The incidence of atherosclerotic cardiovsacular disease (ASCVC) has increased in the developed countries. Dyslipidemia is a primary major risk factor for ASCVD and LDL lowering is one of the main objectives. Although treatment goals for dyslipidemias should be personalized in every patient, statins are cost-effective in primary and secondary prevention of ASCVD. New treatments with higher power and greater decreases in LDL, PSCK9 inhibitors, have made a new breakthrough in ASCVD treatment. The 2019 Guidelines for de Management of Dyslipidaemias: Lipid Modification to reduce Cardiovascular Risk (European Society of Cardiology/European Atherosclerosis Society) with the level of evidence and the strength of the recommendations can facilitate the best decisions and benefits to our patients in clinical practice.
La incidencia de las enfermedades arterioscleróticas ha aumentado en los países desarrollados. La dislipemia es un factor de riesgo cardiovascular mayor y el descenso del LDLc es el objetivo terapéutico. Hay que individualizar los objetivos en cada paciente y las estatinas han demostrado ser un tratamiento coste-efectivo tanto en prevención primaria como en secundaria. La aparición de los inhibidores de PSCK9, más potentes y por tanto consiguiendo un mayor descenso de las cifras de LDLc, son un avance en el tratamiento de la enfermedad cardiovascular. La publicación en 2019 de las Guías de Dislipemias (Sociedad Europea de Cardiología /Sociedad Europea de Arteriosclerosis) con el nivel de evidencia y fuerza de recomendación, pueden ayudar en la toma de decisiones y beneficios para nuestros pacientes en la práctica clínica diaria.
The incidence of cardiovascular disease (CVD) continues to increase in developed countries. Dyslipidaemia plays a key role in the development of atherosclerotic disease. Prospective studies, clinical trials and Mendelian randomisation studies have shown that increased low-density lipoprotein cholesterol (LDL-C) is a cause of CVD. Across the range of elevated LDL-C it is considered that "lower is better" without a lower threshold, at least down to 1mmol/l (40mg/dl). The emergence in recent years of new, more potent drugs and the publication of different studies and meta-analyses has led to changes in international guidelines.
What has not changed is the consideration of LDL-C as the primary therapeutic target to achieve a reduction in this cardiovascular risk (CVR). Lowering LDL-C can generate valuable benefits in patients taking into account total CVR and baseline LDL-C levels. The proportional reduction in CVD risk achieved by lowering LDL-C depends on the absolute reduction in LDL-C, with each 1mmol/l reduction reducing the risk of CVD by 22%.
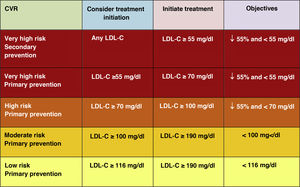
Intensification of treatment targets is important to ensure that treatment of the highest risk patients achieves the greatest possible LDL-C reduction. A minimum LDL-C reduction percentage of 50% and an absolute treatment target of LDL-C<55mg/dl for very high-risk patients and <70mg/dl for high-risk patients are set.
Statins are drugs of choice with high efficacy in lowering plasma LDL-C concentrations and preventing CVD. Combination with ezetimibe or proprotein convertase subtilisin/kexin proprotein convertase type 9 (iPCSK9) inhibitor further reduces the risk of CVD.
The availability of new high-potency lipid-lowering agents, in particular the lPCSK9 monoclonal antibodies, has opened a new horizon in the prevention and treatment of CVD with further reductions in LDL-C.
LDL as a causal agent of cardiovascular diseaseAtherosclerosis is triggered by lipid accumulation in subendothelial arterial cells.1 Intracellular lipid accumulation is caused by LDL circulating in human blood. It has been shown that only modified lipoproteins, but not native LDL, can cause intracellular lipid accumulation in human arterial wall cells.2 Although oxidation remains the most frequently observed atherogenic modification of LDL, other forms of modified LDL have been detected in the blood of atherosclerotic patients.
LDL particles are composed of lipids (80%, predominantly esterified cholesterol) and proteins. The triglyceride (TG) content of LDL is relatively low under normal conditions (5%–10%) but increases with diabetes, obesity, metabolic syndrome, combined familial hypercholesterolaemia (FH) and renal failure.
LDL transports cholesterol in blood and extracellular fluids, and this is largely controlled by interactions of surface proteins with lipid transport proteins, enzymes and cell membrane receptors. Apolipoprotein (Apo) B100 acts as the ligand for LDL receptors and contributes to the interaction between LDL and arterial wall proteoglycans, thus promoting the subendothelial retention of atherogenic LDL in early atherosclerosis.3 Compared to HDL, LDL particles contain relatively few proteins, accounting for approximately 15% of the non-ApoB1004 LDL protein content. This implies that the composition of LDL is mainly associated with its own metabolism.
Proteomic and kinetic studies of LDL reaffirm that LDL-C is the end product of endogenous lipoprotein metabolism. Four of the 5 LDL-C particles are eliminated via the LDL-LDL receptor (LDLR) pathway in the liver.5,6 Because mammalian cells do not have enzymatic systems to degrade cholesterol, the LDL-LDLR pathway is, together with reverse cholesterol transport of high-density lipoprotein cholesterol (HDL-C), the main mechanism for cholesterol removal from the body, the main consequence of a defective LDL-LDLR pathway is an increase in circulating LDL, leading to accumulation of LDL-C in the arterial wall and other peripheral tissues. This is supported by evidence of normalisation of LDL-C levels in patients with homozygous FH after liver transplantation.7
The role of LDL-C in the aetiopathogenesis of atherosclerosis has been demonstrated in numerous epidemiological studies and randomised clinical trials that have not only shown the link between LDL-C and CVD, but also the beneficial effect of lowering LDL-C levels.
Among epidemiological studies, the Framingham study8–10 demonstrated a linear and independent relationship between elevated total cholesterol (TC) levels, elevated LDL-C levels and decreased HDL-C with the risk of CHD. In the MRFIT study, a continuous and gradual relationship (with no threshold for the onset of the relationship) between hypercholesterolaemia and CHD mortality was observed.11 This relationship has also been observed in populations of different socio-cultural and racial backgrounds. 12 Reducing hypercholesterolaemia, by lowering LDL-C, leads to a reduction in incidence and mortality from ischaemic heart disease and CVD in general, both in primary and secondary prevention.13 The importance of dyslipidaemia has also been highlighted in the INTERHEART study,14 where dyslipidaemia accounted for 54% of the population attributable risk of myocardial infarction. However, dyslipidaemia was not defined by LDL-C alone, but as the ApoB/ApoA-I ratio, which is a representative value for the ratio of atherogenic very low-density lipoprotein, intermediate density lipoprotein and atherogenic LDL-C to atheroprotective HDL-C concentrations.15
Adequate control of the different risk factors in general, and of LDL-C in particular, remains the cornerstone for reducing the risk of a new CV event.16 Given the aetiopathogenesis of atherosclerosis, reducing LDL-C levels is the central axis in preventing the occurrence of a CV event. However, unlike with other CVRFs, such as blood pressure in hypertensive patients or glycosylated haemoglobin (HbA1c) in diabetics, where an excessive reduction in these factors could be harmful in certain subgroups of patients such as the frailest subjects, with respect to LDL-C, a figure below which reducing LDL-C could pose a greater risk has not yet been established. Therefore, at present, based on the scientific evidence available for the prevention of CVD, the theory of the lipid hypothesis of LDL-C of "the lower the better"17,18 continues to be supported, even with extremely low concentrations, around 20mg/dl of LDL-C, benefits are still obtained in CV prevention without harmful effects on health.19,20
Randomised clinical trials with statins in both primary prevention (WOSCOP with pravastatin,21 AFCAPS/TexCAPS with lovastatin,22 ASCOT-LLA with atorvasatin,23 ALLHAT-LLT with pravastatin,24 CARDS with atorvastatine,25 MEGA with pravastatine26 and JUPITER27 and HOPE-328 with rosuvastatin, and in meta-analyses of studies in primary prevention29,30), as well as in secondary CV prevention ((4S,31 CARE,32 LIPID33 and HPS,34 carried out in patients with stable coronary heart disease, MIRACL35 PROVE-IT36 and A to Z [phase Z]37 in patients with acute coronary syndrome, GREACE,38 TNT39 and IDEAL40 in patients with stable angina), as well as several meta-analyses,41,42 have shown that the magnitude of the absolute reduction in major vascular events obtained with statin therapy is directly proportional to the absolute reduction in LDL-C achieved, with the additional benefit of more intensive treatment with lipid-lowering therapy, even in those patients in whom LDL-C is already at therapeutic targets.
