In general, both European and American clinical guidelines have addressed the management of atherogenic dyslipidaemia in an unconvincing and even superficial way, largely because of the available therapeutic limitations. Consequently, this type of dyslipidaemia is underdiagnosed, under-treated, and under-controlled. Given the recent presentation of the 2019 guidelines of the European Atherosclerosis Society and the European Society of Cardiology on the management of dyslipidaemias, it seems appropriate to examine its position with respect to atherogenic dyslipidaemia and/or its main components, the increase in triglyceride-rich lipoproteins, and the decrease of high-density lipoprotein cholesterol.
En general, las guías de práctica clínica tanto europeas con americanas han abordado el control de la dislipidemia aterogénica de forma poco convincente e incluso superficial, en gran medida por las limitaciones terapéuticas disponibles. En consecuencia, esta dislipidemia está infradiagnosticada, infratratada e infracontrolada. Dada la reciente aparición de la guía 2019 de la European Atherosclerosis Society y de la European Society of Cardiology sobre el control de las dislipidemias, parece oportuno examinar su posicionamiento con respecto a la dislipidemia aterogénica y/o sus principales componentes, el aumento en las lipoproteínas ricas en triglicéridos y la disminución del colesterol de las lipoproteínas de alta densidad.
The main components of atherogenic dyslipidaemia (AD) are clearly identified, and they are easy to recognise; nevertheless, this type of dyslipidaemia is under-diagnosed and under-treated, as a result of which it is under-controlled.1 The EDICONDIS-ULISEA2 study found that one of every 6 patients treated in lipid and vascular risk units in our country had AD. It is possible that this prevalence is under-estimated, as more than one third of patients had a fall in the concentration of high density lipoprotein cholesterol (HDL) or an increase in triglycerides, and that therefore many of them will have the complete lipid phenotype at another moment of development. More recently, in the EURIKA study of a European population without cardiovascular disease, at least 50 years old and with one or more risk factors,3 approximately 21% had triglyceridaemia ≥ 2.3 mmol/L (200 mg/dL), 22% had low levels of HDL cholesterol (<1 mmol/L [40 mg/dL] in men and <1.3 mmol/L [50 mg/dL] in women), and almost 10% had both lipid alterations. It also revealed that about 55% of patients with high levels of triglycerides, low HDL cholesterol or both, were not receiving any type of hypolipidemiant treatment.
Clinical practice guides are now an essential instrument for our medical work. In 1990 the Institute of Medicine4 l described them as the “set of recommendations set out systematically, to help clinics and patients in the decision-making process, regarding which interventions are the most appropriate to resolve a clinical problem in specific medical circumstances”. They should therefore be considered complementary tools that can never replace clinical judgement. As an example of this, the American cardiovascular prevention guides of 20135 and the European ones of 20126 issue different recommendations based on practically the same scientific evidence. Respecting AD, clinical practice guides have covered this form of dyslipidaemia somewhat unconvincingly,7 and this is largely due to available therapeutic limitations.
Very recently the 2019 guide of the European Atherosclerosis Society (EAS) and the European Society of Cardiology (ESC) was published on the control of dyslipidaemia.8 It therefore seems opportune to examine where it stands respecting AD and/or its main components, the increase in triglyceride-rich lipoproteins and the fall in HDL cholesterol. To this end we performed an exhaustive search for the terms “atherogenic dyslipidaemia”, “apolipoprotein B”, “apolipoprotein B-containing lipoproteins”, “HDL cholesterol”, “non-HDL cholesterol”, “remnant cholesterol”, “triglycerides”, and “triglyceride-rich lipoproteins” in all of the sections of the said 2019 guide.
