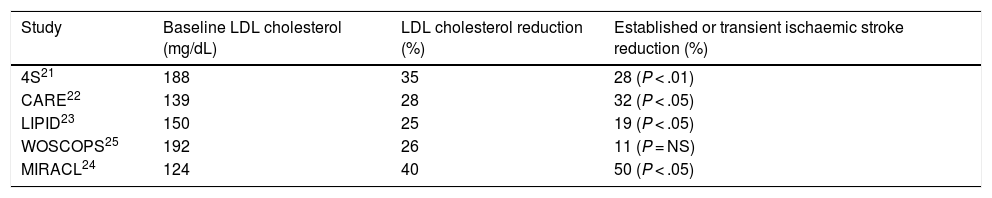Stroke is the second cause of death after myocardial infarction, and the main cause of acquired disability. Patients with ischaemic stroke have a higher risk of future vascular events, including recurrent stroke, myocardial infarction, and death by vascular cause. The initial epidemiological studies demonstrated a weak or non-existent relationship between cholesterolaemia and stroke. Subsequently, statin intervention trials showed a reduction in the risk of recurrence of cerebrovascular events. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL), the first clinical trial designed to assess effects of statin therapy in secondary stroke prevention, highlighted the reduction of stroke recurrence with atorvastatin 80 mg/daily in patients with a recent ischaemic established or transient stroke, with a modest increase in the rate of haemorrhagic stroke. Successive studies have also reported the benefits of statin therapy combined with ezetimibe or PCSK9 inhibitors in primary and secondary ischaemic stroke prevention. Since 80% of recurrent cerebrovascular events could be prevented, it is considered of interest to carry out a narrative review of the benefits of lipid-lowering therapy in the secondary prevention of ischaemic cerebrovascular disease.
El accidente cerebrovascular es la segunda causa de mortalidad después del infarto de miocardio y la principal causa de discapacidad adquirida. Los pacientes con ictus isquémico tienen un elevado riesgo de posteriores episodios vasculares, incluyendo ictus recurrente, infarto de miocardio y muerte de causa vascular. Los primeros estudios epidemiológicos mostraron una relación débil o inexistente entre la colesterolemia y el ictus. Posteriormente, los estudios de intervención con estatinas revelaron una reducción del riesgo de recurrencia de episodios cerebrovasculares. El Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL), primer ensayo clínico diseñado para analizar los efectos de la terapia con estatinas en la prevención secundaria del ictus, demostró que el tratamiento con atorvastatina 80 mg/día reducía la recurrencia de ictus en pacientes con un accidente cerebrovascular isquémico reciente establecido o transitorio, con un modesto aumento en la tasa de ictus hemorrágico. Estudios posteriores han recabado los beneficios de la terapia de estatinas, con ezetimiba o inhibidores de PCSK9 tanto en la prevención primaria como secundaria del accidente cerebrovascular isquémico. Dado que el 80% de los episodios cerebrovasculares recurrentes pueden prevenirse hemos considerado de interés realizar una revisión narrativa de los beneficios de la terapia hipolipemiante en la prevención secundaria de la enfermedad cerebrovascular isquémica.
Data from the World Health Organisation 20161,2 confirm that stroke is the second cause of death after myocardial infarction. It is worth noting that, according to the National Institute of Statistics (INE) 2017, it is already the leading of cause of death in women in Spain.3 The Global Burden of Disease Study 20164 estimated the incidence of stroke and risk of death from all causes excluding stroke to calculate the lifetime risk of a first ischaemic or haemorrhagic stroke in adults aged 25 or more. The risk of ischaemic stroke was 18.3 percent and the risk of haemorrhagic stroke was 8.2 percent, with significant geographical variation.
Patients with ischaemic stroke have a high risk of subsequent vascular events, including recurrent stroke, myocardial infarction and death from vascular causes.5 In this regard, patients with a history of transient ischaemic attack or minor stroke were described to present a sustained risk of cardiovascular events during a 5-year follow-up period, half of which occurred between the second and the fifth year.6 In fact, the risk for the primary objective comprising stroke, acute coronary syndrome or cardiovascular death was 12.9% and for isolated stroke it was 9.5%, both at 5 years, approximately double the rates per year of 6.2% and 5.1%, respectively.
