to identify individuals at risk for cardiovascular disease.1 In fact, cardiovascular risk is linked not only to ectopic fat quantity but also and more importantly to ectopic fat location.2 During the last decade, ectopic fat mass and content have typically been quantified by high precision, non-invasive imaging techniques.3 The specific detection of visceral thoracic fat depots fully enclosed by the pericardial sac in the heart, termed epicardial fat (EAT), is of remarkable clinical interest. EAT covers 80% of the heart's surface4 and constitutes 20% of the total cardiac weight.5,6 EAT has thermoregulatory, metabolic and cardioprotective effects under normal physiological conditions.7 However, in metabolic syndrome and obesity, EAT secretes an altered pattern of adipokines and other modulatory molecules.8 A great number of these EAT-secreted molecules exert endocrine, paracrine, and vasocrine effects on the vasculature and heart9,10 due to the anatomic proximity of EAT to coronary arteries and the myocardium. Considering that cardiovascular diseases are the primary causes of death in obese and diabetic patients11–13 and that functional alterations of EAT contribute to both vascular and myocardial dysfunction in these groups of patients,14,15 it is of great clinical interest to understand the mechanisms linking EAT and cardiovascular diseases. In this review, we summarize and present data on the mechanisms underlying the associations between EAT, metabolic syndrome and cardiovascular diseases and the potential secreted molecules reflecting the relevant mechanisms connecting adipose and cardiovascular tissues.
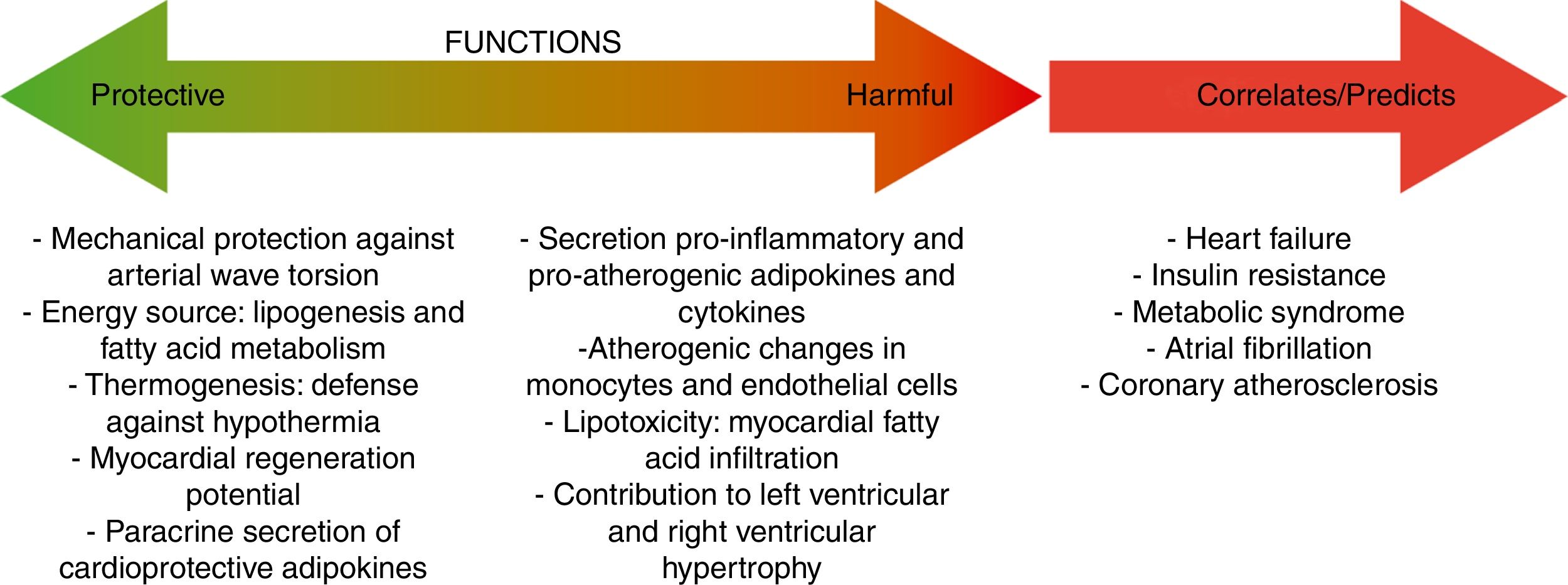
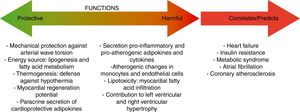
Mechanisms involved in the protective role of epicardial fatA crucial advantage of EAT in front of other fat depots is that, under normal physiological conditions, EAT and pericardial fat have great flexibility to storage or release fatty acids due to their high rates of lipogenesis and lipolysis, serving as a storage depot for fatty acids and protecting the heart and the vasculature against high fatty acid oversupply.16 The heart has a constant demand of fatty acids as energy substrate and, in situations of high energy demand, EAT acts as a local source of fatty acids for the heart, promoting an adequate cardiac function.17 Other interesting characteristic of EAT is that this fat depot express the uncoupling protein-1 (UCP-1), a marker protein for brown fat. These results suggest that EAT could play a significant role in thermogenesis under certain circumstances.18 UCP-1 expression in EAT seems to be higher than in other fat depots, suggesting the presence of brown adipocytes specifically in EAT.19 Other positive EAT characteristic is that contains abundant progenitor cells and therefore, could be a source of myofibroblasts that produce extracellular matrix.20 In this aspect, Bayes-Genis's group supported the use of pericardial-derived fat flaps to cover post-infarction scars and reduce infarct size.21,22 This positive effect seems to be partly due to neovascular connections and cell trafficking at the flap-myocardium interface.