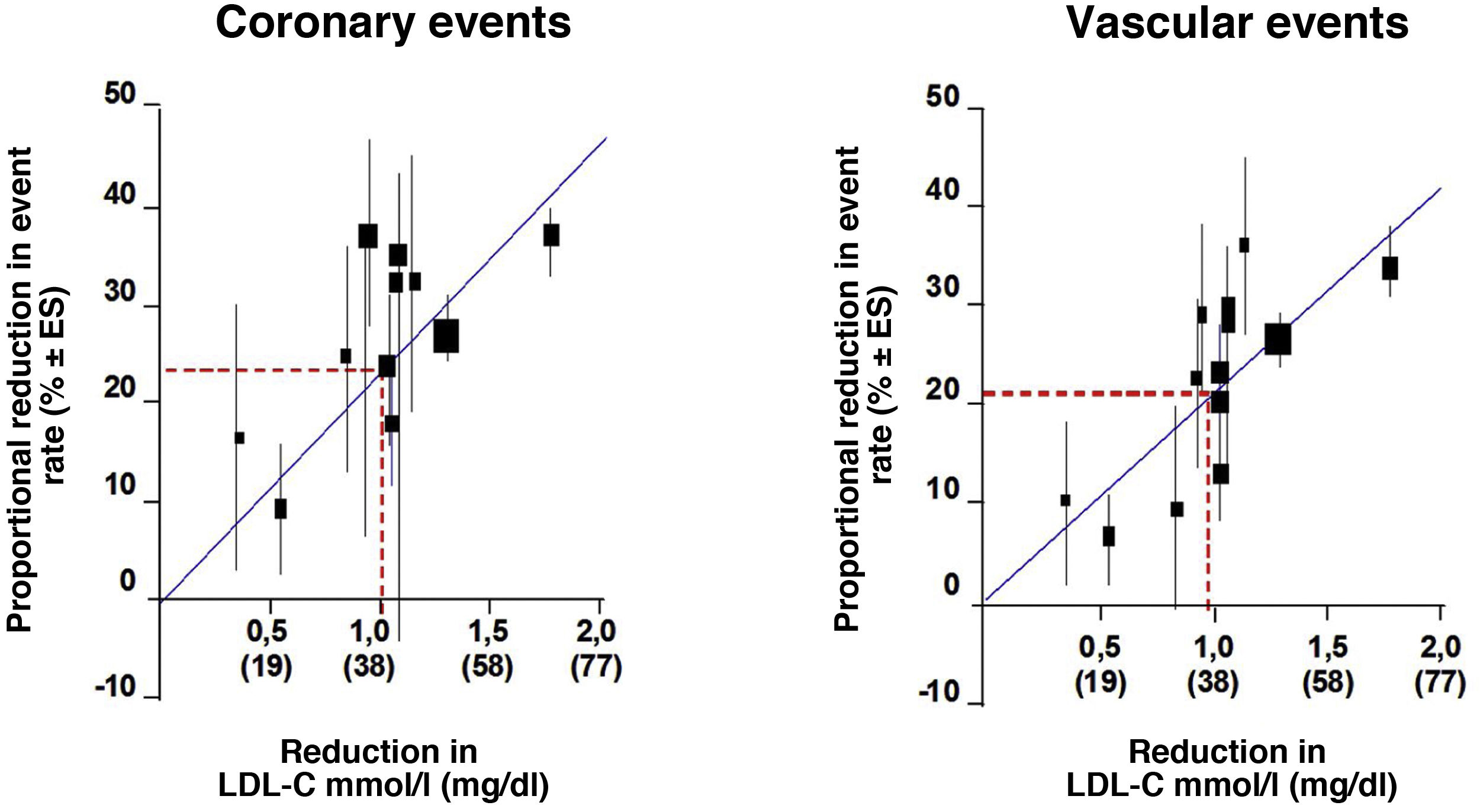
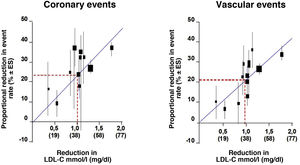
We looked at the relationship between LDL-C and relative risk (RR) for the development of CVD using log-log regression in a prospective meta-analysis using data from 170,000 individuals from 26 statin intervention trials to analyse the relationship between the proportional reduction in the incidence of major coronary events and major vascular events and the mean absolute reduction in LDL-C at one year. Overall, there was a 22% proportional reduction in the risk of major vascular events for each 1mmol/l reduction in LDLc, implying that, at least within the range of LDLc studied to date, a 2mmol/l reduction reduces risk by approximately 40% and a 3mmol/l reduction could reduce risk by approximately 50% (Fig. 1).
RR of coronary events and cardiovascular events.
Modified from Baigent et al.42.
The meta-analysis by Robinson et al.43 shows that the decrease in CVR is proportional to the decrease in LDL-C, irrespective of the therapy used (statins, resins, surgery or diet). The prognostic benefit of lipid-lowering therapy observed in 19 primary and secondary prevention clinical trials is almost exclusively explained by LDL reduction and this supports the hypothesis that more intensive LDL-C reductions could lead to greater clinical benefit. Another recent meta-analysis of different treatment strategies has shown that the use of statin and non-statin therapies that act through up-regulation of LDLR expression to reduce LDL-C was associated with similar RRs of major vascular events due to LDL-C change. Lower levels of LDLc achieved were associated with lower rates of major coronary events.43 In this meta-analysis different treatment strategies have shown that the use of statin and non-statin therapies that act through up-regulation of LDL receptor expression to reduce LDL-C was associated with similar RRs of major vascular events per change in LDL-C. Lower LDL-C levels achieved were associated with lower rates of major coronary events.43
Studies analysing the effects of lipid-lowering therapy on atherosclerotic plaque volume show that as LDL-C levels are reduced, if the reduction is moderate, the treatment is able to slow the progression of atherosclerotic plaque, but when the reduction is more intense, the volume of atherosclerotic plaque is reduced and may even disappear with intensive treatment over time.
Statins have also been shown to be effective in slowing the progression or even promoting regression of coronary atherosclerosis. The REVERSAL study conducted with IVUS in patients with stable coronary artery disease to demonstrate that intensive therapy with atorvastatin 80mg/day, which achieved LDL-C levels of 79mg/dl, significantly slowed the progression of atheromatous lesions at 18 months follow-up, compared to standard therapy with pravastatin 40mg/day, which only reduced LDL-C to 110mg/dl.44
The ASTEROID study showed that intensive treatment with rosuvastatin 40mg/day resulted in LDL-C levels of 61mg/dl, which correlated with a significant reduction in atheromatous plaque size, demonstrating regression of atherosclerosis with intensive lipid-lowering statin therapy.45
Therefore, it is no longer a matter of slowing the progression of atherosclerotic disease, of stabilising atherosclerosis, but of reversing atherosclerotic plaque. And this is only possible with very significant reductions in LDL-C, as demonstrated with intensive treatment at maximum doses with statins, maximum doses of statins with ezetimibe46 and with evolocumab in the GLAGOV study.47
New evidence has confirmed that the key initiating event in atherogenesis is the retention of LDL-cholesterol and other cholesterol-rich ApoB-containing lipoproteins within the arterial wall. Several recent placebo-controlled clinical studies have shown that the addition of ezetimibe or antiprotein convertase subtilisin/kexin type 9 (iPCSK9) monoclonal antibodies19,48 to statin therapy provides an additional reduction in the risk of atherosclerotic CVD (A-CVD), which is directly and positively correlated with the incrementially achieved absolute LDL-C reduction. Furthermore, these clinical trials have clearly indicated that the lower the achieved LDL-C values, the lower the risk of future CV events, with no lower limit for LDL-C values, or no "J" curve effect for LDL-C.
Additionally, studies on the clinical safety of these very low LDL-C values have proven reassuring, although monitoring over longer periods is required. Finally, Mendelian randomisation studies in humans have demonstrated the critical role of LDL-C and other cholesterol-rich ApoB-containing lipoproteins in atherosclerotic plaque formation and subsequent related CV events. Therefore, it has been established that increased LDL-C values are causally related to CVD and that the reduction of LDL particles and other ApoB-containing lipoproteins reduces CV events as much as possible.
Given this new evidence, the new lipid guidelines of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) published in 201949 have adopted a more practical, more simplified and much more aggressive approach with new lower targets for LDL-C reduction for most CVR categories50 than the previous recommendations of the 2016 ESC/EAS guidelines.18 They emphasise the importance of reducing LDL-C levels rapidly and intensively in patients with higher CVR, recommend new treatment strategies in general and in some population subgroups in particular.
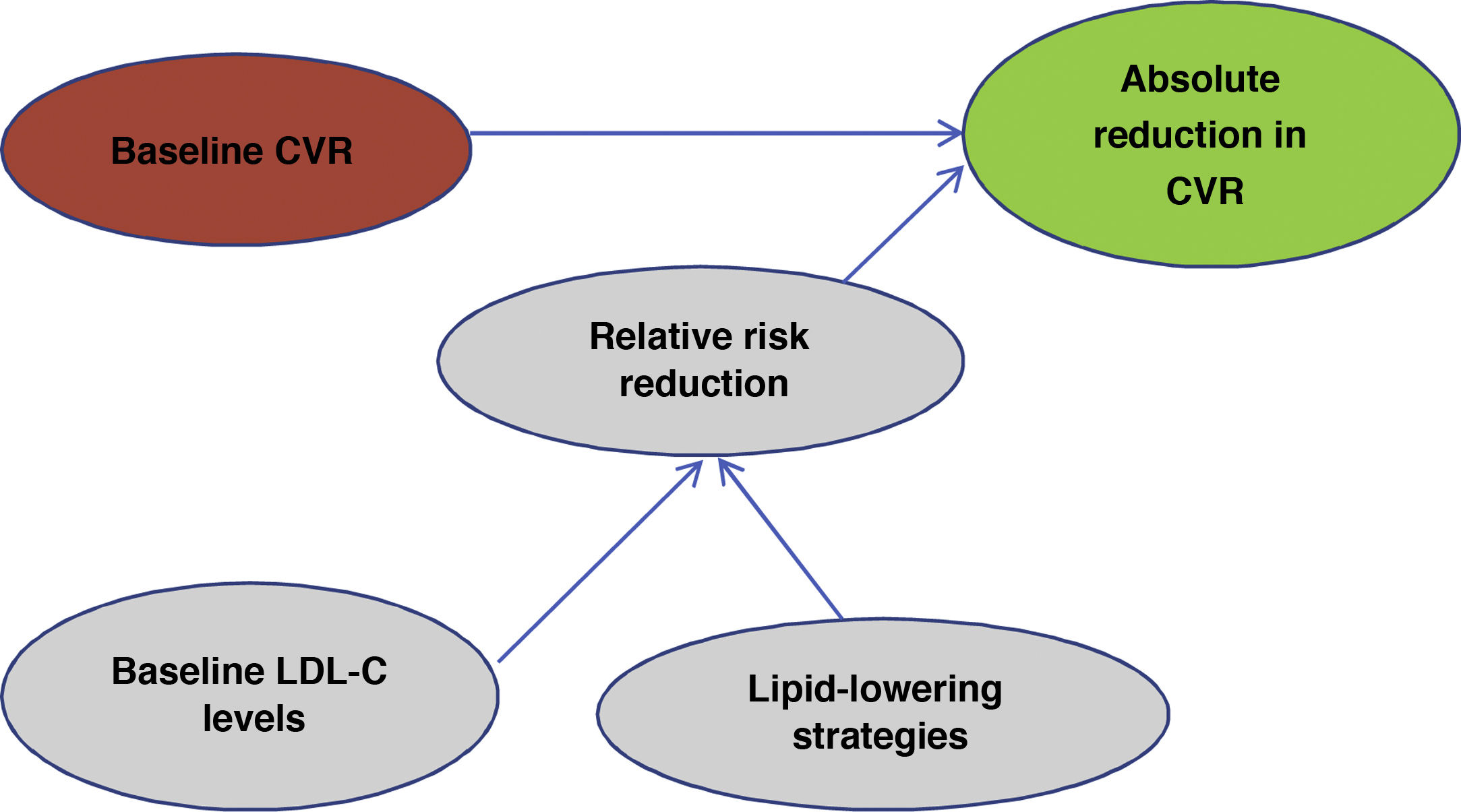
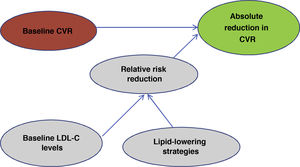
One of the most important and novel concepts that has been emphasised in the publication of these guidelines49 is that when predicting the expected benefit in CVR it is very important to take into account 2factors: baseline CVR and baseline LDL-C levels. Taking into account baseline CVR and baseline LDL-C levels will lead us to use a particular lipid control strategy that will lead to a reduction in LDL-C and in the end it will be these 2factors, i.e., the patient's risk profile and the reduction in LDL-C that we have obtained that will determine the absolute reduction in CVR that we obtain in a given patient (Fig. 2).