Lipids, lipoproteins and atherosclerosisIn agreement with the conjoint EAS/ESC 20169 recommendation, the 2019 guide offers additional data from observational studies, randomised clinical studies and Mendelian randomisation genetic studies. These clearly show the causal effect of cholesterol in the form of low density lipoproteins (LDL) in the development of cardiovascular disease with an atherosclerotic origin. However, it also underlines the importance of apolipoprotein (apo) B-containing lipoproteins in the physiopathology of atherosclerosis. The atherogenic capacity of triglyceride-rich lipoproteins is fundamentally due to 2 factors10: their apoproteic composition, especially apo C-III, and their size. There is therefore an inverse relationship between lipoprotein particle size and their capacity to pass through the endothelium and penetrate the arterial tunica intima, so that lipoproteins containing apo B with a diameter smaller than 70 nm, including small triglyceride-rich lipoproteins and their remnant particles are able to cross the endothelial barrier, especially in the presence of endothelial dysfunction, and they are retained in the arterial wall and lead to lipid deposition.11,12 In an elegant experimental study, Argmann et al.13 showed that very low density lipoproteins (VLDL) and oxidised VLDL are as powerful as oxidised LDL and LDL in increasing the accumulation of cholesterol esters in smooth muscle cells. We must not forget that, from a conceptual viewpoint, AD is defined as the imbalance between proatherogenic lipoproteins that contain apo B (contained in triglyceride-rich lipoproteins) and anti-atherogenic lipoproteins that contain apo A–I (which are contained in HDL).14
Although epidemiological data link low concentrations of HDL cholesterol with the risk of cardiovascular episodes,15 as the result of genetic studies that do not support the protective role of HDL cholesterol in humans,16 and pharmacological intervention trials designed to increase the concentration of HDL cholesterol, as these too showed no cardiovascular benefits,17–21 the guide does not consider low HDL cholesterol to be a therapeutic objective. On the other hand, it underlines the resurgence of triglyceride-rich lipoproteins and their remnant particles, for which triglyceride levels are the main biomarker.22,23 Triglyceride-rich lipoproteins and their remnants contain triglycerides and cholesterol, and their atherogenic power is probably the result of these lipoprotein particles becoming enriched in cholesterol. This is what is known as the “remnant cholesterol” which is estimated in clinical practice as total cholesterol minus the sum of LDL and HDL cholesterol.
It must be said that observational studies as well as those based on Mendelian randomisation support the causal role of remnant cholesterol transported by triglyceride-rich lipoproteins in cardiovascular disease, even independently of HDL cholesterol.24,25 Moreover, high postprandial levels of remnant cholesterol are associated with a higher risk of mortality due to all causes in primary26 as well as in secondary prevention.27 These findings emphasise the role of postprandial lipidaemia in atherogenesis, as during a normal day people are in a postprandial state for longer than they are fasting.28,29 On the other hand, remnant cholesterol atherogenicity is also associated with inflammation,30 as the Copenhagen studies show that plasma concentrations of C-reactive protein >2 mg/dL are generally accompanied by triglyceride concentrations ≥1.7 mmol/L (150 mg/dL).31
The studies which have analysed the impact of mutations in the genes involved in triglyceride-rich lipoprotein metabolism have consolidated their association with cardiovascular risk. Thus variants with loss of function in the genes that code apo A–V and lipoprotein lipase (LpL) are associated with lifelong higher plasma levels of triglycerides and a greater risk of coronary cardiac disease.32–35 Genetic polymorphisms with loss of function in APOC3 and ANGPTL4 are associated with lower levels of triglycerides in plasma from birth and reduced risk of coronary cardiac disease.36–39 A recent Mendelian randomisation study proved that LpL variants which lead to a fall in triglycerides and variants of the LDL receptor that lead to a fall in LDL cholesterol had the same effect on the risk of cardiovascular disease due to change of apo B unit, indicating that all of the lipoproteins which contain apo B have the same effect on cardiovascular risk.40 These studies indicate that the causal effect of triglyceride-rich lipoproteins and their remnants in cardiovascular disease is fundamentally determined by the circulating concentration of particles that contain apo B, rather than the content of triglycerides in itself.
Lipoprotein determinationGiven the causal role of apo B-containing lipoproteins at the start and during the progression of atherogenesis, the guide describes the direct measurement of apo B-containing atherogenic lipoprotein concentrations as “ideal” to estimate risk and guide therapeutic decisions. Due to the fact that all apo B-containing lipoproteins, including VLDL, remnant particles and LDL, have a single apo B molecule, quantifying this apoprotein directly estimates the total number of atherogenic particles in plasma.