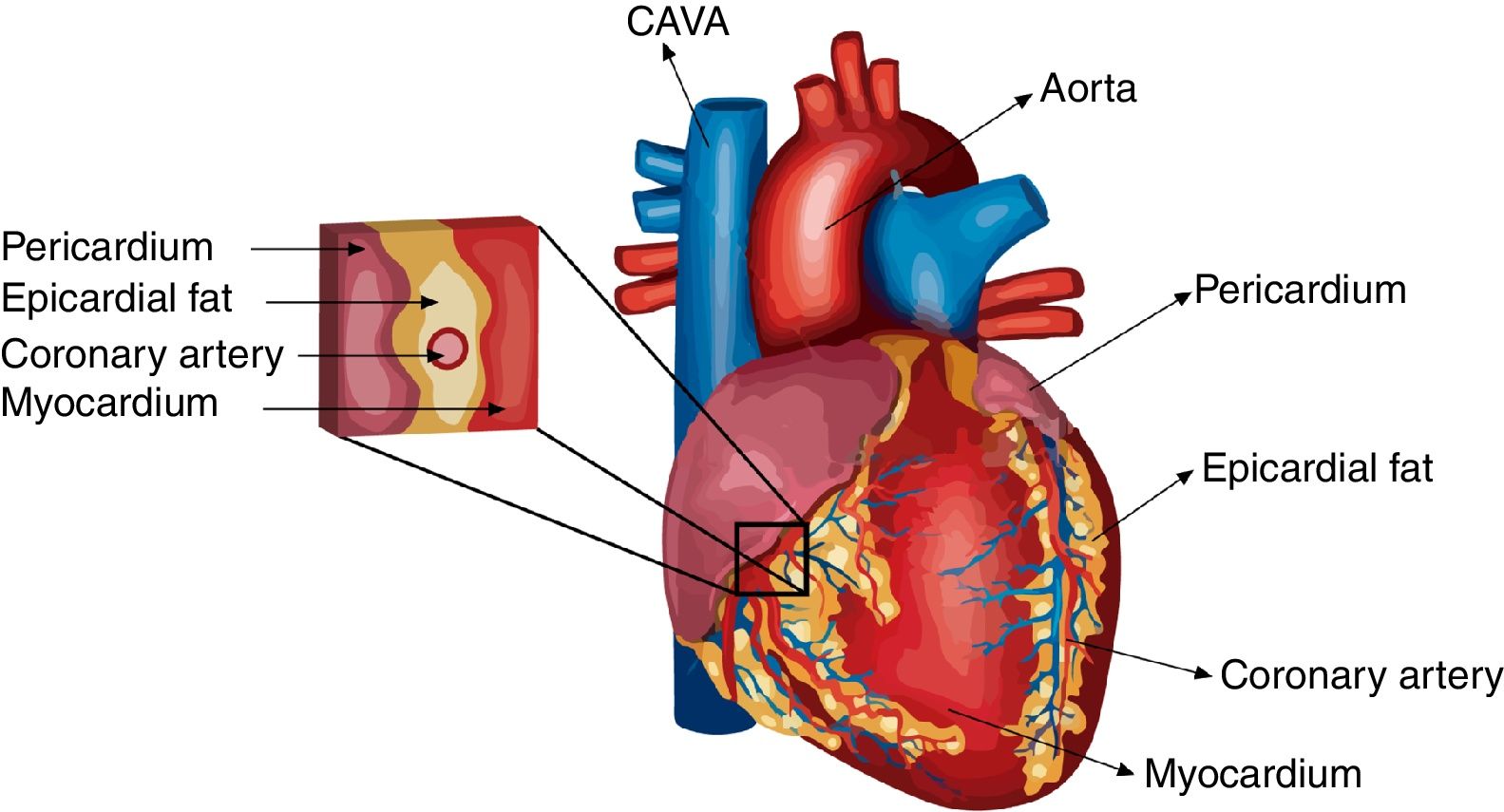
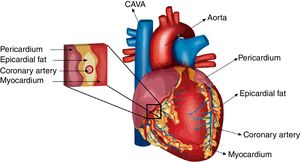
Differences in eat extension and weight between normal and pathological conditionsUnder normal circumstances, the biggest mass of EAT is localized on the lateral and anterior walls of the right atrium, the apex, the atrioventricular and the interventricular sulci, the entire surface of the right ventricle and the greater coronary vessels from their origin.23 Under pathological conditions, EAT enlarges and it usually also accumulates on the left atrium surface and along the vessel's adventitia with spreading into the myocardium (Fig. 1). It is important to remark that factors such as age, sex, body weight and ethnic origin are key factors to take into account previous evaluation of the prognostic value of EAT determination.24,25 It has been reported that EAT extension correlates with age and it is usually larger in men than in women. Threshold values for high risk echocardiographic EAT extension are those over 9.5 and 7.5mm in men and women, respectively. Therefore, echocardiographic EAT measurements could help for cardiometabolic risk stratification in obese and T2DM subjects.17
Epicardial fat, endocrine and metabolic diseasesSeveral clinical studies have demonstrated associations between EAT extension, insulin resistance syndrome (i.e., metabolic syndrome), type 2 diabetes (T2D) and non-alcoholic fatty-liver disease (NFLAD).26–28 The association between EAT thickness and metabolic syndrome has been documented in a meta-analysis29 and, currently, EAT is considered a novel therapeutic target in the management of patients with metabolic syndrome. In moderate and severe obese patients, weight loss induced by low-calorie diet and exercise directly impact EAT extension. Importantly, EAT shrink was significantly associated with cardio-protection.30,31 Several drugs, including statins and pioglitazone, as well as incretin-based drugs, have been proposed as potential EAT modulators.32,33 Statins, also known as HMG-CoA reductase inhibitors, are efficient for treating patients with hypercholesterolemia, reducing cardiovascular risk.34 In particular, atorvastatin significantly reduces EAT extension compared to combined simvastatin/ezetimibe treatment.35 Like simvastatin, pioglitazone and pioglitazone with simvastatin therapy reduces the increased inflammatory EAT markers present in metabolic syndrome and T2DM patients. Interestingly, the reduction in EAT inflammation was associated with reduced coronary atherosclerosis progression.36,37 Modulators of incretin axis such as exenatide, sitagliptin (GLP-1 agonists) or liraglutide significantly decrease EAT volume in T2DM even in short-term treatment.33,38–40