Evaluation of expected benefit.
Own elaboration based on Mach et al.49
All current guidelines on CVD prevention in clinical practice recommend assessment of total CVD risk. CVD prevention in a given individual should be related to his or her total CV risk: the higher the risk, the more intense the action should be.
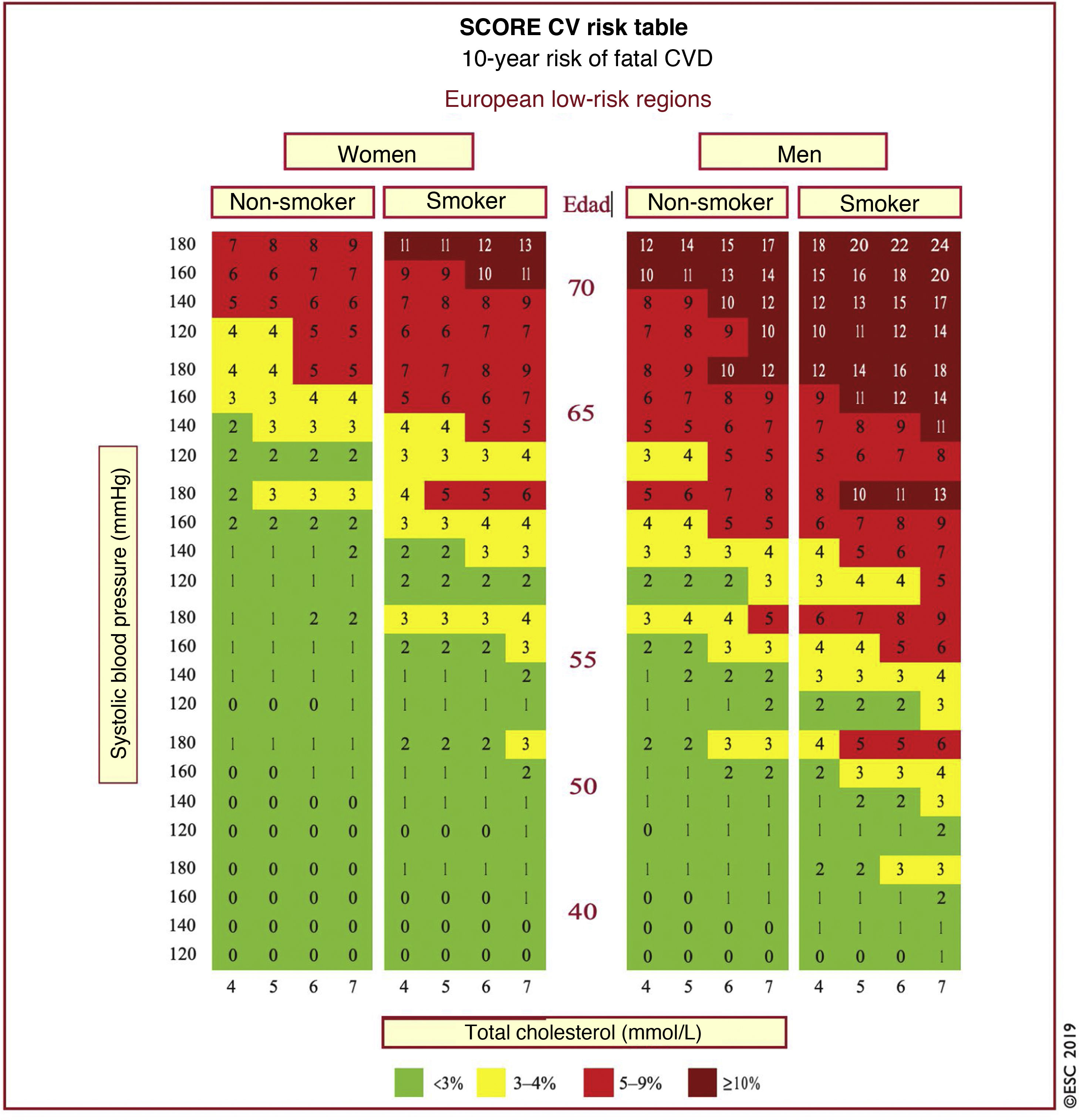
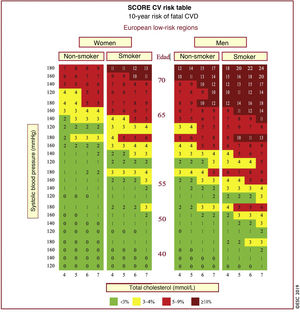
In Spain, the SCORE table is recommended for use in low CVR countries. The SCORE risk charts included in the ESC/EAS Guidelines 201949 differ slightly from those in the EAS/ESC Guidelines 201618 for the treatment of dyslipidaemias, in that (Fig. 3):
- 1
The age has been extended from 65 to 70 years.
- 2
The interaction between age and each of the other risk factors has been incorporated, thereby reducing the overestimation of risk in older people in the original systematic coronary risk estimation tables.
- 3
The 8mmol/l cholesterol band has been removed since such persons will qualify for further assessment in any case.
SCORE table for calculation of CVR in low-risk countries. SCORE table for estimating coronary risk for European populations at low risk of cardiovascular disease. The 10-year risk of fatal cardiovascular disease in populations at low risk of cardiovascular disease according to the following risk factors: age, sex, smoking, systolic blood pressure and total cholesterol. To convert risk of fatal cardiovascular disease to risk of total cardiovascular disease (fatal or non-fatal), multiply by 3 in men and 4 in women, and slightly less in the elderly. Note: The SCORE table is used in people without overt cardiovascular disease, diabetes (type 1 and 2), chronic kidney disease, familial hypercholesterolaemia or very high levels of individual risk factors because these people are already at high risk and need intensive risk factor management. Cholesterol: 1mmol/l=38.67mg/dl.
SCORE: Systematic Coronary Risk Estimation.
Modified from Mach et al.49
People with documented CVD, type 1 diabetes mellitus (DM) or long-standing type 2 DM, very high levels of individual risk factors, chronic kidney disease (CKD), HF, carotid or femoral plaques, coronary artery calcium score >100 or extreme elevation of lipoprotein (a) (Lp[a]), generally have a very high or very high total CV risk and risk estimation models are not needed for such people. Everyone needs active management of all risk factors.
In apparently healthy individuals who have not experienced a CV event, the use of a risk estimation system such as SCORE, which estimates the 10-year cumulative risk of a first fatal atherosclerotic event, is recommended to estimate total CV risk, as many individuals have several risk factors that, in combination, can result in high levels of total CVR. This is the basis for the estimation and management of total CVR. Screening for risk factors, including lipid profile, should be considered in men >40 years and in women >50 years or postmenopausal.