Although the vascular risk factors are the same for any site of atherosclerotic disease, the impact of each is different according to the arterial territory affected. Traditionally, and unlike coronary heart disease, the relationship between plasma cholesterol and stroke has been weak or non-existent, with high blood pressure being the main risk factor for stroke7 (Fig. 1). However, more recent clinical evidence has shown an increased risk of cerebrovascular disease with high levels of cholesterol linked to low density lipoproteins (LDL), as well as a decrease in this risk with lipid lowering treatment.8,9 Along these lines, the meta-analysis by Amarenco and Labreuche10 described that each mmol/L decrease in LDL cholesterol was accompanied by a reduction in the relative risk (RRR) of stroke of 21.1% (95% confidence interval [CI]: 6.3–33.5; P = .009).
Together with mortality data, we should not forget that cerebrovascular disease is the leading cause of acquired disability,11 and therefore has substantial socioeconomic and health repercussions. However, it is important to note that up to at least 80% of recurrent cerebrovascular events could be prevented with appropriate therapeutic measures. For this reason, we considered it appropriate to conduct a narrative review of the impact of lipid-lowering therapy on secondary prevention of ischaemic stroke.
Hypercholesterolaemia, cerebrovascular disease and mortalityOne of the first epidemiological studies to analyse the relationship between cholesterolaemia and cardiovascular mortality was published in 1989 by Iso et al.12 in the context of the Multiple Risk Factor Intervention Trial (MRFIT) involving 350,977 men aged 35–57 years, with no previous history of coronary heart disease. The risk of death at 6 years of follow-up due to intracranial haemorrhage was 3 times higher in men with total cholesterol levels <160 mg/dL than in those with higher levels (P = .05). The inverse relationship between cholesterol levels and risk of death from haemorrhagic stroke was confined to patients with diastolic blood pressure ≥90 mmHg. As for non-haemorrhagic stroke, a positive and significant association was observed between cholesterol levels and risk of death from stroke.
Along the same lines, the findings of the Prospective Studies Collaboration (PSC) of 199513 are worth noting, which included 45 prospective cohorts comprising 450,000 individuals with 13,387 recorded strokes and 16 years of follow-up. After adjusting for education level, age, sex, diastolic blood pressure, presence of coronary heart disease and ethnicity, no association between cholesterolaemia and stroke risk was documented. However, it should be noted that in this study the results were not categorised according to different types of stroke (ischaemic or haemorrhagic), unlike the MRFIT study.12
Lipid-lowering treatment and reduction of risk of cerebrovascular diseaseIn contrast to the findings of epidemiological studies, the clinical evidence for the favourable effect of LDL cholesterol reduction in ischaemic cerebrovascular disease is more conclusive. The role of lipid lowering therapy in the secondary prevention of ischaemic stroke before and after the statin era will be described below.