Epicardial fat and coronary artery diseaseEAT shares many of the pathophysiological properties of other visceral fat deposits,41 including developmental origin.16 However, EAT shows unique characteristics, in particular a unique transcriptome involved in inflammation and endothelial function.42 This EAT-specific transcriptome explains why EAT has the capacity to secrete and release pro-inflammatory cytokines that potentially contribute to coronary atherosclerosis development.9,10,43 Moreover, epicardial fat, beyond the contribution of visceral fat, may exert important roles in the pathogenesis of coronary atherosclerosis due to close anatomic relationships with vascular structures. EAT surrounds the coronary arteries and, therefore, establishes an outside-to-inside inflammatory signal.9 In particular, the direct physical contact and communication of EAT with the adventitia of the coronary arteries, without the interposition of a fascial layer, facilitates modulatory effects of EAT on angiogenesis.44,45 Angiogenesis is a crucial determinant of future clinical coronary events.46,47
During the pathological evolution of EAT, it becomes hypoxic and dysfunctional, and macrophages and T lymphocytes invade EAT, producing a shift in its metabolic profile.48 This creates an inflammatory environment propitious for atherosclerosis development.43 A typical proinflammatory characteristic is the reduced secretion of anti-atherosclerotic adipokines, such as adiponectin.16 In line with these findings, EAT has been proposed as an independent predictor of coronary atherosclerosis.1 Two epidemiological studies, Framingham and MESA (Multiethnic Study of Atherosclerosis), have demonstrated an association between EAT and coronary artery calcification. This association remained significant even after adjusting for traditional cardiovascular risk factors.49,50 Furthermore, EAT volume has been reported to be similarly elevated in patients with exclusively non-calcified plaques than in those with mixed and calcified plaques.51 These authors proposed that EAT extension may precede plaque calcification and the development of mature atherosclerotic plaques. Thus, EAT volume quantification may be used in addition to calcium scoring to identify patients with coronary artery disease (CAD) even in the absence of coronary calcium. In the same direction, EAT volume seems to be comparable among patients with stenotic and non-stenotic plaques, suggesting that EAT accumulation is associated with early stages of coronary atherogenesis.52 EAT has also been independently associated with the thrombolysis and myocardial infarction (TIMI) risk score in patients with non-ST-elevation myocardial infarction (NSTEMI) and unstable angina pectoris.53
Cross-talk interactions between epicardial fat and myocardial tissueAs is the case on coronary arteries, the impact of EAT on cardiac function relies on the lack of an anatomical boundary between EAT and the myocardium. EAT and the heart share an unobstructed microcirculation that facilitates interaction through EAT-released molecules that have vasocrine and paracrine effects on the myocardium.10,42 Additionally, EAT exerts cardioprotective effects by acting as a depot for intravascular free fatty acids (FFAs) (Fig. 2).
