In parallel with the improvement of living standard, Non-alcoholic fatty liver disease (NAFLD) becomes the most common liver disease around the world. Huazhi Fugan Granules (HZFGG) is a formula which is used to treating of fatty liver, Based on the data we studied, HZFGG may have potential as a therapeutic formula for the alleviation of NAFLD.
ObjectivesThe aim of our study was to identifying the improvement of HZFGG on NAFLD and exploring the potential mechanisms.
MethodsMCD diet fed C57BL/6 mice once a day for 4 weeks to induce NAFLD model, HZFGG (10, 15, 20g/kg) orally administered simultaneously. The serum levels of TC, TG, ALT, AST were detected. H&E and Oil Red O staining were used to observed the liver sections. TNF-α, IL-1β and Gpx were also detected. The expression levels of TLR4, MyD88, p-NF-κB, NF-κB, p-IκBa were measured by western blotting assay. The apoptosis of the liver tissues were detected by TUNEL assay.
ResultsHZFGG decreased the serum levels of TC, TG, ALT, AST in MCD-diet mice. HZFGG alleviated inflammation by decreasing the levels of TNF-α and IL-1β and ameliorated oxidative stress through increased the level of Gpx. HZFGG Attenuates MCD-induced liver steatosis and injury in mice. Hepatocyte apoptosis was decreased after HZFGG treatment. Furthermore, HZFGG also suppressed the expression levels of TLR4 and MyD88, subsequently, inhibited the phosphorylation of NF-κB and IκBa.
ConclusionHZFGG can improved MCD induced hepatic injury through inhibited TLR4/NF-κB signaling pathway in NAFLD model.
En paralelo con la mejora de la calidad de vida, la enfermedad de hígado graso no alcohólico (EHGNA) se ha convertido en la enfermedad hepática más común a nivel mundial. Los gránulos de Huazhi Fugan (HZFGG) son una fórmula utilizada para tratar el hígado graso. Basándonos en los datos que estudiamos, HZFGG puede tener potencial de fórmula terapéutica para el alivio de EHGNA.
ObjetivosEl objetivo de este estudio fue identificar la mejora de EHGNA con el uso de HZFGG y explorar los mecanismos potenciales.
MétodosSe alimentó con dieta deficiente en metionina y colina (MCD) a ratones C57BL/6 una vez al día durante cuatro semanas, para inducir el modelo EHGNA, administrándose simultáneamente HZFGG oral (10, 15, 20 g/kg). Se detectaron los niveles séricos de TC, TG, ALT y AST. Se utilizaron tinciones de hematoxilina-eosina y rojo aceite para observar las secciones hepáticas. También se detectaron TNF-α, IL-1β y Gpx. Se midieron los niveles de expresión de TLR4, MyD88, p-NF-κB, NF-κB y p-IκBa, mediante la técnica de Western blot. Se detectó la apoptosis de los tejidos hepáticos utilizando la técnica TUNEL.
ResultadosHZFGG redujo los niveles séricos de TC, TG, ALT, AST en los ratones con dieta MCD. HZFGG alivió la inflamación reduciendo los niveles de TNF-α e IL-1β, y mejoró el estrés oxidativo a través del incremento del nivel de Gpx. HZFGG atenúa la esteatosis y lesión hepáticas inducidas por MCD. La apoptosis hepatocítica se redujo tras el tratamiento con HZFGG. Además, HZFGG suprimió también los niveles de expresión de TLR4 y MyD88, y posteriormente inhibió la fosforilación de NF-κB e IκBa.
ConclusiónHZFGG puede mejorar la lesión hepática inducida por MCD, mediante la inhibición de la vía de señalización de TLR4/NF-κB en un modelo de EHGNA.