In general, concentrations of LDL, non-HDL cholesterol and apo B show a high degree of correlation, and they supply very similar information about cardiovascular risk.41,42 Nevertheless, under certain circumstances, such as in subjects with hypertriglyceridaemia, diabetes, obesity or very low levels of LDL cholesterol, the calculated values or directly measured levels of LDL cholesterol may under-estimate the concentration of cholesterol transported by the LDL as well as, more importantly, under-estimating the total concentration of apo B-containing lipoproteins, thereby under-estimating the risk of cardiovascular disease. Due to this, in clinical situations, the guide recommends measuring apo B as well as non-HDL cholesterol as routine parts of the lipid profile to evaluate risk in patients with high levels of triglycerides. As apo B gives an accurate estimation of the total concentration of atherogenic particles under all circumstances, it is the measurement of choice to refine the estimation of cardiovascular risk even further.
On the other hand, it is recommended that HDL cholesterol be determined first to then estimate LDL cholesterol LDL using Friedewald’s formula43 and, secondly, to improve risk estimation by using systematic coronary risk estimation (SCORE).
Therapeutic lipid targetsThis guide centres therapeutic targets on the concentration of LDL cholesterol as the main aim of treatment, and it includes a higher level of demand on the basis of new clinical evidence. At the same time it recommends very active treatment for dyslipidaemia in patients at high or very high cardiovascular risk, including those with family hypercholesterolaemia and diabetes.
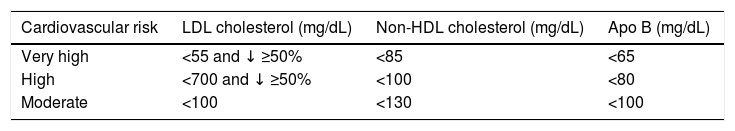
It also sets secondary objectives for non HDL cholesterol and in apo B for individuals at very high, high and moderate risk, as shown in Table 1. On the contrary, it does not set specific objectives for HDL cholesterol or triglycerides. For the latter it states that a concentration of <1.7 mmol/L (150 mg/dL) indicates a lower risk, while higher levels indicate the need to look for other risk factors.
Therapeutic objectives according to the 2019 European Guide for Dyslipidaemia Control.
| Cardiovascular risk | LDL cholesterol (mg/dL) | Non-HDL cholesterol (mg/dL) | Apo B (mg/dL) |
|---|---|---|---|
| Very high | <55 and ↓ ≥50% | <85 | <65 |
| High | <700 and ↓ ≥50% | <100 | <80 |
| Moderate | <100 | <130 | <100 |
Apo: Apolipoprotein; HDL: High density lipoprotein; LDL: Low density lipoprotein.
Source: Mach et al.8
The guide repeats that all of the measures which can improve sensitivity to insulin, such as weight loss and regular physical exercise, help to correct hypertriglyceridaemia and increase HDL cholesterol concentrations. As it should, it emphasises the negative impact of drinking alcohol on triglyceride levels, particularly in individuals with hypertriglyceridaemia. Additionally, it reminds us that the harmful effects of a diet high in carbon hydrates in cases of triglyceridaemia chiefly occur when foods rich in refined carbon hydrates are consumed, while they are far less outstanding when the diet is based mainly on fibre-rich low glycaemic index foods, as indicated for diabetes and metabolic syndrome.44 It also states that the habitual consumption of significant amounts (>10% of all energy) of fructose in the diet contributes to raising triglyceride levels, above all in individuals with hypertriglyceridaemia or abdominal obesity, and that these effects are dose-dependent. Regarding this it points out that saccharose, a disaccharide that contains glucose and fructose, is a major dietary source of fructose.
Lastly, it underlines the importance of ceasing to smoke, as this may contribute to raising HDL cholesterol, on condition that weight increase is avoided.
Hypolipimiant drugsIn this section we will only refer to the aspects included in the guide that cover the hypolipimiant drugs that are currently approved for use in Europe and their effect on the components of AD.
- 1)
Statins are well-known to reduce triglyceride concentrations by from 10%–20%; in practice the most powerful statins such as atorvastatin, rosuvastatin and pitavastatin usually have a greater reducing effect on triglycerides, especially at high doses and in patients with basal hypertriglyceridaemia. Their effect on levels of HDL cholesterol varies from an increase of from 1% to 10%, depending on the dose.