When the ability of EAT to buffer fatty acids is exceeded, EAT overextension is associated with increased arrhythmogenic right ventricular cardiomyopathy, atrial fibrillation,20,54 and heart failure.10,55 A crucial mediator of these effects is activin A, a member of the transforming growth factor beta (TGF-β) family. In the hallmark of heart failure or diabetes, activin is actively produced by EAT having marked fibrotic effects on atrial myocardium.56,57 Other key factor causing myocardial dysfunction is the infiltration of adipocytes into the atrial myocardium. This process contributes to disorganization of the depolarization wave front and the blockage of local conduction.20 To remark that it has been recently reported that the atrial epicardium is a source of adipocytes that can contribute to the accumulation of EAT in adult atria and that atrial natriuretic peptide (ANP) secreted by the myocardium is a trigger of this process.58 EAT accumulation in adult atria is a slow process that could occur in response to chronic alterations of atrial myocardium workload and metabolic conditions. These results support that EAT and myocardium may influence each other and that this influence depends, at least in part, on the cardiometabolic status.
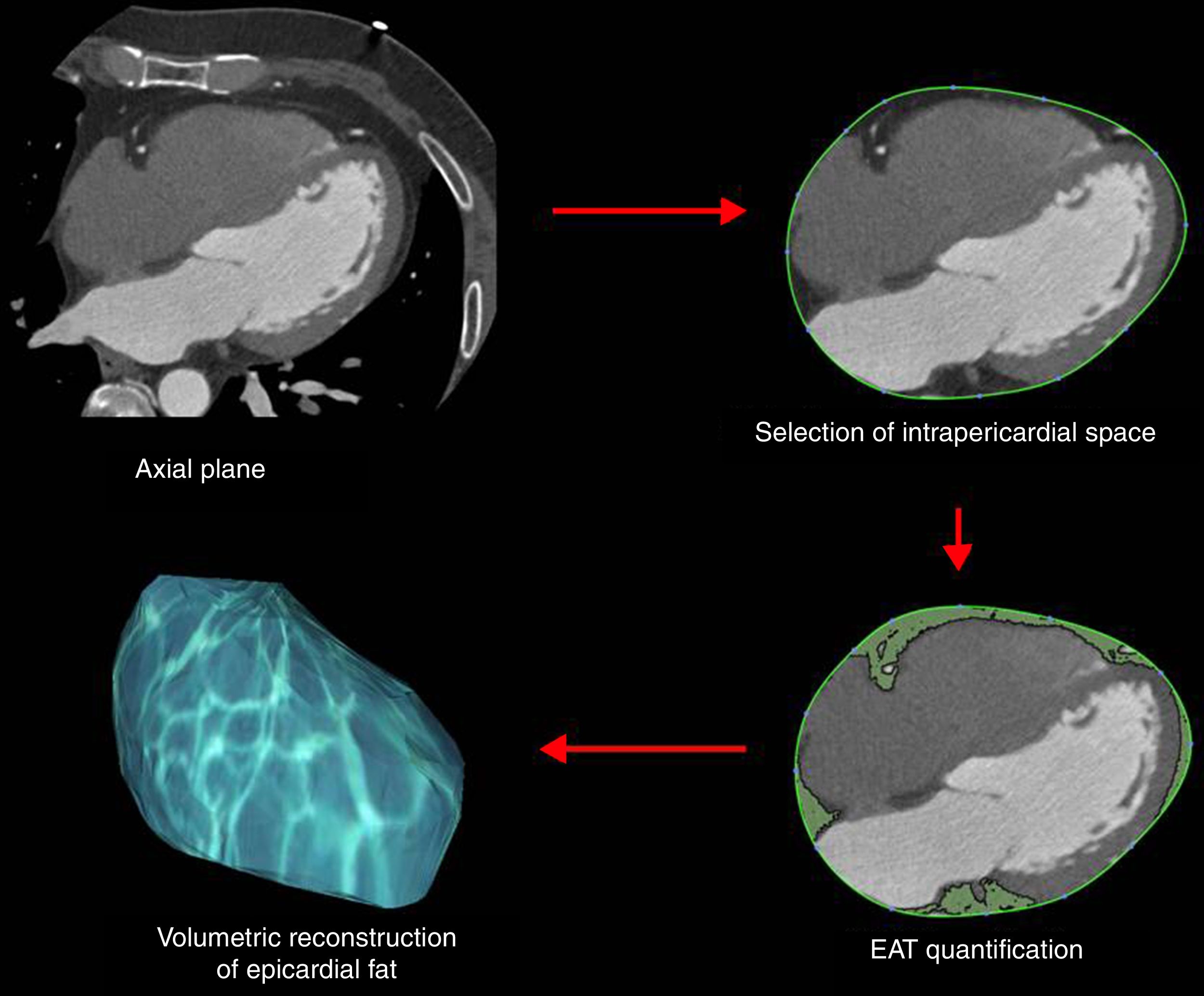
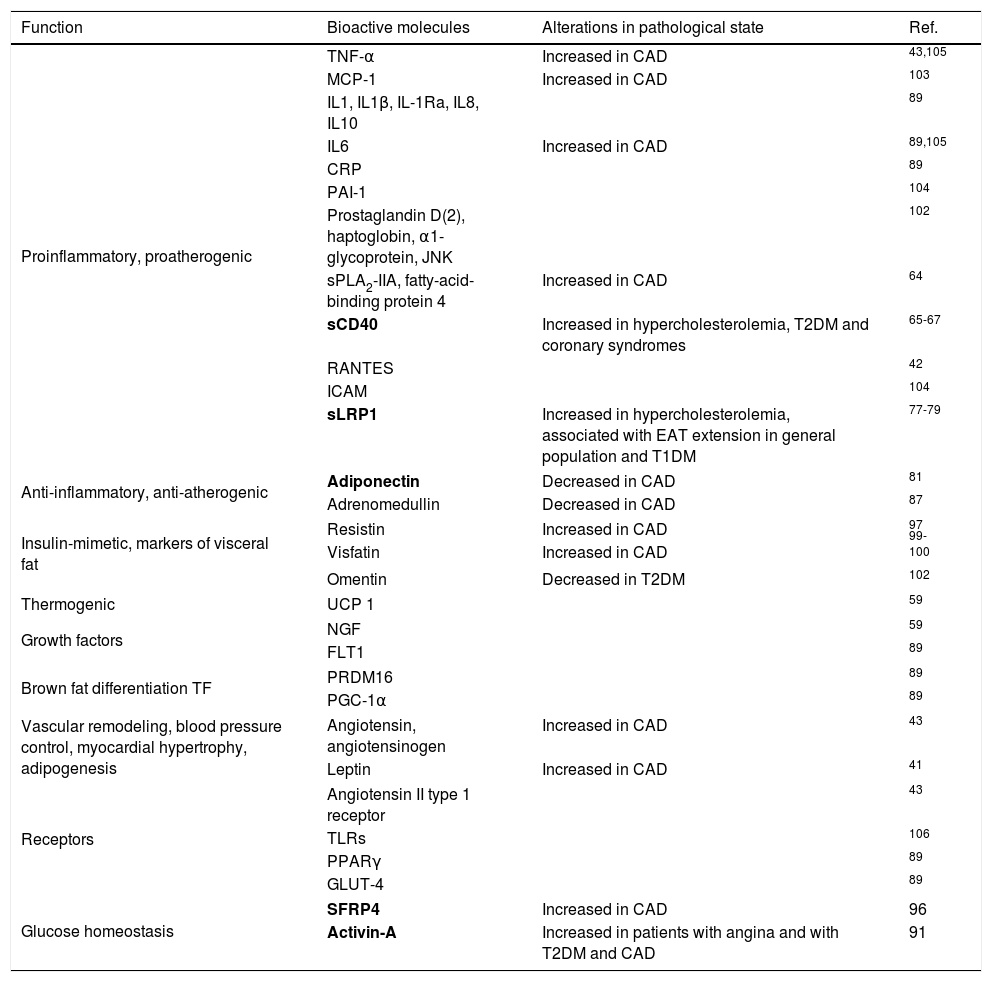
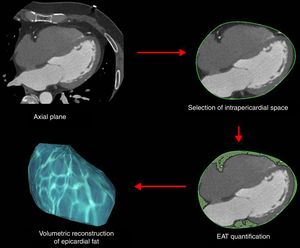
Epicardial fat as a secretory tissue and source of new potential biomarkers for cardiovascular diseasesEAT is an endocrine organ that synthesizes, produces and secretes several metabolically bioactive molecules, including adipokines and vasoactive factors that diffuse between epicardial fat and cardiovascular tissues (e.g., coronary arteries and myocardium), influencing vascular and myocardial functions59–61 (Table 1). EAT evaluation is currently performed by computed tomography (CT) as shown in Fig. 3. However, these measurements are not practical for large-scale population screening and presents several disadvantages including radiation exposure, limited availability, and elevated cost.62 Thus, blood-based biomarkers emerge as non-invasive and early accessible EAT indicators. Here, we review the roles of EAT-secreted molecules in cardiovascular diseases according to their functions associated with CAD, remarking those that have a potential use as biomarkers.
Bioactive EAT-secreted molecules. Potential biomarkers have been marked in bold.
| Function | Bioactive molecules | Alterations in pathological state | Ref. |
|---|---|---|---|
| Proinflammatory, proatherogenic | TNF-α | Increased in CAD | 43,105 |
| MCP-1 | Increased in CAD | 103 | |
| IL1, IL1β, IL-1Ra, IL8, IL10 | 89 | ||
