Lipoprotein (a) [Lp(a)] is a lipoprotein defined by presenting a specific apolipoprotein, ApoA, linked to the ApoB-100 by different types of chemical bonds, including a disulfide bridge. Despite their atherogenic mechanism is not fully understood, its importance has been demonstrated in the development of premature aterosclerosis. Multiple studies have shown its role as a cardiovascular risk factor associated with heart disease and stroke. We report the case of a patient with a diagnosis of Takayasu arteritis in which a massive elevation of Lp(a) was detected. We emphasise its diagnostic and therapeutic implications.
La lipoproteína (a) [Lp(a)] es una lipoproteína definida por presentar una apolipoproteína específica, la apoA, unida a la apoB-100 por diversos tipos de enlaces químicos, entre ellos un puente disulfuro. A pesar de que su mecanismo aterogénico no es completamente conocido, está demostrada su importancia en el desarrollo de ateroesclerosis prematura, mostrando múltiples estudios su papel como factor de riesgo cardiovascular asociado a enfermedad coronaria e ictus. Presentamos el caso de una paciente con diagnóstico de arteritis de Takayasu en la que se detectó una elevación masiva de Lp(a), y abordamos las implicaciones diagnósticas y terapéuticas que tuvo este hallazgo.
Lipoprotein(a) [Lp(a)] has been one of the main areas of focus in cardiovascular pathology in recent decades. Despite not fully understanding its atherogenic role, its importance in the development of premature atherosclerosis and its role as a risk factor for developing cardiovascular disease (CVD) have been proven.1 The case that we present shows how atherosclerosis stimulated by a massive Lp(a) elevation may cast doubt on a clinical diagnosis of vasculitis.