Non-alcoholic fatty liver disease (NAFLD), characterized by excessive deposition of intrahepatic lipids and no history of alcohol abuse, is a common metabolic disease in most countries including the Middle East, Europe, Asia, and the United States.1 It is estimated that the prevalence of NAFLD worldwide up to 25%.2–5 The obesity, diabetes, hyperlipidemia, and metabolic syndrome elevated the trend of the NAFLD.6–8 Metabolic parameter disorders, endotoxin-induced cytokine release, inflammation and oxidative stress contribute to the development and progression of NAFLD, and almost 20% patients will progress to nonalcoholic steatohepatitis (NASH), which have a higher chance of developing fibrosis, cirrhosis, and hepatocellular carcinoma due to the liver steatosis, more severe lobular and portal inflammation, and ballooning.9 However, there is still no effective drugs for effective management of NAFLD and NASH, currently, some preventive strategies, such as lifestyle modification and an energy-restricted diet, have achieved limited success including improved steatosis and decreased aberrant aminotransferases.10 Therefore, it is important to find effective drugs for NAFLD treatment.
Zhang Ruixia, a well-known herbalist doctor in Shaanxi Provincial Hospital of Traditional Chinese Medicine, has more than 60 years of clinical experience. She has rich experience for the treatment of fatty liver. Based on clinical practice, she developed the Huazhi Fugan Granules (HZFGG), which mainly consists of Salvia miltiorrhiza (Danshen), Artemisiae Scopariae Herba (Yinchen), Rhizoma Alismatis (Zexie), Scutellaria baicalensis Georgi (Huangqin), Crataegus pinnatifida (Shanzha) to treat the fatty liver. It was reported that Zhang Xin and Li Xiaorui conducted a observation of a certain number of patients using HZFGG before and after treatment, and the total effective rate reached 86.9% and 86.7% respectively.11,12 In the present study, the aim was to determine the role of HZFGG in the development of methionine and choline deficient (MCD) diet-induced NAFLD in mice. We hypothesized that HZFGG may have a hepatoprotective effect during progression of NAFLD and prevent the progression from NAFLD to NASH. According to the “parallel, multiple-hit” hypothesis, the progression from NAFLD to NASH is the consequence of multiple interactive factors, such as lipotoxicity, oxidative stress, insulin resistance, endoplasmic reticulum stress and even endotoxin from gut microbiota, which converge on intracellular inflammatory signaling,13–17 which might be potential therapeutic targets. Inflammatory responses is a key step in the prevention of NAFLD and NASH, Toll-like receptor-4 (TLR4), which downstream adaptor molecules is myeloid differentiation factor 88 (MyD88) plays a pivotal role in the inflammatory response.18 It has been reported that TLR4 is also implicated in hepatic steatosis and NAFLD pathogenesis,19 MyD88 activates NF-kappa B (NF-kB) transcription factor, subsequently results in production of proinflammatory cytokines.19,20 In the present study, we investigated the effects and potential mechanisms of HZFGG on MCD diet-induced NAFLD mice, which is a classic model and has been used for over 40 years.21
MethodsAnimals and treatmentsC57BL/6 mice (male, 6-week-old, 18–22g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). The mice were accommodated in a pathogen-free environment under a 12-h light/dark cycle and the temperature ranged between 20°C and 25°C, humidity was maintained between 55% and 65%. All mice had free access to food and water and were weighed at weekly intervals. All mice were randomly divided into six groups (10 mice per experimental group) including the control group, HZFGG group (15g/kg), MCD group, and HZFGG (10, 15, 20g/kg) with MCD-diet groups. The HZFGG was provide by Shaanxi Provincial Hospital of Traditional Chinese Medicine. The NAFLD model was induced by MCD diet in mice, orally administered simultaneously with HZFGG once a day for four weeks. At the end of four weeks, The blood sample were collected and then centrifuged at 3000×g for 15min at 4°C. The fresh liver tissues were collected and stored at −80°C for molecular biological assays and partial hepatic tissues were fixed with 4% paraformaldehyde for histological examination.
Determination of serum biochemical parametersThe mouse serum was separated from blood to detect biomarkers, including total cholesterol (TC), triglyceride (TG), alanine aminotransferase (ALT) and aspartate aminotransferase (AST), with reagent kits from Nanjing Jiancheng Bioengineering Institute, (Nanjing, China), tumor necrosis factor (TNF)-α, interleukin (IL)-1β levels were measured by an ELISA kit (Nanjing Jiancheng Biotech Co., Nanjing, China), glutathione peroxidase (GPx) activity was detected to analyze oxidative stress by commercial kit according to the manufacturer's instructions.