| IL6 | Increased in CAD | 89,105 | |
| CRP | 89 | ||
| PAI-1 | 104 | ||
| Prostaglandin D(2), haptoglobin, α1-glycoprotein, JNK | 102 | ||
| sPLA2-IIA, fatty-acid-binding protein 4 | Increased in CAD | 64 | |
| sCD40 | Increased in hypercholesterolemia, T2DM and coronary syndromes | 65-67 | |
| RANTES | 42 | ||
| ICAM | 104 | ||
| sLRP1 | Increased in hypercholesterolemia, associated with EAT extension in general population and T1DM | 77-79 | |
| Anti-inflammatory, anti-atherogenic | Adiponectin | Decreased in CAD | 81 |
| Adrenomedullin | Decreased in CAD | 87 | |
| Insulin-mimetic, markers of visceral fat | Resistin | Increased in CAD | 97 |
| Visfatin | Increased in CAD | 99-100 | |
| Omentin | Decreased in T2DM | 102 | |
| Thermogenic | UCP 1 | 59 | |
| Growth factors | NGF | 59 | |
| FLT1 | 89 | ||
| Brown fat differentiation TF | PRDM16 | 89 | |
| PGC-1α | 89 | ||
| Vascular remodeling, blood pressure control, myocardial hypertrophy, adipogenesis | Angiotensin, angiotensinogen | Increased in CAD | 43 |
| Leptin | Increased in CAD | 41 | |
| Receptors | Angiotensin II type 1 receptor | 43 | |
| TLRs | 106 | ||
| PPARγ | 89 | ||
| GLUT-4 | 89 | ||
| Glucose homeostasis | SFRP4 | Increased in CAD | 96 |
| Activin-A | Increased in patients with angina and with T2DM and CAD | 91 | |
Proinflammatory and proatherogenic EAT-secreted molecules. Secretion of epicardial inflammatory bioactive molecules contributes to the metabolic and inflammatory milieu that promotes atherogenesis. In fact, many of these molecules changed their levels in the pathological state. Soluble CD40 ligand (sCD40L) is mainly expressed by platelets63 but also by T-cells, macrophages and mast cells that infiltrate into EAT. Circulating sCD40L levels have been reported to be elevated in patients with hypercholesterolemia, T2DM, and other coronary syndromes.64–67 Interestingly, EAT reduction is associated with decreased circulating sCD40L levels in obese men.68 Thus, sCD40L has been proposed to be used as a predictor of cardiovascular disease in patients with psoriasis69 and in healthy women.70
Low-density lipoprotein receptor-related protein 1 (LRP1) is a lipoprotein receptor belonging to the LDL receptor family that regulates adipocyte energy homeostasis.71 In addition, LRP1 plays a crucial role in the cardiovascular system. Our group has previously shown that cardiomyocyte LRP1 overexpression is associated with intracellular cholesterol accumulation and calcium-handling alterations.72,73 LRP1 overexpression is induced by prevalent cardiovascular risk factors, including hypercholesterolemia, hypertension and hypoxia.72,74,75 It has been reported that LRP1 upregulation in EAT is associated with increased EAT volume in patients with type 2 diabetes.76 In addition, our group demonstrated the relationship between epicardial LRP1 overexpression and hypertriglyceridemia in T2DM