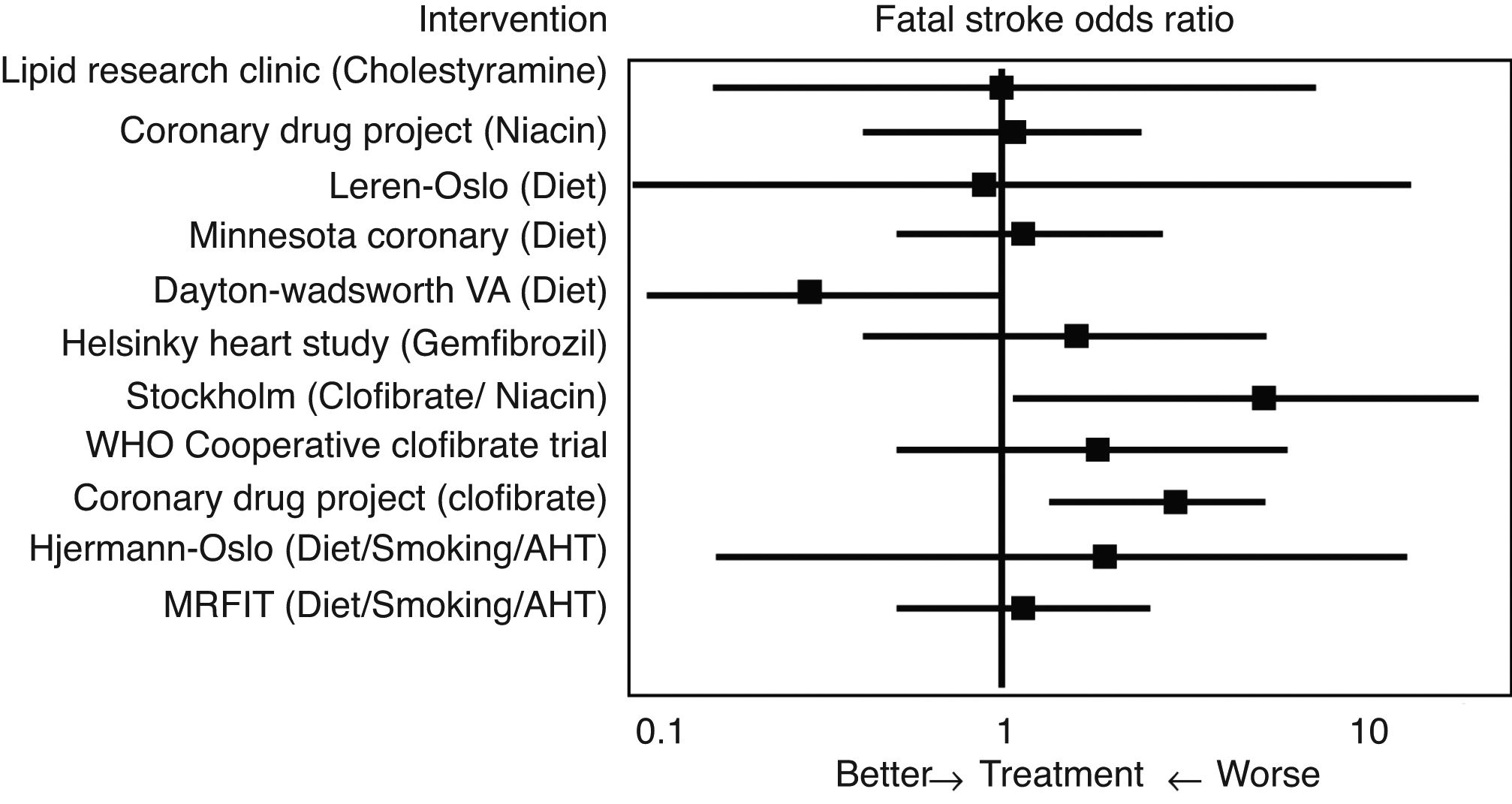
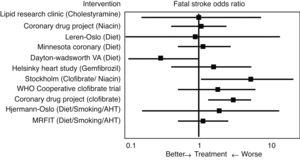
Initial studies of the “pre-statin” eraIn 1993 Atkins et al.14 conducted one of the first meta-analyses to evaluate the effect of lipid lowering treatment prior to the introduction of statins, including dietary hygiene measures, cholestyramine, niacin or clofibrate, among others, on the risk of cerebrovascular disease (Fig. 2). With reference to fatal stroke, the overall odds ratio (OR) associated with therapeutic strategies to reduce plasma cholesterol levels was 1.32 (95% CI .94–1.86), and the odds ratio (OR) for the 10 single intervention trials was 1.34 (CI .91–1.96). Among the eight studies that included non-fatal episodes, the likelihood rate of non-fatal stroke for participants in the active treatment arm compared to controls was 0.88 (CI .70–1.11), and the likelihood rate of total stroke was 0.98 (CI .80–1.19). In the three clofibrate trials, this fibrate significantly increased the risk of fatal stroke (OR 2.64, CI 1.42–4.92) but not non-fatal stroke (OR 0.87, CI .61–1.26). Finally, logistic regression analysis did not reveal a significant association between the magnitude of cholesterol reduction and the risk of fatal stroke. Therefore, the main conclusion of this meta-analysis was that lowering cholesterol levels with hygiene-dietary measures or with lipid lowering drug treatment other than statins did not decrease cerebrovascular disease-related morbidity and mortality in middle-aged males.
Intervention studies with statinsSince the introduction of lovastatin in 1987 as the first 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitor approved for use in humans,15 statins have become the most widely used lipid-lowering drugs, with proven efficacy in cardiovascular prevention in all age groups,16–19 mainly by reducing LDL cholesterol levels. In the following years, different studies have assessed the role of statins, and their consequent lowering of cholesterol, with the risk of a cerebrovascular event, showing much more promising results than those obtained in the "pre-statin" era. In fact, statin therapy has been considered one of the most important advances in stroke prevention since the advent of aspirin or antihypertensive treatment.20
Table 1 shows the main studies that have assessed the possible relationship between LDL cholesterol reduction with statins and the risk of stroke or transient ischaemic attack. In most secondary prevention of cardiovascular disease studies, such as the Simvastatin Survival Study Group (4S),21 Cholesterol and Recurrent Event (CARE),22 and Long Term Intervention with Pravastatin in Ischemic Disease (LIPID),23 a significant reduction in stroke risk in patients with ischaemic heart disease was found, which reached 50% in the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study24 in patients with recent acute coronary syndrome. In contrast, in the West of Scotland Coronary Prevention Study (WOSCOPS),25 a primary prevention study of cardiovascular disease, showed a non-significant 11% decrease in the risk of stroke during the 5 years of follow-up.