- 2)
The intestinal cholesterol absorption inhibitor, ezetimibe, gives rise to a significant increase of 3% in HDL cholesterol and a significant fall of 8% in triglyceridaemia in comparison with a placebo.
- 3)
Bile acid sequestrants have no effect on HDL cholesterol, and in some patients triglyceride levels may increase.
- 4)
PCSK9 inhibitors, as well as their powerful effect on LDL cholesterol, also reduce the levels of triglycerides by about 25%, and they increase levels of HDL cholesterol and apo A–I by 10% and 5%, respectively.
- 5)
In patients with homozygous familial hypercholesterolaemia (HFHo), Lomitapide, a microsomal triglyceride transfer protein inhibitor, reduces concentrations of total cholesterol, LDL, apo B and triglycerides with or without another hypolipimiant, including aphaeresis. In patients without HFHo with moderate hypercholesterolaemia and hypertriglyceridaemia, Lomitapide also has favourable effects on LDL cholesterol and triglycerides. However, patients with and without HFHo experience a fall in HDL cholesterol and apo A-1.
- 6)
Respecting the fibrates, these achieve a fall of up to 50% in the level of triglycerides and an increase in HDL cholesterol of ≤20%. The magnitude of their effect largely depends on the basal levels, and it is notably less (∼5% and ∼20%, respectively) in studies of long-term interventions in patients with type 2 diabetes without AD. Pemafibrate was launched recently, and it is a new selective PPAR-α modulator, which at relatively low doses is markedly effective in reducing triglyceride-rich lipoproteins.45 We will have to wait for the results of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study,46 which is a designed and controlled clinical study with cardiovascular objectives. It is evaluating the efficacy of Pemafibrate in approximately 10,000 diabetic patients with AD and high cardiovascular risk.
- 7)
Omega 3 fatty acids reduce triglyceride levels according to the dose used (2−4 g/day), with a marginal effect on the other lipoproteins. In the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT), using high doses of icosapent ethyl (4 g/day) in comparison with a placebo, found a significant 25% reduction in the relative risk of severe cardiovascular episodes.47 Two studies are currently in progress to determine whether reducing triglyceride-rich lipoproteins and their remnants in patients treated with statins brings about an additional fall in cardiovascular risk: Outcome Prevention on Cardiovascular Events by Antihyperlipidemic Therapy With N3-fatty Acid in Japan (OCEAN3) and Outcomes Study to Assess STatin Residual Risk Reduction with EpaNova in HiGh CV Risk PatienTs with Hypertriglyceridemia (STRENGTH).
Respecting triglycerides, the guide mentions evinacumab, an anti-ANGPTL3 monoclonal antibody that reduces concentrations of LDL cholesterol, triglycerides and lipoprotein(a) [Lp(a)] in patients with HFHo.48 Two recent phase 1 studies with hypertriglyceridaemic subjects with the same drug found similar results to those observed with mutations involving loss of ANGPTL3 function.49 Likewise, the guide also offers information about a new antisense oligonucleotide against ANGPTL3, IONIS-ANGPTL3-LRx, which reduces triglycerides by 85%. Finally, it describes the safety and efficacy results of volanesorsen, a second generation antisense oligonucleotide which targets the ARNm of apo C-III, giving rise to a 70% fall in triglycerides and an 80%–90% fall in apo C-III. More recently, the beneficial effects of volanesorsen have been described when used as a complement to diet in adults with genetically confirmed familial chylomicronemia syndrome. In this study the active treatment arm achieved an 84% reduction in levels of apo C-III reduction and a 77% fall reduction in triglycerides at 3 months.50
Regarding HDL cholesterol, the guide centres on mimetic peptides of apo A–I and recombinant forms of HDL, with the potential to remodel HDL particles in vivo and greater cardioprotective activity.51 On the other hand, it also refers to volanesorsen, which as well as its hypotriglyceridaemiant effect also leads to a 40% rise in HDL cholesterol in subjects with hypertriglyceridaemia.