patients.60 LRP1 has a soluble form, sLRP1, that could influence the activity of LRP1 ligands. Our group has previously shown that circulating sLRP1 concentrations are higher in severe hypercholesterolemia compared with moderate hypercholesterolemia or normocholesterolemia and that sLRP1 is significantly associated with established pro-atherogenic lipid parameters in two different hypercholesterolemic populations. sLRP1 concentrations decrease after statin treatment and increase after statin withdrawal. Interestingly, circulating sLRP1 concentrations are independently associated with the occurrence of carotid atherosclerosis in the hypercholesterolemic population.77 We also showed that circulating sLRP1 levels are associated with EAT volume in general population78 and in type 1 diabetes mellitus (T1DM) patients79 Using multivariate linear regression analyses, we demonstrated that the association between EAT volume and circulating sLRP1 was independent of potential confounding factors, including age, sex, body mass index, CRP, HbA1c and LDL-C.78,79 Taken together, these results point to sLRP1 as a new potential biomarker in the evaluation of cardiometabolic diseases.
Others bioactive molecules are tumor necrosis factor alpha (TNFα), monocyte chemotactic protein-1 (MCP-1) and interleukin-6 (IL-6).80 The presence of inflammatory mediators near coronary arteries cause amplification of vascular inflammation, plaque instability through apoptosis (TNF-α) and neovascularization (MCP-1).43 However, the local inflammation may not correlate with plasma concentrations of these circulating cytokines. Therefore, these bioactive molecules, although increased locally at EAT, could not be used as biomarkers in blood.43
Anti-inflammatory and anti-atherogenic EAT-secreted molecules. The inflammatory process is characteristic of all stages of atherosclerosis, and, thus, the molecules involved in anti-inflammatory processes in CAD patients should be found below normal levels.81 Adiponectin is exclusively produced by adipocytes and is stable in plasma at very high concentrations.82 Low levels of adiponectin are linked with the pathogenesis of cardiovascular disease (CAD, diabetes, and myocardial infarction).81,83 Interestingly, EAT adiponectin expression is lower in patients with CAD than in patients without CAD.84 These studies indicate that low levels of EAT-secreted adiponectin could be associated with cardiovascular risk. One of the proposed mechanisms behind this association is the capacity for adiponectin to promote vessel wall repair by decreasing vascular adhesion molecule expression. The physiological blood concentrations of adiponectin are sufficient to inhibit the expression of adhesion molecules during atherosclerosis progression.82,85,86 Adrenomedullin has vasodilating, angiogenic, antioxidative and anti-inflammatory properties, as well as adiponectin.87 Chronic CAD influences local and systemic adrenomedullin plasma levels and adrenomedullin synthesis and production by EAT, causing a reduction in plasma levels.88 An increased intracoronary adrenomedullin levels are only detected when hemodynamic conditions have been improved (i.e. after coronary revascularization).89