Case reportWe present the case of a 38-year-old female patient with no known cardiovascular risk factors, diagnosed in 2008 at the age of 31 with Takayasu's arteritis (TAK) at another centre. The symptoms began with chest pain and severe hypertension. The diagnosis was based on a difference in blood pressure (BP) in both arms along with radiological abnormalities observed in the MR and CT angiography considered compatible. Stenosis of the left common carotid artery and the left subclavian artery was observed. The patient received first-line therapy of intravenous cyclophosphamide per protocol and corticosteroids. She was then placed on maintenance therapy with methotrexate 10mg administered once weekly for 5 years. The patient was seen at our centre for a second opinion. She did not bring any imaging tests except for an echocardiogram, which showed severe, non-obstructive septal hypertrophy. The last imaging test was in 2008, the year of diagnosis. Her family history included a parent who had a stroke at the age of 49 and a sister with severe LP(a) elevation, with figures at 156mg/dL.
The physical examination showed a left arm BP: 105/70mmHg; right arm BP: 175/85mmHg. Bilateral ankle-brachial index of 0.95. HR: 85bpm. Cardiopulmonary auscultation was normal. No murmurs were present in the abdomen. There was no oedema in the lower limbs, with the pulses present and symmetric.
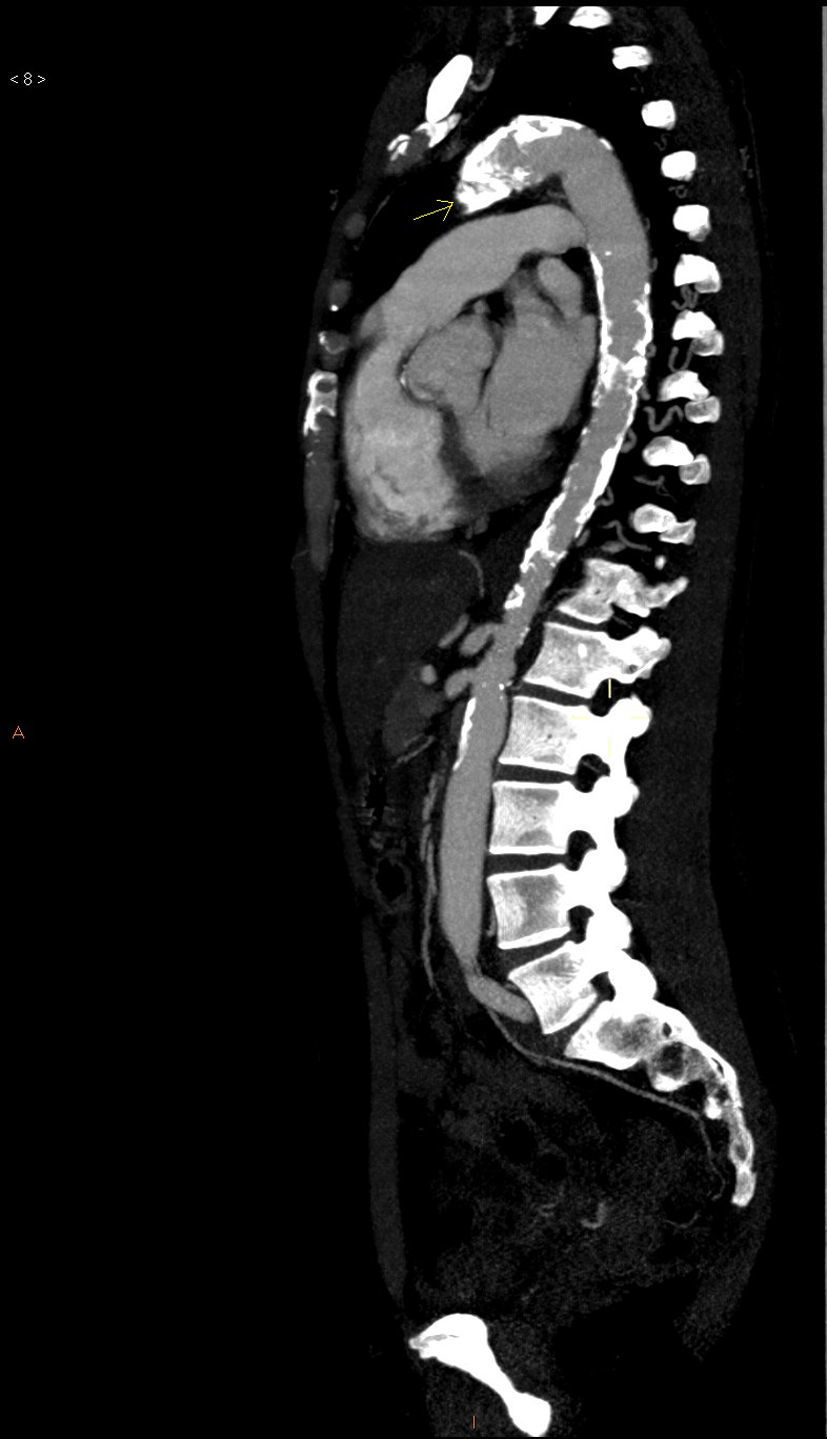
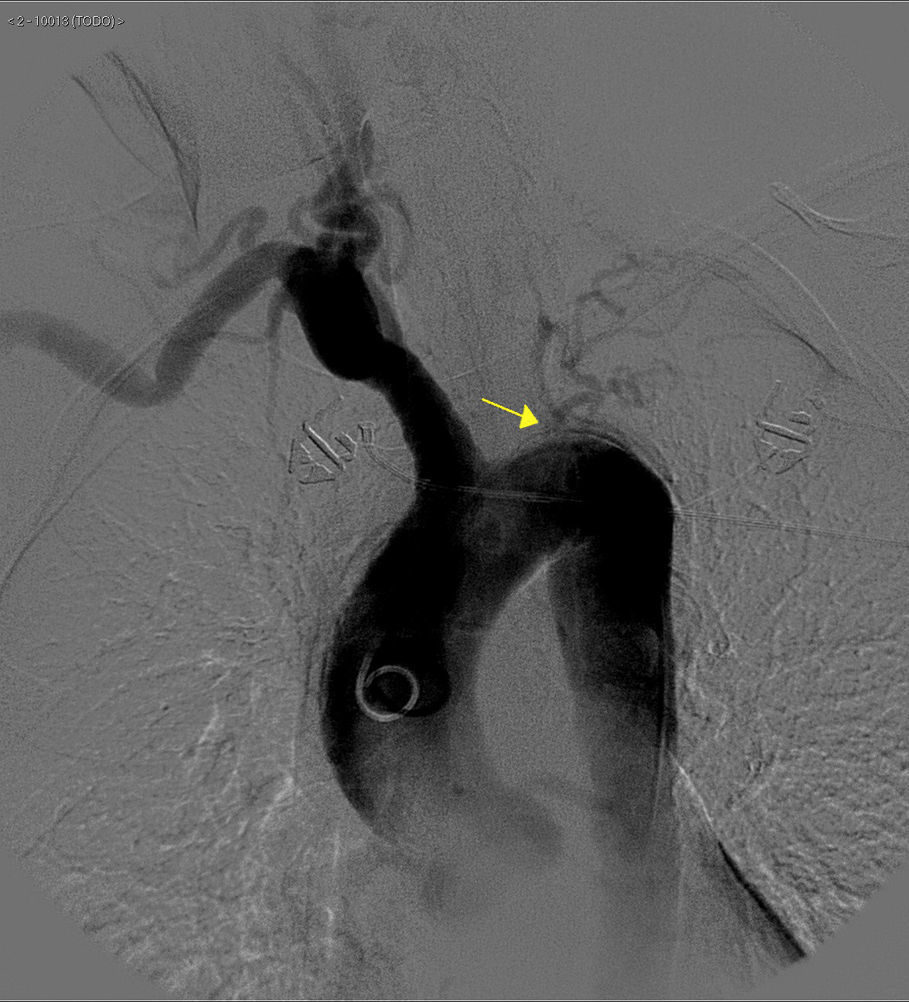
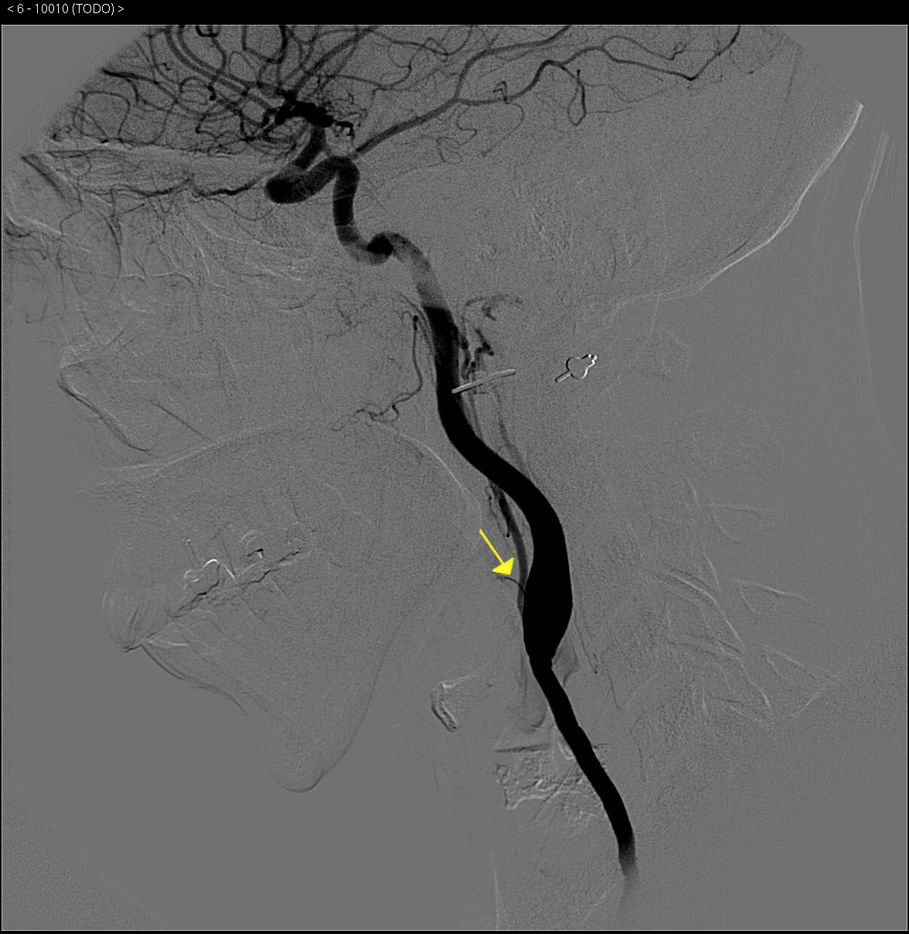
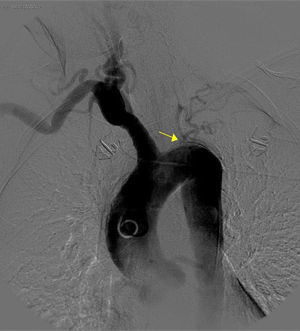
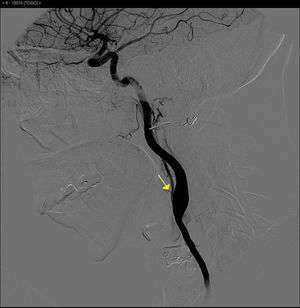
The basic blood work and chest X-ray were normal. A thoracic CT angiogram was performed which showed complete blockage of the left subclavian and carotid arteries along with calcified atherosclerosis of the thoracic and abdominal aorta (Fig. 1). The MRA showed stenosis of the left internal carotid artery, likely affecting the cervical carotid artery. The supra-aortic vessels were unable to be visualised