In older patients, where CVD morbidity and mortality is very high, specific tables have been developed for them, achieving a reduction in false positives and therefore a reduction in overtreatment in this vulnerable population with significant comorbidities. Importantes.51
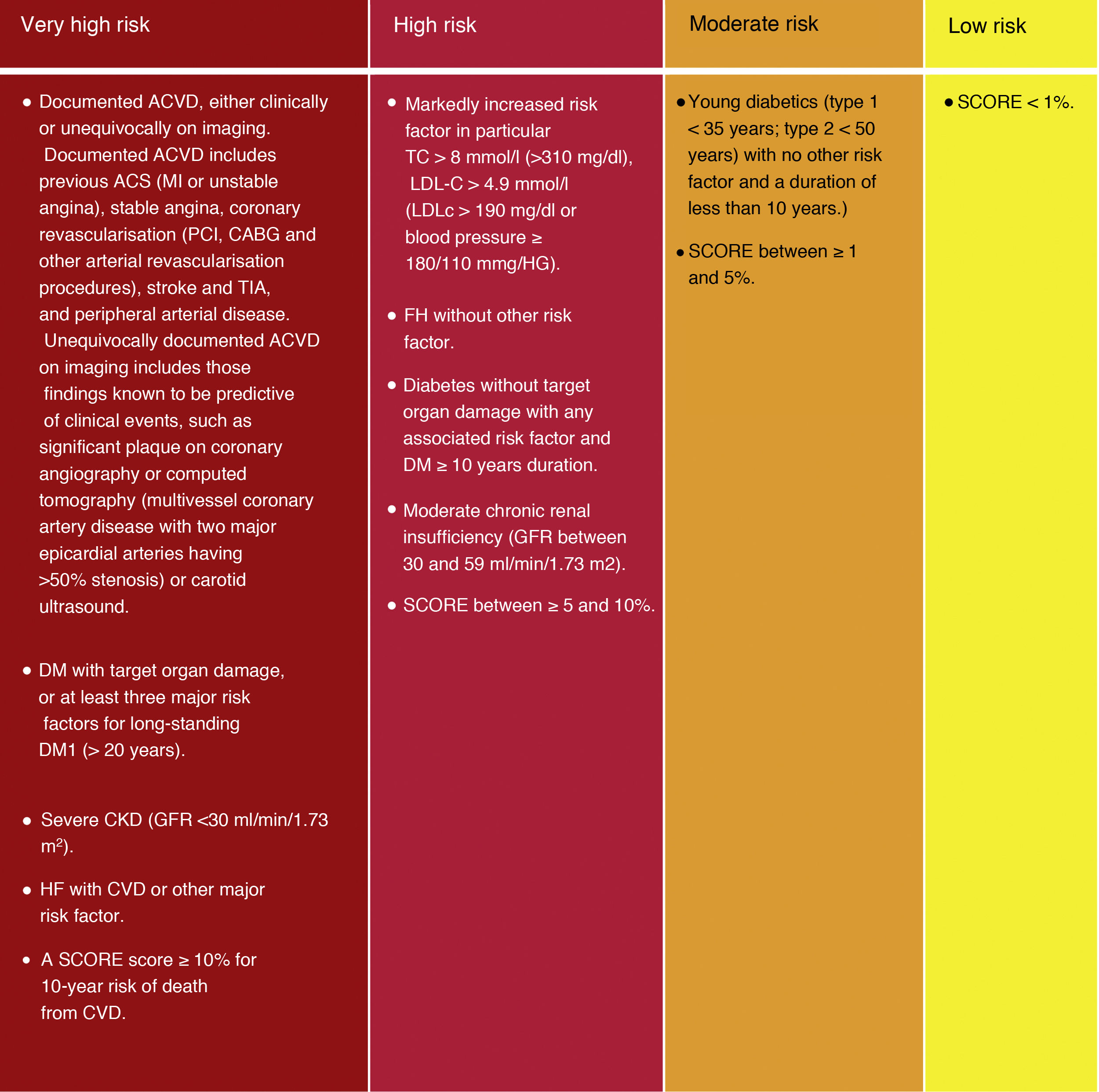
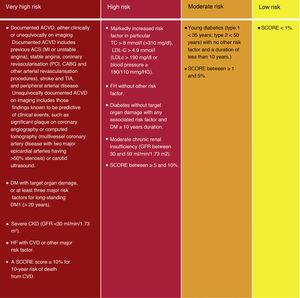
Although the risk categories published in the ESC/EAS 2016 guidelines18 are maintained, some modifications have been introduced in this latest version of 2019.49 Thus, 4 categories of CVR are determined (Fig. 4).
Cardiovascular risk categories.
ACVKD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HF, familial hypercholesterolaemia; LDL-C, low-density lipoprotein cholesterol; Non-HDL-C, non-HDL cholesterol ; TC, total cholesterol; Modified from Mach et al.49
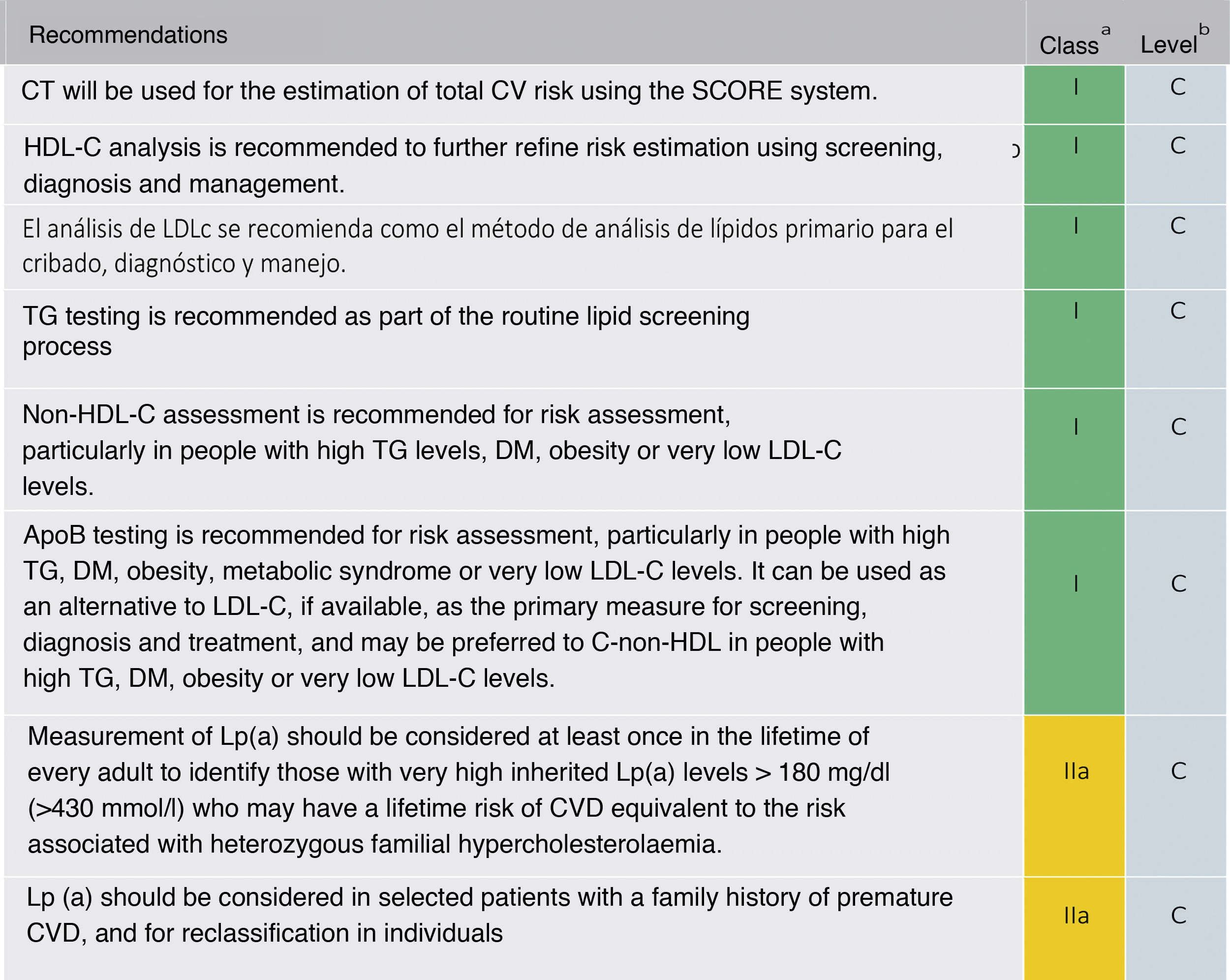
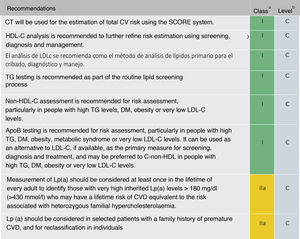
The recommendation to order a complete lipid profile (TC, LDL-C, TG, HDL-C, and calculation of non-HDL cholesterol [non-HDL-C]) is maintained (Fig. 5). If available, ApoB may be a better measure of an individual's exposure to atherosclerotic lipoproteins and, therefore, its use may be particularly useful for risk assessment in individuals where measurement of LDL-C underestimates this burden, such as those with high TG, diabetes, obesity or very low LDL-C.
Lipid analysis to estimate the risk of cardiovascular disease.