EAT-released molecules related with glucose homeostasis. Activin A, a member of the transforming growth factor beta (TGF-β) family, has important functions in glucose homeostasis and in inflammatory responses. In addition, TGF-β plays a key role in angiogenesis, vascular remodeling and atherosclerosis.90 Activin A is expressed by a large number of cells and tissues, especially by EAT.91 Chen and collaborators demonstrated that patients with cardiovascular disease and patients with stable and unstable angina have higher EAT activin A levels than healthy patients. Additionally, EAT activin A levels are elevated in T2DM patients with CAD compared to those without CAD.92 Greulich et al. showed for the first time the presence of activin A in EAT secretoma from T2DM patients.93 These authors also found that activin A-exposed rat cardiomyocytes showed reduced contractile function and decreased insulin-mediated Akt phosphorylation.93 These results suggest that T2DM produces alterations in the EAT secretion profile that could play a role in the development of metabolic and cardiac derangements in T2DM patients.93
Secreted frizzled related protein 4 (SFRP4) is a circulating modulator protein that binds to Wnt receptors to block Wnt signaling, thereby impairing insulin sensitivity.94 It has been shown that SFRP4 alters pancreatic islet functionality causing a reduction in glucose-induced insulin secretion.95 These authors showed that high SFRP4 circulating levels are indicative of low grade inflammation in T2DM. SFRP4 is currently considered a biomarker of β-cell dysfunction, insulin resistance and other metabolic disorders.95 SFRP4 levels have been found increased in the EAT and plasma of CAD patients.96 SFRP4 expression levels are lower in EAT than in SAT. However, high SFRP4 expression levels in EAT are sufficient to increase circulating levels of SFRP4 in patients with CAD. Therefore, SFRP4 is considered a novel CAD biomarker; however, the precise role of SFRP4 in atherosclerosis development remains unknown.
EAT-released molecules acting as insulin-mimetics. There are other adipokines that are markers of adipose tissue, such as resistin, visfatin and omentin. Resistin is a secreted factor associated with insulin resistance and high resistin plasma levels are linked with a positive history of previous myocardial infarction.97 Langheim et al. conclude that EAT resistin production is higher in patients with acute coronary syndrome than in patients with stable CAD or individuals with angiographically normal coronary arteries.98