Established or transient ischaemic stroke reduction in the main statin intervention studies.
| Study | Baseline LDL cholesterol (mg/dL) | LDL cholesterol reduction (%) | Established or transient ischaemic stroke reduction (%) |
|---|---|---|---|
| 4S21 | 188 | 35 | 28 (P < .01) |
| CARE22 | 139 | 28 | 32 (P < .05) |
| LIPID23 | 150 | 25 | 19 (P < .05) |
| WOSCOPS25 | 192 | 26 | 11 (P = NS) |
| MIRACL24 | 124 | 40 | 50 (P < .05) |
LDL: low density lipoproteins; NS: not significant.
Focussing on secondary prevention of cerebrovascular disease with statins, the Heart Protection Study (HPS)26 was the first study to assess the effect of simvastatin treatment on secondary prevention of stroke in patients with previous cerebrovascular disease. A total of 20,536 patients at high risk of vascular events were included with a 5-year follow-up. Treatment with simvastatin significantly reduced the risk of vascular events (RRR of 24%, P < .00001) and stroke (RRR of 27%, P < .00001). This same study included 3280 randomly selected stroke patients (none with transient ischaemic attack) and 1822 stroke patients without established coronary heart disease. The RRR of serious cardiovascular events was 19% in all the stroke patients, which rose to 23% in the stroke patients without coronary heart disease. However, this study did not show a reduction in the risk of stroke among the patients with recurrent stroke (10.4% of patients in the statin group had recurrent stroke compared to 10.5% of the patients in the placebo group). Therefore, in the patients with a previous stroke, statins probably reduced the incidence of coronary events, but there was no evidence that statins also reduced the incidence of recurrent stroke.
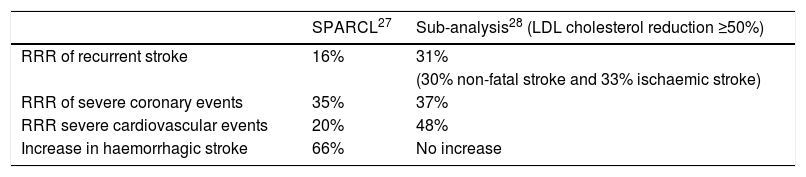
Stroke prevention by aggressive reduction of cholesterol levels (SPARCL)As a result of the findings of the MIRACL study,24 the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL)27 study was conducted, the first clinical trial designed to confirm the benefits of statin therapy in the secondary prevention of stroke. We included 4731 patients >18 with a history of established or transient ischaemic stroke in the previous 1–6 months and who did not have hypercholesterolaemia or coronary heart disease. Most were ischaemic strokes, and some were haemorrhagic. The patients received either atorvastatin 80 mg/day (n = 2365) or placebo (n = 2366) and were followed for almost 5 years. The primary objective of the study was to assess the incidence of fatal and non-fatal stroke. A 16% RRR was observed in recurrent stroke, a 35% RRR in severe coronary events and a 20% RRR in severe cardiovascular events. The superiority of atorvastatin over placebo was mainly in the patients with fatal stroke, who experienced a 43% reduction (P = .03). It would be unfair not to mention here the 66% increase in the relative risk of haemorrhagic stroke in the patients who were randomised to atorvastatin.
On careful analysis of the results of the SPARCL study, there are certain aspects worth noting. Twenty-five percent of the patients who were assigned to the placebo arm ended up receiving statin treatment at some point in the study. Therefore, it could be argued that the results obtained underestimate the true clinical impact of high-dose statin therapy, given that atorvastatin was compared to a control group where one in four patients also received the same treatment. Therefore, one might ask whether the SPARCL findings underestimate the real effect of intensive statin therapy, or whether they underestimate the increased risk of intracerebral haemorrhage with statin therapy.