Apo: apolipoprotein; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; Non-HDL-C: non-HDL cholesterol; CV: cardiovascular; CVD: cardiovascular disease; ACVD: atherosclerotic cardiovascular disease; DM: diabetes mellitus; Lp (a): lipoprotein (a); TG: triglycerides; TC: total cholesterol
Modified from Mach et al.49
It also recommends a single Lp(a) measurement in all individuals at least once in a lifetime (values ≥ 180mg/dl determine a risk equivalent to FH). A single measurement of Lp(a) may help to identify individuals with very high inherited levels of Lp(a) who may have a substantial lifetime risk of CVD. It may also be useful to further optimise risk stratification of high-risk patients, in patients with a family history of premature CVD, and to determine treatment strategies in individuals whose estimated risk is at the borderline of the risk categories.52–54
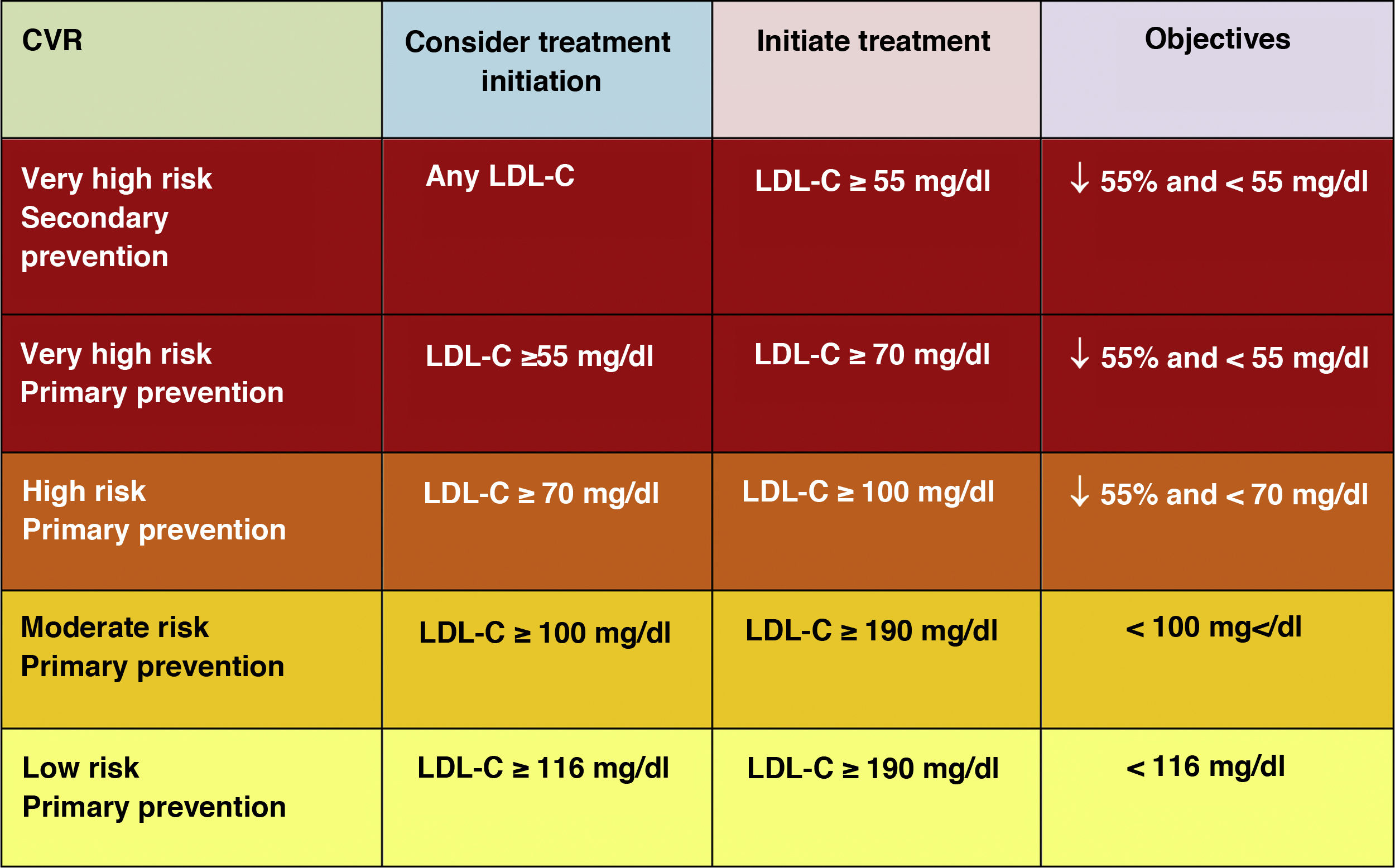
Lipid objectives for LDL-C controlLDL-C is recommended as the primary lipid testing method for screening, diagnosis and management and is considered by all dyslipidaemia treatment guidelines as the primary control target. The new guidelines determine new LDL-C targets (lower lipid values) compared to the previous recommendations of the 2016 guidelines.18 (Fig. 6)
The new guidelines emphasise the importance of reducing LDL-C levels rapidly and intensively in patients with elevated CVR. The key strategy in these guidelines is "lower LDL-C levels are better at preventing CVD", and while this has been generally recommended in the ESC/EAS 2016 guidelines,18 the new guidelines are recommending more strongly that the benefit still remains at very low LDL-C levels, with a simpler approach than before, and in higher risk patients they are recommending that LDL-C should be reduced as much as possible without really having a lower limit.
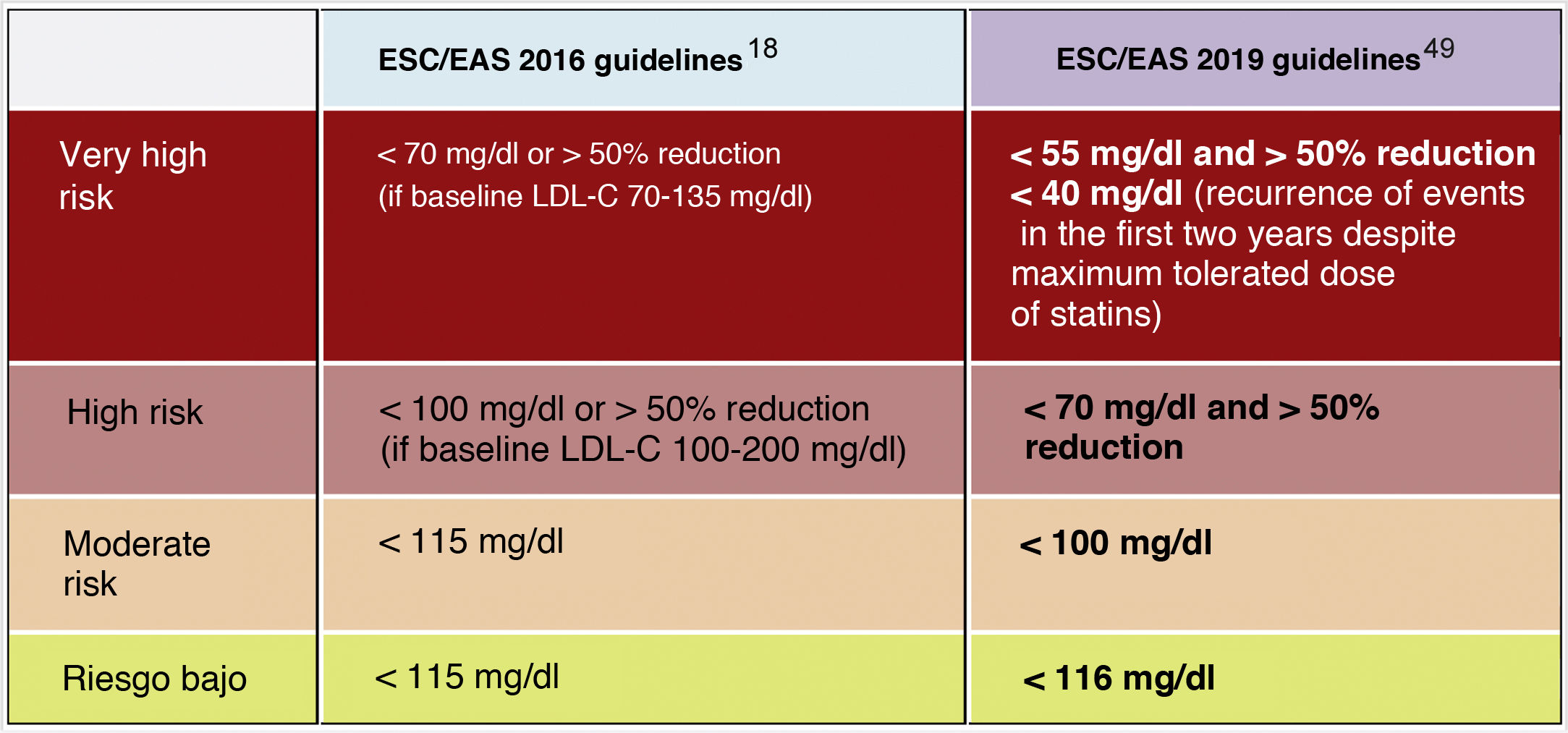
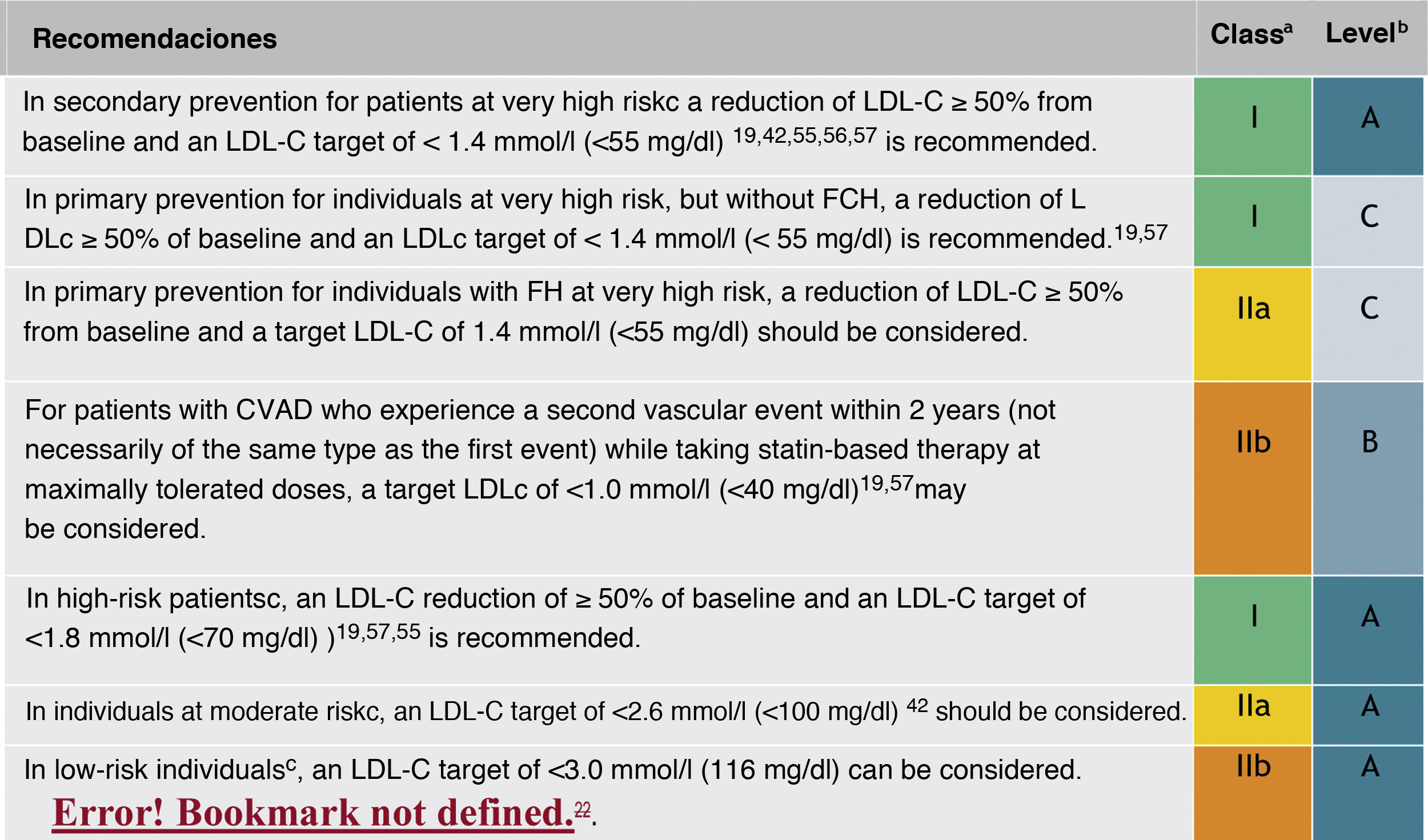
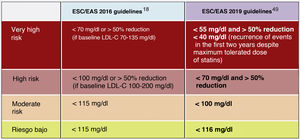
In this regard, the ESC/EAS 2019 guidelines49 recommend a LDL-C target of less than 1.4mmol/l (<55mg/dl) for patients at very high risk and an even lower target of less than 1.0mmol/l (<40mg/dl) for patients at the highest risk, those with multiple recent events (Fig. 7)
Recommendations for LDL-C cholesterol treatment targets.