as the patient did not tolerate the test. An abdominal and supra-aortic vessel arteriography was performed which showed diffuse atherosclerosis of the abdominal aorta, 50% stenosis of the left renal artery, occlusion from the origin of the left common carotid, subclavian, and vertebral arteries, reduced calibre of the common carotid artery, and obstruction of the right external carotid artery (Figs. 2 and 3). No lesion was considered to be suitable for endovascular treatment.
Given these findings, the diagnosis of TAK was called into question, and we began to investigate causes for premature vascular disorder. Tests for autoimmune disorders, thrombophilia, and antiphospholipid antibodies were normal. Homocysteine 11.7μmol/L. Urine sediment was normal. A lipid profile was ordered which showed the following values: total cholesterol 197mg/dL; triglycerides 74mg/dL; HDL-c 68mg/dL; LDL-c 114mg/dL; Lp(a) 277mg/dL; apoA 176mg/dL; apoB 102mg/dL.
With the aim of discerning whether we were faced with premature atherosclerosis due to a massive elevation of Lp(a) or TAK with residual established lesions, a PET-CT was ordered which showed no signed of active inflammatory disease. Lastly, a coronary CT angiogram was ordered, which showed diffuse mixed atherosclerosis along the entire aorta trajectory with discontinuous calcification in the ascending and descending trajectory, involvement of both coronary ostia, with an estimated 70% stenosis of the right ostium and marked calcified atherosclerosis of the rest of the trajectory of the right coronary artery. In line with the rest of the additional tests ordered, the findings were interpreted as having an atherosclerotic origin, although the TAK diagnosis could not be definitively ruled out. The patient was included in a lipoprotein apheresis programme with sufficient tolerance. Immunosuppressant treatment was withdrawn and the patient is currently asymptomatic. The clinical judgement according to the findings was premature atherosclerosis due to massive Lp(a) elevation affecting the aorta, coronary, carotid, and renal arteries, without being able to rule out Takayasu's arteritis in remission.