H&E and oil red O staining analysisLiver tissues embedded in paraffin were sectioned into 4μm slices and then stained with hematoxylin and eosin (H&E), briefly, the nuclei were stained hematoxylin and the cytoplasm was stained with the eosin after liver sections were dewaxed. For oil red O staining, the frozen liver sections were cuted into 8-μm-thick and were fixed in 4% paraformaldehyde for 10min, washed 3 times with deionized water and stained with oil red O working solution (Sigma-Aldrich) for 10min at room temperature, washed in deionized water and stained with hematoxylin.
TUNEL assaysThe apoptosis of the liver tissues were detected by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay according to the manufacturer's instructions of a commercial kit (Roche Diagnostics GmbH, Mannheim, Germany). Images were acquired on an optical microscope at 20× magnification.
Western blotting assayLiver tissues stored at −80°C were homogenized with stainless steel beads in RIPA lysis buffer by Tissue Lyser II (QIAGEN, Hilden, Germany) and centrifuged for 15min at 14,000rpm. The BCA protein assay kit (Thermo Scientific, Pierce, MA, USA) was used to estimated protein concentrations in different groups. Equal amounts of protein and molecular weight markers were loaded on sodium dodecyl sulfonate polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to a polyvinylidene difluoride (PVDF) membrane at room temperature for 2h, subsequently, blocked in 5% nonfat milk soluted with TBST (0.5% Tween-20) for 2h, the membranes were incubated with primary antibodies (1:1000) against Cleaved caspase-3, caspase-3, MyD88, TLR4, phosphorylated IκBα, total NF-κB, phosphorylated NF-κB, and β-actin overnight at 4°C. Then the membranes were washed 3 times and each time for 10min in TBST (1× Tris-buffered saline with 0.5% Tween-20), incubated with a secondary antibody conjugated with HRP including anti-rabbit immunoglobulin G (IgG, 1:5000) or anti-mouse immunoglobulin G (IgG, 1:5000) for 2h at room