ACVD: atherosclerotic cardiovascular disease; FHD: familial hypercholesterolaemia; LDL-C: low-density lipoprotein cholesterol.
aClass of recommendation.
bLevel of evidence.
cFor definitions, see Cardiovascular risk categories.
dThe term "baseline" refers to the LDL-C level in a person who is not taking any LDL-C-lowering medication. In individuals taking LDL-C-lowering medications, projected baseline (untreated) LDL-C levels should be estimated based on the average LDL-C lowering efficacy of the medication or combination of medications administered.
Modified from Mach et al.49
This recommendation is much more aggressive than previous guidelines, which had a target of 1.8mmol/l (<70mg/dl) or a 50% reduction. The difference between "and" or "or" may seem like a subtle change, but it could make a big difference for some patients. For example, if a very high-risk patient has an untreated LDL-C level of 1.5mmol/l, which is just above the target of 1.4; then the new recommendation of needing a 50% reduction in addition to being below 1.4 would require the LDL-C level to be reduced much further, to 0.75mmol/l.
Also, secondary targets for non-HDL-C (<85mg/dl, <100mg/dl and <130mg/dl) and ApoB (<65mg/dl, <80mg/dl and <100mg/dl and <100mg/dl) are set for the very high, high and moderate risk categories, respectively.
The American Association of Endocrinology, in an update of the Recommendations for the Treatment of Dyslipidaemia and Prevention of CVD in 2017, had already created the category of extreme CVR (progressive CVD and unstable angina in patients with LDL-C <70mg/dl, established CVD in patients with DM2, CKD stages 3–5, HF and history of premature CVD) for which it set targets of LDL-C <55mg/dl,58 categories that the 2019 European guidelines encompass in the high CVR category with targets of LDL-C <50% and <55mg/dl.49
Treatment for lowering LDL-CAs in the 2016 guidelines,18 we should consider the patient's risk and baseline LDL-C level to choose the lipid-lowering strategy to follow. In these 2019 guidelines,49 in addition to significantly lowering the therapeutic targets, the intervention strategies for achieving LDL-C targets have changed. As in previous guidelines, lifestyle modification and individualised pharmacological treatment based on total CVR and baseline LDL-C are still recommended. The estimation of total CVR does not imply that all patients should be treated pharmacologically,59 but it does identify those who benefit from better control of their lipid profile.
These guidelines have been developed for healthcare professionals to advise individuals about their CV risk and the benefits of adopting and maintaining a healthy lifestyle; to discuss early lipid-related CVR modification with patients on an individual basis, applying the guidelines' recommendations for patient management; which should enable more clinicians to efficiently and safely reduce CV risk through lipid modification.
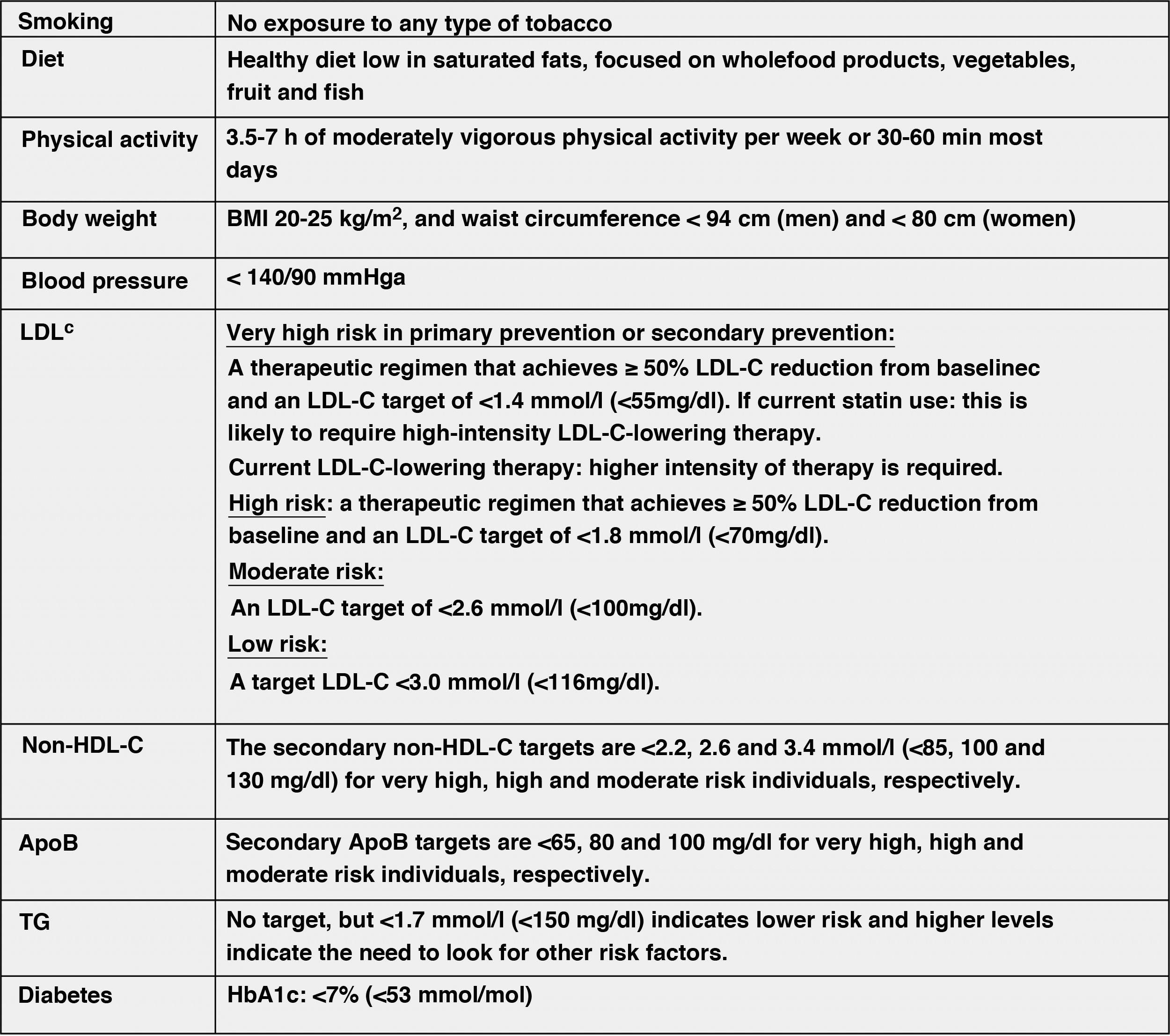
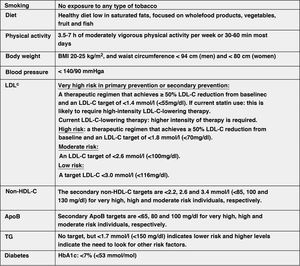
Modification of lifestyle and comprehensive control of all CVRFThe lifestyle modifications recommended in this new guideline to improve the plasma lipid profile for CV prevention, which are basically smoking cessation, adequate diet, regular physical activity, weight control, reduction of alcohol consumption, avoidance of stress and comprehensive control of all CVRFs, have been made taking into account the latest evidence available in this field, from which it establishes a series of targets for CVD prevention (Fig. 8).
Comprehensive treatment targets for the prevention of CVD.
Apo, apolipoprotein; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HbA1c, glycosylated haemoglobin; non-HDL-C, non-HDL cholesterol;
aLower treatment targets are recommended for most treated hypertensive patients, provided that treatment is well tolerated.
bThe term "baseline" refers to the LDL-C level in a person not taking any lipid-lowering medication, or the extrapolated reference value for those on current treatment.
Modified from Mach et al.49
The lifestyle interventions to reduce TC and LDL-C levels with the highest level of evidence and greatest impact are avoiding trans fats in the diet, reducing saturated fats in the diet, increasing dietary fibre, consuming functional foods enriched with phytosterols, consuming red yeast rice nutraceuticals, reducing excess body weight, reducing dietary cholesterol and increasing regular physical activity.49
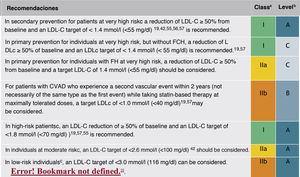
Pharmacological treatment to reduce LDL-CIn addition to the lifestyle modification and recommendation of healthy habits described above, initiation of pharmacological treatment is recommended in the situations described in Fig. 9 for each CVR level.