DiscussionThe presented case report raised the question of whether we were faced with premature atherosclerosis due to massive Lp(a) elevation, residual TAK with no signs of activity, or both diagnoses. Attempts were made to address this issue by using PET-CT. Its utility has been demonstrated for diagnosing and monitoring large-vessel vasculitis, including TAK. A pathological uptake has also been demonstrated in developing atheroma plaques. Using this test we were able to attempt to discern both pathologies by the degree, topography, and morphology of the radiotracer uptake.2 In this case, it is a difficult task to differentiate the two entities given the absence of uptake on the PET-CT. The time the patient has remained asymptomatic, the normality of the acute-phase reactants, the intense vascular calcification, and the coronary artery involvement, rare in TAK, are data that in our judgement do not support the TAK diagnosis. Hypertrophic cardiomyopathy, sometimes related to TAK, is justified by the sustained effects in the time the left ventricular outflow tract was obstructed, or by prolonged high BP figures. The latter is the most plausible in our patient.3 We believe that this is independent of TAK. Furthermore, it is true that the patient does not have any other cardiovascular risk factors (although she has a first-degree family history of premature cerebrovascular disease). Thus it may be argued that the extensive vascular involvement is due only to the massive LP(a) elevation. This means that the possibility of TAK in remission with residual lesions cannot be ruled out entirely.