temperature after washing with Tris-buffered saline Tween 20 (TBST) for 3 times and 10min each time. The protein sample signals were detected by ECL detection kit (GE Healthcare, Milwaukee, WI, USA) and quantified with ImageJ software (National Institute of Health). All primary antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA) without particular stated.
Statistical analysisAll the results are expressed as the mean±standard deviation. Statistical analyses were achieved using Graph Pad Prism (version 7.0; Graph Pad software Inc. San Diego CA, California, USA). Every two groups were analyzed by Student's t-test. The values of p<0.05 were deliberated as statistically significant.
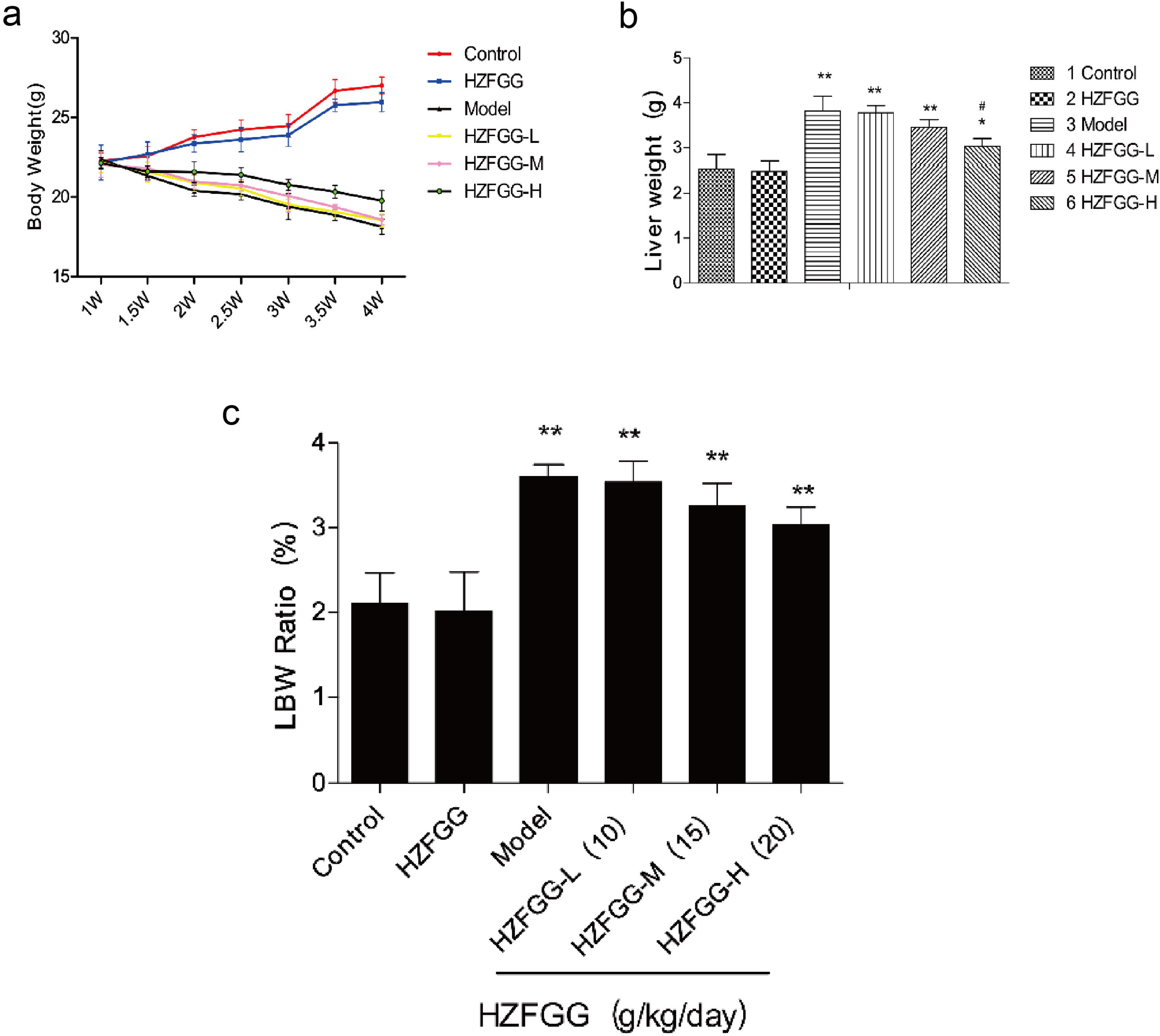
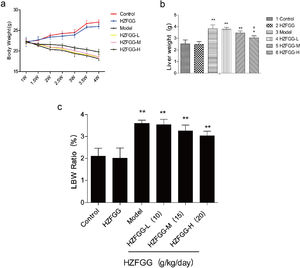
ResultsHZFGG decreases liver weight mice with MCD dietTo investigate the potential effect of HZFGG on NAFLD mice, we fed C57BL/6 mice with a normal or MCD diet for 4 weeks, and simultaneously orally administered with HZFGG at three different doses of 10, 15, or 20g/kg (weight of mice). As expected, compared with the control group, mice exhibited a significant reduction in body weight and an increase both in weight of livers and the Liver-to-Body Weight Ratio (LBW) in MCD-induced group, administered with HZFGG-L (10g/kg) and HZFGG-M (15g/kg) did not influence the mice with chow diet, however, HZFGG markly reduced the liver weight of MCD-diet mice in HZFGG-H (20g/kg) group and had no significance influence in body weight and the Liver-to-Body Weight Ratio (LBW) of mice (Fig. 1).
HZFGG attenuates the progress of NAFLD mice with MCD diet. (a) The changement of body weight every half week of mice in different group. (b) The liver weight in chow diet or MCD diet mice with or without HZFGG treatment. (c) The body weight and the Liver-to-Body Weight Ratio in chow diet or MCD diet mice with or without HZFGG treatment. The data presented are the mean±SD and **P<0.01 vs control group, #P<0.05 vs model group, ##P<0.01 vs model group.