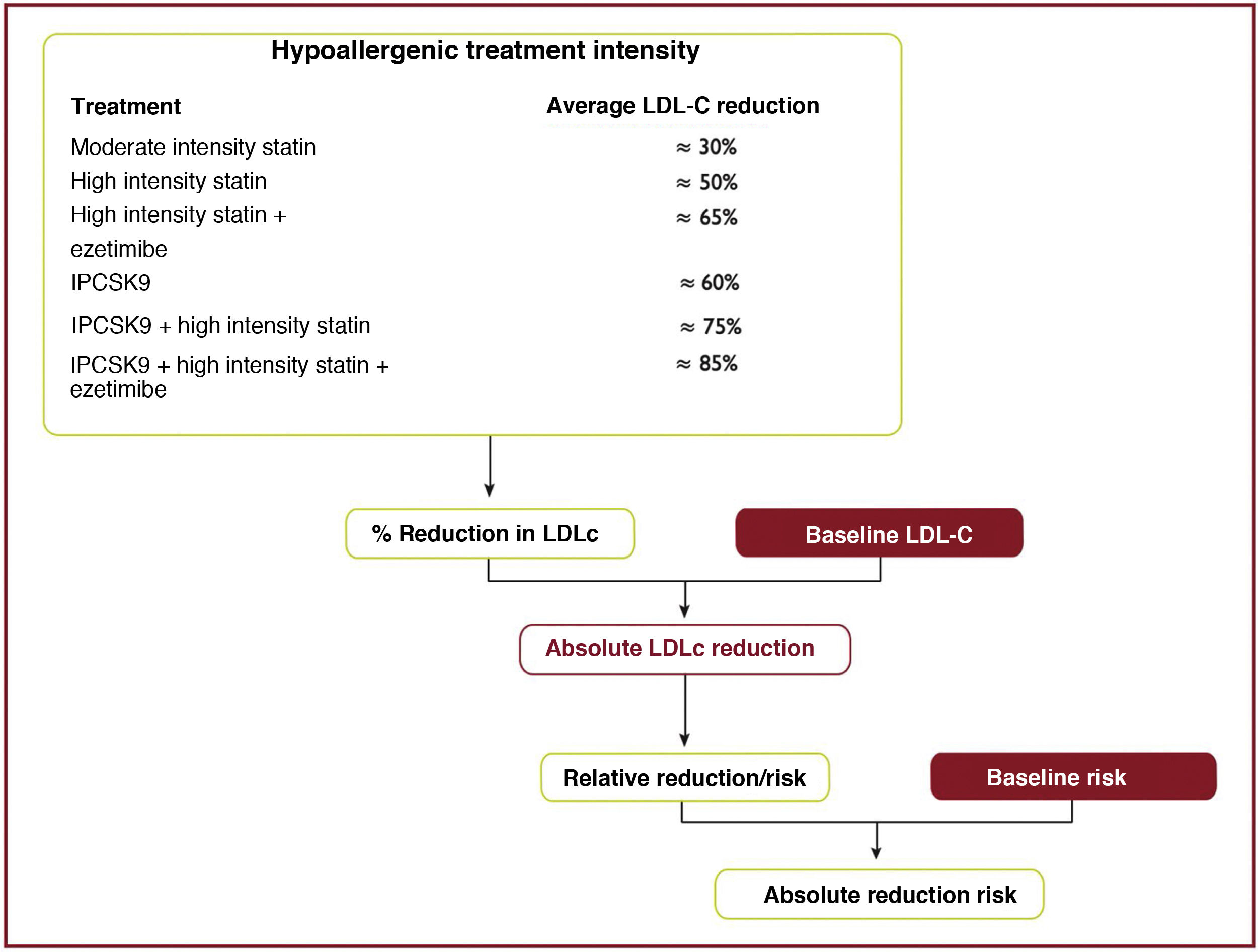
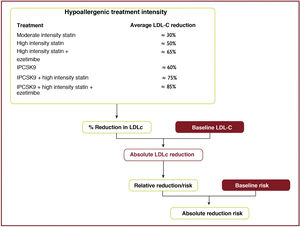
The expected clinical benefits of LDL-C-lowering treatment can be estimated for any individual49 (Fig. 10); it depends on the intensity of therapy, the baseline LDL-C level, the expected absolute reduction in LDL-C achieved and the estimated risk of atherosclerotic CV disease.
Expected clinical benefits of low-density lipoprotein cholesterol-lowering therapies.
LDL-C, low-density lipoprotein cholesterol; iPCSK9, inhibitor of proprotein convertase subtilisin/kexin type 9.
Modified from Mach et al.49
The intensity of therapy should be selected to achieve the recommended proportional reduction in LDL-C based on the individual's estimated risk of atherosclerotic CVD. Multiplying the proportional reduction in LDL-C by a person's baseline LDL-C level estimates the expected absolute reduction in LDL-C cholesterol likely to be achieved by that therapy. Because each 1.0mmol/l (40mg/dl) absolute reduction in LDL-C is associated with a 20% reduction in the risk of CV events, larger absolute reductions in LDL-C lead to larger proportional reductions in risk.
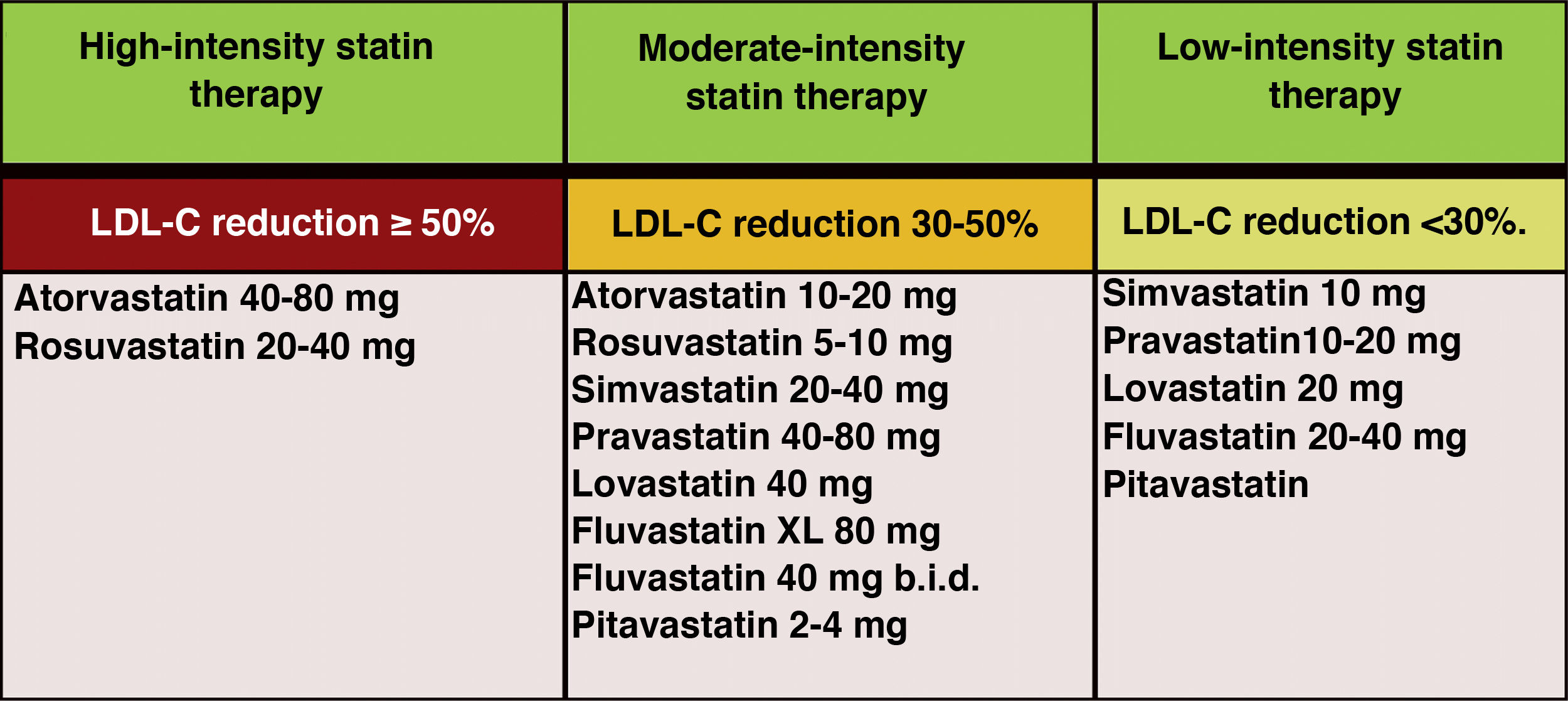
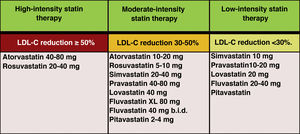
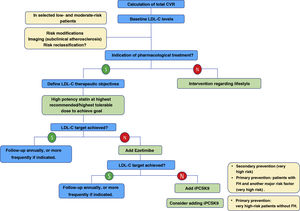
In the new ESC/EAS 2019 guidelines49 after calculating the total CVR in an individual and knowing the baseline LDL-C levels, control targets are determined, the patient is involved in decisions about the management of their CVR, and a statin treatment regimen is chosen with the intensity necessary to achieve the control targets (Fig. 11). As the response to statins is variable, it may be necessary to increase the dose of statins before prescribing additional drugs for more intensive LDL-C lowering. If the target is not met with the maximum tolerated dose of statins, ezetimibe should be administered.
Intensity of lipid-lowering statin therapy.