Recommendations for the pharmacological treatment of hypertriglyceridaemiaFor the therapeutic approach to be used in cases of hypertriglyceridaemia, the guide recommends treatment with statins as the drug of choice, to reduce cardiovascular risk in high-risk individuals with triglycerides >2.3 mmol/L (>200 mg/dL).This recommendation is based on the sub-analysis of the Treating New Targets (TNT) study by Vallejo-Vaz et al.52 This concludes that the cholesterol of triglyceride-rich lipoproteins is an independent cardiovascular risk marker, and that it offers evidence for the cardiovascular benefit of statins in patients with secondary prevention with triglyceride-rich lipoproteins with a high cholesterol content.
In patients at high/very high risk with triglyceride levels from 1.5 to 5.6 mmol/L (135−499 mg/dL) in spite of treatment with statins, the guide considers the use of polyunsaturated n-3 fatty acids (icosapent ethyl 4 g/day) in combination with a statin, due to the results of the REDUCE-IT47 study. Afterwards, when the authors undertake a sub-analysis of the results according to triglyceride terciles, they conclude that the cardiovascular benefits of icosapent ethyl are chiefly associated with basal risk, and other effects unconnected with triglycerides.53 Finally, the guides consider that in patients with primary prevention or at high risk, with LDL cholesterol at target levels and triglycerides >2.3 mmol/L (>200 mg/dL), fenofibrate or bezafibrate should be used in combination with statins.
Control of dyslipidaemia in different clinical situationsCombined familial hyperlipidaemiaThree aspects are underlined of this mixed type of dyslipidaemia which runs with high levels of LDL cholesterol, triglycerides or both: its high rate of prevalence, which is a frequent cause of early onset ischemic cardiopathy, and it shows superposition of type 2 diabetes and metabolic syndrome lipid phenotypes. The guide still proposes the combination of apo B > 120 mg/dL and triglycerides >1.5 mmol/L (133 mg/dL) with a family history of premature cardiovascular disease as the criteria to identify this situation. It repeats that treatment with statins reduces cardiovascular risk individuals with and without hypertriglyceridaemia.
Familial dysbetalipoproteinaemiaThe guide advises that the treatment and control of patients with familial dysbetalipoproteinaemia must take place in a lipids clinic or specialised unit, and it states that the majority of cases respond well to treatment with a statin, or, if hypertriglyceridaemia predominates, with a fibrate; it states that combined treatment with a statin and a fibrate is often necessary.
Genetic causes of hypertriglyceridaemiaAs we pointed out above, the concentration of triglycerides in plasma is a biomarker for triglyceride-rich lipoproteins and remnant particles. The guide emphasises that the majority of hypertriglyceridaemias are usually multigenic or polygenic, with DNA accumulation of common variants with little impact, and rare variant DNA with major repercussion in triglyceridaemia. Additionally, in susceptible individuals hypertriglyceridaemia is aggravated with the concurrence of environmental factors such as lifestyle, being overweight and alcohol consumption.
Severe single gene hypertriglyceridaemias which cause chylomicronemia are due to mutation with loss of function in the genes that regulate the catabolism of triglyceride-rich lipoproteins such as LpL, APOC2, APOA5, LMF1, GPIHBP1 and GPD1, and the most common mutations are of LpL and secondly GP1HBP1.54
For LpL deficiency, the manufacturer of gene therapy with tiparvovec, a vector associated with subtype 1 of adenovirus carrier of the LpL functional gain variant p.S447X, decided not to apply for renewal of the authorisation for commercialisation in 2017.55 Recently the results of one year of treatment of the first patient with LpL deficit with this gene therapy have been published.56
This section of the guide includes a chapter on the prevention of acute pancreatitis in cases of severe hypertriglyceridaemia. It insists here on the importance of restricting calorie intake and dietary fat, as well as alcohol abstinence. Therapy with fibrates (fenofibrate) has to commence, with n-3 fatty acids (2−4 g/day) as a complementary therapy. It states that lomitapide may be considered in severe cases.57 In patients with diabetes, insulin therapy should be initiated to achieve good glycaemic control. In an acute context, plasmapheresis may swiftly reduce triglyceridaemia.58 Lastly, it recalls the recent approval by the European Medicines Agency (EMA) of volanesorsen as a complement to diet in adult patients with familial chylomicronemia who are at high risk of pancreatitis.