In order to answer these questions, and address the issue of the actual benefits and risks of statin therapy in stroke patients, Amarenco et al.28 re-analysed the data, but this time reclassified the patients not according to the assigned treatment arm, but according to the level of LDL cholesterol reduction. This was possible because the methodological aspects of the study included 4 serial tests per patient and year. The patients were classified into 3 categories: no change in their LDL cholesterol, a <50% decrease or a ≥50% decrease in LDL cholesterol, hypothesising that the latter group would indicate the true biological effect of intense LDL cholesterol reduction in recent stroke patients. As expected, most patients with a dramatic decrease in LDL cholesterol (≥50%) were in the atorvastatin group, although not all participants in the atorvastatin group had such a marked therapeutic response. Approximately one-third of them had a <50% decrease in LDL cholesterol, and the study confirms that there were some people in the placebo group who took statins when an intense reduction in LDL cholesterol was noted. Comparing the patients with a reduction in LDL cholesterol levels ≥50%, it was found that instead of an RRR of 16% for recurrent stroke, an RRR of 31% was achieved (for any subtype). There were also reductions in the risk of serious coronary events (RRR of 37%), and an RRR of 48% in the need for any revascularisation procedure. The main results of the SPARCL27 study as well as the subsequent subanalysis28 stratified by reduced LDL cholesterol levels are detailed in Table 2.
SPARCL study results and subsequent sub-analysis.
| SPARCL27 | Sub-analysis28 (LDL cholesterol reduction ≥50%) | |
|---|---|---|
| RRR of recurrent stroke | 16% | 31% |
| (30% non-fatal stroke and 33% ischaemic stroke) | ||
| RRR of severe coronary events | 35% | 37% |
| RRR severe cardiovascular events | 20% | 48% |
| Increase in haemorrhagic stroke | 66% | No increase |
LDL: low density lipoproteins; RRR: relative risk reduction.
The second question in analysing the results of the SPARCL27 study was whether the final results underestimated the increased risk of intracerebral haemorrhage from statins. In this regard, it should be noted that in the SPARCL28 sub-analysis described in the above section, no increase in haemorrhagic stroke was observed in the group with reduced cholesterol levels LDL > 50%, despite the fact that the atorvastatin group in the original analysis27 presented an increased risk of haemorrhagic stroke.
These results are consistent with those subsequently described by Goldstein et al.29 in 2008, where risk factors for intracerebral haemorrhage were analysed in the SPARCL study. In this case, male sex (hazard ratio [HR]: 2.21, 95% CI: 1.20–4.09; P = .01), age (HR: 1.40, 95% CI: 1.08–1.81; P = .01) and intracerebral haemorrhage as the form of presentation (HR: 8.38, 95% CI: 3.78–18.56; P < .001), in addition to poor blood pressure control, were the factors associated with the risk of a haemorrhagic event. Notably, no quartile of LDL cholesterol was associated with an increased risk of intracerebral haemorrhage. More recently, Gaist et al.30 confirmed that statin use was not associated with an increased risk of intracranial haemorrhage in patients with a history of established or transient ischaemic stroke.
In a recent Mendelian randomisation study,31 each mmol/L of genetically engineered LDL cholesterol was associated with RRRs of 0.75 (95% CI .60–.95) for ischaemic stroke and 1.13 (95% CI .91–1.40) for intracranial haemorrhage. In the same study, analysis of pharmacological reduction in LDL cholesterol confirmed that each mmol/L decrease was accompanied by an RRR of 0.80 (95% CI: 0.76−0.84) for ischaemic stroke and 1.17 (95% CI: 1.03–1.32) for intracerebral haemorrhage.
In short, the available evidence has not been able to demonstrate a real increased risk of intracranial haemorrhage secondary to statin therapy.
Combined treatmentThe current joint guideline 2019 of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS)32 for the management of dyslipidaemia recommends with a degree of evidence A intensive lipid lowering therapy to reduce LDL cholesterol levels in established or transient ischaemic stroke patients. Although the therapeutic goals for LDL cholesterol can be achieved with statins in monotherapy in a substantial number of cases, a significant proportion of patients at high/very high risk or with elevated LDL cholesterol levels require additional drug therapy. In this clinical situation, where despite statin therapy at the maximum tolerated dose the therapeutic goal is not achieved, the combination with ezetimibe is recommended, and if still not achieved, the addition of a PCSK9 inhibitor is recommended.