Modified from Stone et al.60.
For very high-risk primary prevention patients without FH, if targets are not met with the maximum tolerated dose of statins and ezetimibe, iPCSK9 could be considered.
For patients with very high-risk FH (with a history of vascular disease or associated major risk factors) and for patients in secondary prevention, if targets are not achieved with the maximum tolerated dose of statins and ezetimibe, it is recommended that iPCSK9 be added to treatment to achieve treatment targets (percentage and absolute value).
If a statin-based regimen is not tolerated (any dose), ezetimibe or iPCSK9 should be considered. If the target is not met, a statin-resin combination could be considered.
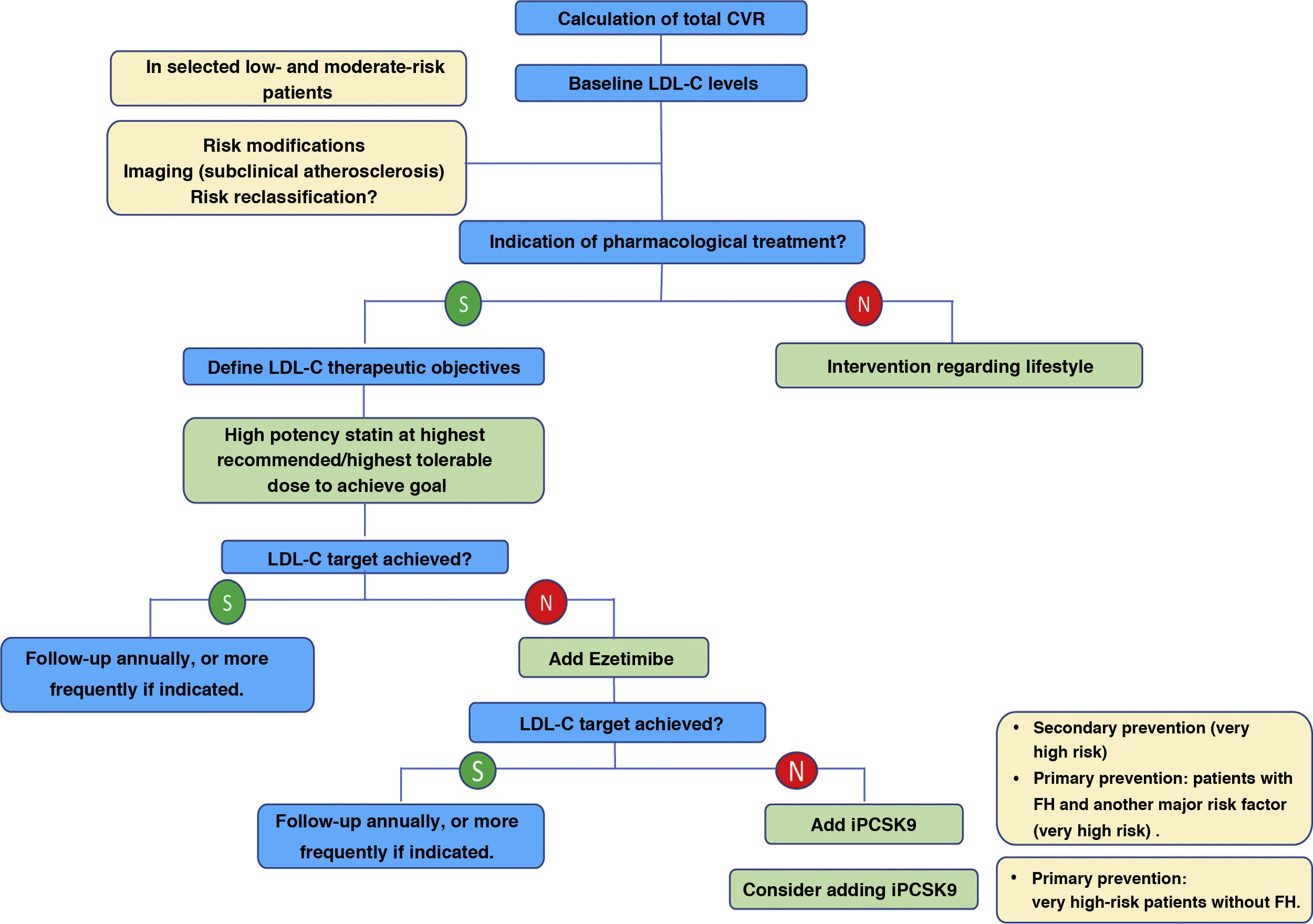
Overall, the new ESC/EAS 2019 guidelines49 reinforce the importance of reducing lipid levels rapidly and intensively in patients with high CVR and highlight the role of iPCSKs9 in achieving this, for which the algorithm shown in Fig. 12 is proposed. The recommendations of the latest guidelines represent a major advance in lipid-lowering therapy, moving from the concept of using high-intensity statins to the use of high-intensity lipid-lowering therapy,61 which includes, in addition to statins, ezetimibe and iPCSK9.
Treatment algorithm for pharmacological LDL-C lowering.
LDL-C: low-denpsity lipoprotein cholesterol; iPCSK9: inhibitor of proprotein convertase subtilisin/kexin proprotein convertase type 9; CVR: cardiovascular risk.
Modified from Mach et al.49
The implementation of clinical practice guidelines is of vital importance, with the discordance between recommendations and current clinical practice being disappointing at present due to the low percentage of LDL-C targets being achieved.62
For this reason, all guidelines insist on the importance of reaching a consensus with the patient on treatment and targets, in which LDL-C plays a fundamental role. Prioritising above all those at very high risk, with powerful and intensive treatment, taking into account the comorbidities of the individual patient.
The ACC/AHA Guidelines63 published in 2018 made a series of recommendations whose main messages to reduce the risk of CVD through cholesterol control, are similar recommendations to the ESC/EAS Guidelines 2019,49 but much less aggressive in the high and very high CV risk levels:
- 1
A heart-healthy lifestyle needs to be recommended for all people throughout the life course.
- 2
In patients with clinical CVD, LDL-C should be reduced by treating with high-intensity statins or statin therapy at the maximum tolerated dose to reduce LDL-C levels by ≥50%.
- 3
In very high-risk CVD, use a LDL-C threshold of 70mg/dl to consider the addition of non-statin drugs to statin therapy. In very high-risk patients whose LDL-C level remains ≥70mg/dl with statins at the maximum tolerated dose and ezetimibe, adding a PCSK9 inhibitor is reasonable.
- 4
In patients with severe primary hypercholesterolaemia (LDL-C level ≥190mg/dl), without calculating the 10-year CVD risk, start high-intensity statin therapy. If the LDL-C level remains ≥100mg/dl it is reasonable to add ezetimibe. If the LDL-C level on statin plus ezetimibe remains ≥100mg/dl and the patient has multiple factors that increase subsequent risk of CVD events, iPCSK9 may be considered.
- 5
In patients aged 40–75 years with DM and LDL-C ≥70mg/dl, it is advised to start treatment with moderate-intensity statins without calculating the 10-year CVD risk. In patients with DM at higher risk, especially those with multiple risk factors or those aged 50–75 years, it is reasonable to use a high-intensity statin to reduce the LDL-C level by ≥50%.
- 6
In adults aged 40–75 years in primary prevention of CVD, patients need to be counselled about the potential benefits of lifestyle modification therapies and statins; the potential for adverse effects and drug interactions; consideration of the costs of statin therapy; and patient preferences and values in shared decision-making.
- 7
In adults aged 40–75 years without DM and with LDL-C levels ≥70mg/dl, with a 10-year CVD risk of ≥7.5%, it is reasonable to start with a moderate-intensity statin. If risk status is uncertain, consider coronary artery calcium (CAC) to improve specificity. If statins are indicated, reduce LDL-C levels by ≥30%, and if 10-year risk is ≥20%, reduce LDL-C levels by ≥50%.
- 8
In adults aged 40–75 years without DM and 10-year risk of 7.5%–19.9% (intermediate risk), factors that increase risk favour initiation of statin therapy. These factors include: family history of premature CVD; persistently elevated LDL-C levels ≥160mg/dl; metabolic syndrome; CKD; history of pre-eclampsia or premature menopause (age <40 years); chronic inflammatory disorders (eg, rheumatoid arthritis, psoriasis or chronic human immunodeficiency virus); high-risk ethnic groups (eg, South Asian); persistent elevations of TG≥175mg/dl and, if measured in selected individuals, ApoB ≥130mg/d, high-sensitivity C-reactive protein ≥2.0mg/l, ankle-brachial index <0.9 and Lp(a) ≥50mg/dl or 125nmol/l, especially at higher Lp(a) values. Factors that increase risk may favour statin therapy in patients with a 10-year risk of 5–7.5 % (borderline risk).
- 9
In adults aged 40–75 years without DM and with LDL-C levels ≥70mg/dl to 189mg/dl (≥1.8–4.9mmol/l), with a 10-year CVD risk ≥7.5%–19.9%, if the decision about statin treatment is uncertain, consider measuring CAC. If CAC is zero, statin treatment can be stopped or delayed, except in smokers, those with DM and those with a strong family history of premature CVD. A CAC score of 1–99 favours statin therapy, especially in those ≥55 years of age. For any patient, if the CAC score is ≥100 Agatston units or ≥75th percentile, statin therapy is indicated unless clinical-patient risk negotiation suggests otherwise.
- 10
Assess compliance and percentage response to LDL-C lowering medications and lifestyle changes with repeated lipid measurement 4–12 weeks after statin initiation or dose adjustment, repeat every 3–12 months as needed. Define responses to lifestyle and statin therapy by percentage reductions in LDL-C levels compared to baseline. In very high-risk CVD patients, criteria for associating non-statin drug therapy are defined by threshold levels of LDL-C ≥70mg/dl in patients on maximum-dose statin therapy.
The authors have no conflict of interests to declare.