Other metabolic lipoprotein genetic disordersWithin the genetic hypolipidaemias hypobetalipoproteinaemia stands out, together with abetalipoproteinaemia, Tangier disease (analphalipoproteinemia) and lecithin-cholesterol-acyl-transferase deficiency, which cause very low or non-existent levels of HDL cholesterol. Very high levels of HDL cholesterol are detected in patients with cholesteryl ester transfer protein deficiency.
Lysosomal acid lipase deficiency or disease caused by cholesteryl ester storage is a rare cause of high LDL cholesterol and low HDL, accompanied by hepatomegalia and microvesicular steatosis. Treatment with statins reduces the levels of LDL cholesterol and may therefore prevent cardiovascular disease in these patients, although it will not halt the progression of liver damage. Enzyme replacement therapy with sebelipase alfa may offer a treatment solution in the near future.59
Metabolic syndrome and type 2 diabetes dyslipidaemiaThe guide repeats that this type of dyslipidaemia is accompanied by a set of lipoprotein alterations, including raised triglyceride levels, small dense LDL and apo B and low levels of HDL cholesterol and apo A–I. It also states that non-HDL cholesterol or apo B are good markers of triglyceride-rich lipoproteins and their remnants, and that they are a secondary therapeutic objective (Table 1).
Finally, it underlines the well-known fact that AD is one of the main cardiovascular risk factors in individuals with type 2 diabetes, abdominal obesity and insulin-resistance or glucose intolerance.
The clinical benefits deriving from the treatment of AD that are often seen in diabetes are still the cause of debate, as the effects of fenofibrate on the core aim of the studies Fenofibrate Intervention and Event Lowering in Diabetes (FIELD)60 and Action to Control Cardiovascular Risk in Diabetes (ACCORD) were negative.61 In a sub-analysis of the FIELD study, fenofibrate reduced cardiovascular episodes by 27% in patients with high triglyceride levels (2.3 mmol/L [200 mg/dL]).62 The ACCORD study confirmed that patients who had triglyceride level in the upper third (2.3 mmol/L [200 mg/dL]) and a level of HDL cholesterol in the lower third (≤0.4 mmol/L (34 mg/dL]), benefited from the addition of fenofibrate to simvastatin.61 Prolongation of the clinical follow-up of the participants in ACCORD confirmed the beneficial effect of fenofibrate in the subjects with AD at the start of the study.63 In agreement with these findings, 3 practically simultaneous meta-analyses of fibrates in cardiovascular prevention corroborated that the cardiovascular benefits of fibrates fundamentally occur in patients with AD or its components.64–66
According to the available data, the guide indicates that diabetic patients with AD may obtain clinical benefits from triglyceride reduction therapy to complement treatment with statins.67,68 The PROMINENT study with pemafibrate will therefore be decisive if it confirms this.46
Respecting type 1 diabetes, the lipid profile of these patients is usually normal with correct control of glycaemia, due to the effect of insulin on LpL activity in the adipose tissue and skeletal muscle. Nevertheless, in spite of the apparent “normality” of the lipid profile, qualitative changes in the composition of HDL and LDL are potentially atherogenic.
Lipids and chronic kidney diseaseAD is the characteristic form of dyslipidaemia in the early stages of chronic kidney disease, including predominance of small dense LDL particles. Studies also indicate that the kidney plays a crucial role in Lp(a) catabolism, and that its levels rise in association with kidney disease. All of these lipoprotein alterations may be reversed by kidney transplant or the remission of nephrosis.
TransplantAlterations in the lipid mechanism are frequent in patients subjected to bone marrow, heart, lung or liver transplant, and they involve higher cardiovascular risk and risk of transplant arterial vasculopathy. Immunosuppressor drugs may also have adverse effects on the lipid mechanism, leading to increases in total cholesterol, VLDL and triglycerides, as well as in the size and density of LDL particles. For the treatment and control of dyslipidaemia in transplanted patients, the guide recommends following the same guidelines as those for patients at high/very high cardiovascular risk.