With regard to combination treatment with statins and ezetimibe, the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT)33 provided clear evidence that reinforces the importance of reducing LDL cholesterol in cardiovascular prevention. In this regard, it should be stressed that the additional reduction in LDL cholesterol achieved with ezetimibe is of the same quality in terms of cardiovascular risk reduction, as that obtained with statins in monotherapy. Each mmol/L (38.7 mg/dL) reduction in LDL cholesterol obtained with statins in monotherapy or with the statin plus ezetimibe combination is associated with an approximate reduction in the RR of cardiovascular disease of 20%, findings which are in fully in line with those of the Cholesterol Treatment Trialist Collaboration.17 In a complementary analysis of IMPROVE-IT, Bohula et al.34 showed that simvastatin and ezetimibe combination therapy in patients stabilised after an acute coronary syndrome reduced the frequency of ischaemic stroke, especially in patients with previous stroke.
Finally, there is recent evidence regarding the beneficial effects of treatment with PCSK9 inhibitors in cardiovascular prevention. The publication of the results of Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER)35 with evolocumab and ODYSSEY Outcomes: Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment with Alirocumab36 support the theory that the lower the LDL cholesterol the better in patients at high/very high cardiovascular risk, and together with IMPROVE-IT33 point to the use of high-intensity lipid-lowering therapies.37 These two studies were included in a recent systematic review and meta-analysis by Guedeney et al.38 which documented an RRR of ischaemic stroke of 22% with treatment with PCSK9 inhibitors.
The challengesDespite the availability of effective and potent therapeutic tools, real-life clinical practice studies show that patients at high/very high cardiovascular risk are under-treated and under-controlled. For example, the EUROASPIRE V registry39 conducted in 27 countries with patients in secondary prevention found that 71% had LDL cholesterol ≥1.8 mmol/L (70 mg/dL) despite the fact that just over 80% of the patients were treated with statins. This highlights that undertreatment is one of the main barriers to overcome towards improving therapeutic performance. Almost contemporaneously, similar results were described in the PALM registry.40 Less use and intensity of statins was observed in subgroup analyses by age and LDL cholesterol levels, although differences were not statistically significant in older patients and those with LDL ≥ 100 mg/dL. On the other hand, no statistically significant differences in statin use and intensity of statin therapy were observed among patients with coronary artery disease and stroke versus coronary artery disease alone. In this same study, the median (p25–p75) LDL cholesterol levels were 90 mg/dL (73–114), 88 mg/dL (69–111) and 83 mg/dL (66–107) for patients with cerebrovascular disease alone, coronary artery disease and cerebrovascular disease, or coronary artery disease alone, respectively (P < .001). It should be noted that only 59.2% of patients with cerebrovascular disease presented LDL cholesterol levels <100 mg/dL, and therefore, most of the patients did not achieve the therapeutic goals.
Advances in analytical magnetic resonance imaging and mass spectrometry platforms have made substantial contributions to the field of metabolomics and lipidomics in general.41 These emerging technologies have become increasingly sophisticated through the development of new statistical methods, bioinformatic tools and database resources. More recently, systems biology approaches have been applied to decipher biological and clinical complexities with the emergence of computational and mathematical models. Thus, the inclusion of metabolomics and lipidomics, along with other "omics", is possible.42 Furthermore, fluxomics is a relatively new and ever-expanding field that characterises the dynamic metabolic profile of the cell phenotype and involves a more comprehensive assessment of complex metabolic networks related to disease. Fluxoma, the set of metabolic flows in a metabolic system, is a direct manifestation of the metabolic phenotype, and therefore is key to understanding any disease with a strong metabolic component. However, more research is needed to unravel the causal mechanisms of ischaemic stroke and pave the way for new drug treatments to help us achieve the therapeutic goals set for our patients.
ConclusionsCerebrovascular disease is one of the main causes of mortality in our population, with hypercholesterolaemia being one of the modifiable risk factors described. Previous studies have shown that lipid-lowering treatment, both with statins and in combination with ezetimibe or PCSK9 inhibitors, are effective in secondary prevention of ischaemic stroke, by lowering LDL cholesterol levels. However, the main barrier in clinical practice remains the failure to achieve therapeutic goals in LDL cholesterol, the result of undertreatment. It has been documented that prior therapeutic planning43 using updated Masana and Plana tables44 and the use of computerised tools included in the clinical history45 significantly improve the rate that therapeutic goals are met and thus achieve effective vascular prevention.
FundingThis research study received no specific subsidies from public sector agencies, the commercial sector or non-profit organisations.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Climent E, Benaiges D, Pedro-Botet J. Tratamiento hipolipemiante en la prevención secundaria de la enfermedad cerebrovascular isquémica. Clin Investig Arterioscler. 2020;32:175–182.