As for the Lp(a), multiple studies have demonstrated its role as a vascular risk factor associated with both heart disease and stroke. This includes a meta-analysis of 36 prospective studies with a total of 126,634 patients published in JAMA, which concluded that there is a continuous, independent correlation of the Lp(a) concentration and the risk of heart disease and stroke.4 Our patient had very high Lp(a) levels, but the threshold from which the Lp(a) concentration increases cardiovascular risk has not yet been fully established, and many studies do not estimate the vascular risk given extreme levels of Lp(a), as in our case. Nevertheless, the published analysis performed on a cohort study of heart disease in Copenhagen, an increased risk of myocardial infarction in proportion to the Lp(a) levels was observed. A threshold effect was not found and there was a three- to four-fold increase of the risk of acute myocardial infarction at extreme levels (≥120mg/dL) of Lp(a).5 We have not found a case in the literature as explicit as this one that shows as extensive a level of involvement. The mechanism by which Lp(a) induces the development of atherosclerosis is still unknown. Given its similarity with the plasminogen domain, a prothrombotic mechanism has been suggested for the development of atherosclerosis. The VLDL receptor expressed in macrophages present in the atheroma plaque may be implicated in its metabolism, enabling LP(a) endocytosis and promoting deposits in the lesion zone.6 The wide heterogeneity in the size of the gene coding for apoA would be responsible for the wide range of Lp(a) concentrations found in different individuals, and the size of the apoA isoforms. There is a correlation between the size of the isoforms and cardiovascular risk, with the smaller forms conferring greater atherogenic power.7
The European Consensus for handling patients with LP(a) elevation suggests it be determined in the following cases: premature CVD, familial hypercholesterolaemia, family history of premature CVD or LP(a) elevation, recurrent CVD despite statin treatment, ≥3% and ≥10% risk at 10-years of fatal CVD according to European and American clinical practice guidelines, respectively.8
There are currently few available therapeutic options. Hygiene and dietary measures have not been shown to influence the Lp(a) levels and statins do not lower its concentration. One of the drugs that has been demonstrated to reduce the LP(a) level is nicotinic acid (niacin), with a reduction of up to 40% at the highest tolerated dose. Anti-PCSK9 monoclonal antibodies can reduce LP(a) levels by up to 30%, although its main effect is to reduce LDL-c levels. Mipomersen, an antisense oligonucleotide against apoB100, may reduce the Lp(a) concentrations by 19–31%.9 Studies on an antisense oligonucleotide against apoA are currently starting Phase 2 trials. The last currently available step is one of the most effective measures for reducing Lp(a) levels, lipoprotein apheresis. This method achieves decreases up to 70%. Leebmann et al.10 included 170 patients with increased LP(a) levels and progressive CVD with the maximum tolerated doses of lipid-lowering therapy. The study proved not only that lipoprotein apheresis is safe and effective for reducing Lp(a) levels, but it also reduced the incidence of cardiovascular episodes. It should be taken into account that LDL-c levels were reduced in parallel, which may explain the reduction of episodes as LDL-c also decreased. In our case, LP(a) levels were reduced by 71% (pre- and post-apheresis levels were 257 and 86mg/dL, respectively). The desired LP(a) level established by European consensus is <50mg/dL.8
ConclusionIncreased blood LP(a) values represent an independent cardiovascular risk factor, with an important role in the development of premature atherosclerosis. It may be advisable to measure it in patients who present or atypically develop large-vessel vasculitis. A useful tool for differentiating vascular involvement due to atherosclerosis or vasculitis is PET-CT. In this case, given the absence of uptake and symptoms, it is not possible to label the patient with a concrete diagnosis.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were conducted on human beings or animals for this research.
Confidentiality of dataThe authors declare that they have followed the protocols of their work site regarding the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data is contained in this article.
AuthorshipAll the authors mentioned have contributed to this manuscript, both by providing ideas as well as in preparing the draft and its final approval.
Conflicts of interestThe authors of this manuscript declare that they have no conflicts of interest.
Please cite this article as: Alarcón García JC, Rodríguez Suárez S, Muñiz Grijalvo O, García Morillo S. Lesiones vasculares en paciente diagnosticada de arteritis de Takayasu y elevación masiva de lipoproteína (a). ¿Afectación residual o arterioesclerosis prematura? Clin Invest Arterioscler. 2017;29:98–102.