Visfatin is an adipokine with a possible role as a compensatory response in diet-or obesity-induced insulin resistance.99 Patients with T2DM have elevated visfatin plasma levels.100 In patients with CAD, visfatin levels are increased producing pro-inflammatory effects that directly impact atherosclerotic plaque activity and stability.10
EAT-released molecules involved in vascular remodeling, blood pressure control, myocardial hypertrophy and adipogenesis. The adipokines associates with vascular remodeling, blood pressure control, myocardial hypertrophy and adipogenesis are angiotensin, angiotensinogen and leptin. These adipokines are increased in CAD patients.10 Leptin have higher concentrations in EAT from patients with critical CAD who underwent CABG surgery than in that from non-CAD subjects.101
All of these bioactive molecules are expressed in EAT and in other tissues, especially in other adipose tissues such as SAT or VAT. Due to the close anatomical proximity of EAT with coronaries and myocardium, the levels of EAT-released molecules are crucial in the development of CAD and other cardiovascular diseases.102
Conclusions and perspectivesMetabolic syndrome, T2DM, NFLAD and CAD are associated with high EAT volume. EAT shows certain particularities in front of other fat depots that confer to EAT a protective role including higher flexibility in the storage/release of fatty acids and capacity of thermogenesis, Under pathological conditions, EAT, through paracrine and vasocrine effects, contributes to the onset and development of coronary atherosclerosis. In addition, EAT causes direct deleterious effects on myocardial function, promoting arrhythmias and heart failure. From a clinical point of view, lifestyle changes and useful treatments should be introduced to modulate EAT volume and thus improve the management of patients with metabolic diseases. Some of the EAT-secreted molecules are specifically synthesized and/or released only by this fat depot, others not. Nevertheless, we consider that the close proximity between coronaries/heart and EAT, by facilitating a direct impact of EAT-released molecules on myocardial function and vice versa, enhance the potential value of these molecules as biomarkers of cardiometabolic alterations.
Conflict of interestNone.
Our work was supported by FIS PI14/01729 from the Instituto Salud Carlos III, co-financed by the European Fund for Regional Development (E.F.R.D.) and Fundació Marató TV3 (201521 10). CIBER Cardiovascular (CB16/11/00403) is an Instituto de Salud Carlos III Project. VLL-C is the IP of the Quality Research Group 2017 SGR 946 from Generalitat de Catalunya. We would like to thank Dr. David Vilades (Image Cardiac Unit, Department of Cardiology, Hospital de la Santa Creu i Sant Pau) by the generous gift of EAT representative CT images and of the scheme used to quantify EAT extension in such CT images.