Retinal vascular diseaseThe guide recognises that atherosclerotic changes in the retinal arteries are correlated with concentrations of total cholesterol, LDL, triglycerides and apo B, as well as with coronary heart disease, and that fenofibrate reduces the progression of diabetic retinopathy.
Gaps in the evidenceThe guide ends with this section, which underlines those aspects which are insufficiently covered by scientific evidence. It therefore emphasises the lack of comparative studies based on the results of LDL cholesterol versus apo B as primary measurement methods for screening, diagnosis and control.
It also states that there is a need for more clinical evidence regarding the seemingly adverse association between extremely high levels of HDL cholesterol and cardiovascular clinical objectives, and it also highlights the lack of clinical impact of therapies that modify HDL particle function.
ConclusionsThe physiopathological complexity of AD hinders implementing treatment that would increase cardiovascular protection. In this context it is essential to promote awareness of AD and its associated risk, as well as disseminating existing knowledge and the implementation of measures for its correct identification, treatment and control. Table 2 shows the key points in the position of the 2019 European Guide for the control of dyslipidaemia8 respecting AD and/or its main components. It underlines that high levels of triglycerides are associated with higher risk of severe cardiovascular episodes and mortality, and this is aggravated even more by high concurrent levels of LDL cholesterol or low levels of HDL cholesterol. Non-HDL cholesterol, which depends mainly on the levels of triglyceride-rich lipoproteins, should be the chief therapeutic objective in the control of patients with AD. Although quantifying apo B is the most exact and stable measurement of cardiovascular risk, its limited availability is the main barrier against broader routine use of this method.
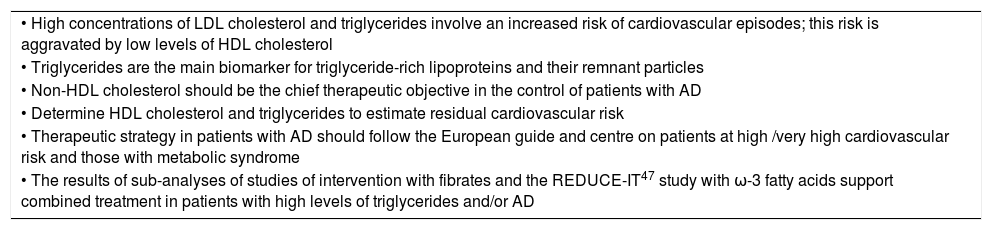
Key points of the 2019 European Guide for Dyslipidaemia Control8 for atherogenic dyslipidaemia and/or its components.
| • High concentrations of LDL cholesterol and triglycerides involve an increased risk of cardiovascular episodes; this risk is aggravated by low levels of HDL cholesterol |
| • Triglycerides are the main biomarker for triglyceride-rich lipoproteins and their remnant particles |
| • Non-HDL cholesterol should be the chief therapeutic objective in the control of patients with AD |
| • Determine HDL cholesterol and triglycerides to estimate residual cardiovascular risk |
| • Therapeutic strategy in patients with AD should follow the European guide and centre on patients at high /very high cardiovascular risk and those with metabolic syndrome |
| • The results of sub-analyses of studies of intervention with fibrates and the REDUCE-IT47 study with ω-3 fatty acids support combined treatment in patients with high levels of triglycerides and/or AD |
AD: atherogenic dyslipidaemia; HDL: High density lipoprotein; LDL: Low density lipoprotein.
AD treatment should comply with current European recommendations, centring on patients at high/very high cardiovascular risk and those with metabolic syndrome. Although statins reduce cardiovascular risk in all patients, the specific differences of patients with AD may require an individualised approach.
Conflict of interestsThis study is by a work group of the Spanish Arteriosclerosis Society, and it receives financing from Mylan.
Please cite this article as: Pedro-Botet J, Ascaso JF, Blasco M, Brea Á, Díaz Á, Hernández-Mijares A, et al. Triglicéridos, colesterol HDL y dislipidemia aterogénica en la guía europea para el control de las dislipidemias 2019. An Pediatr (Barc). 2020;32:209–218.