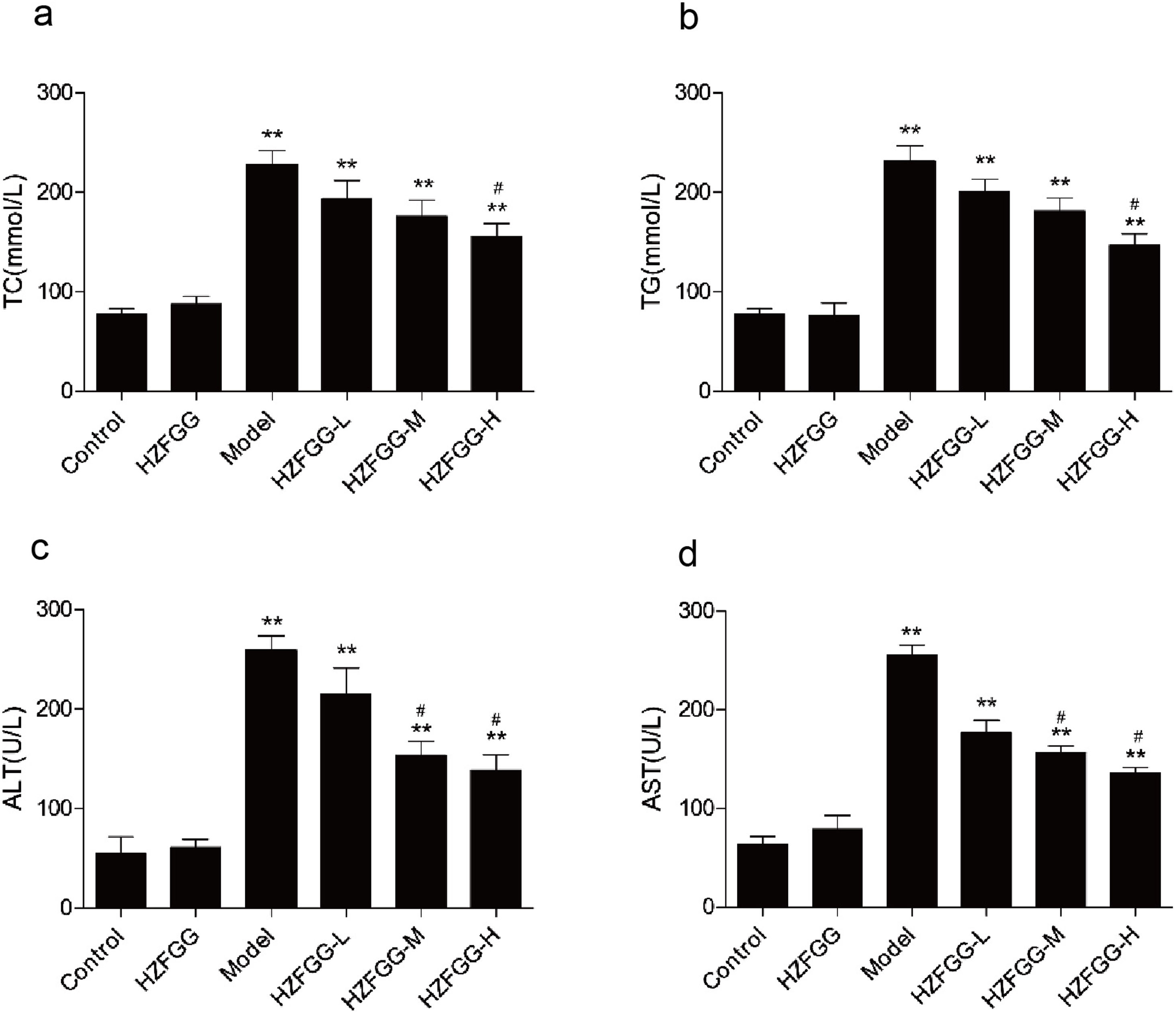
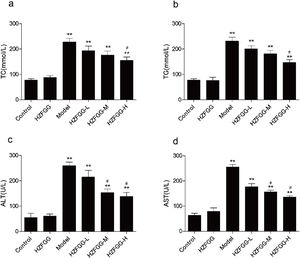
To further assess the effect of HZFGG on the NAFLD model induced by MCD diet, serum levels of TG, TC, ALT and AST were detected. As shown in Fig. 2, the serum levels of TG, TC, ALT and AST were significantly increased in MCD-diet group compared to those in the normal-diet group, however, HZFGG treatment significantly lowered the levels of TG and TC in high dose group, the levels of ALT and AST were markly decreased by HZFGG both in middle and high dose group, which indicated that HZFGG shows a protective effect in MCD-induced NAFLD.
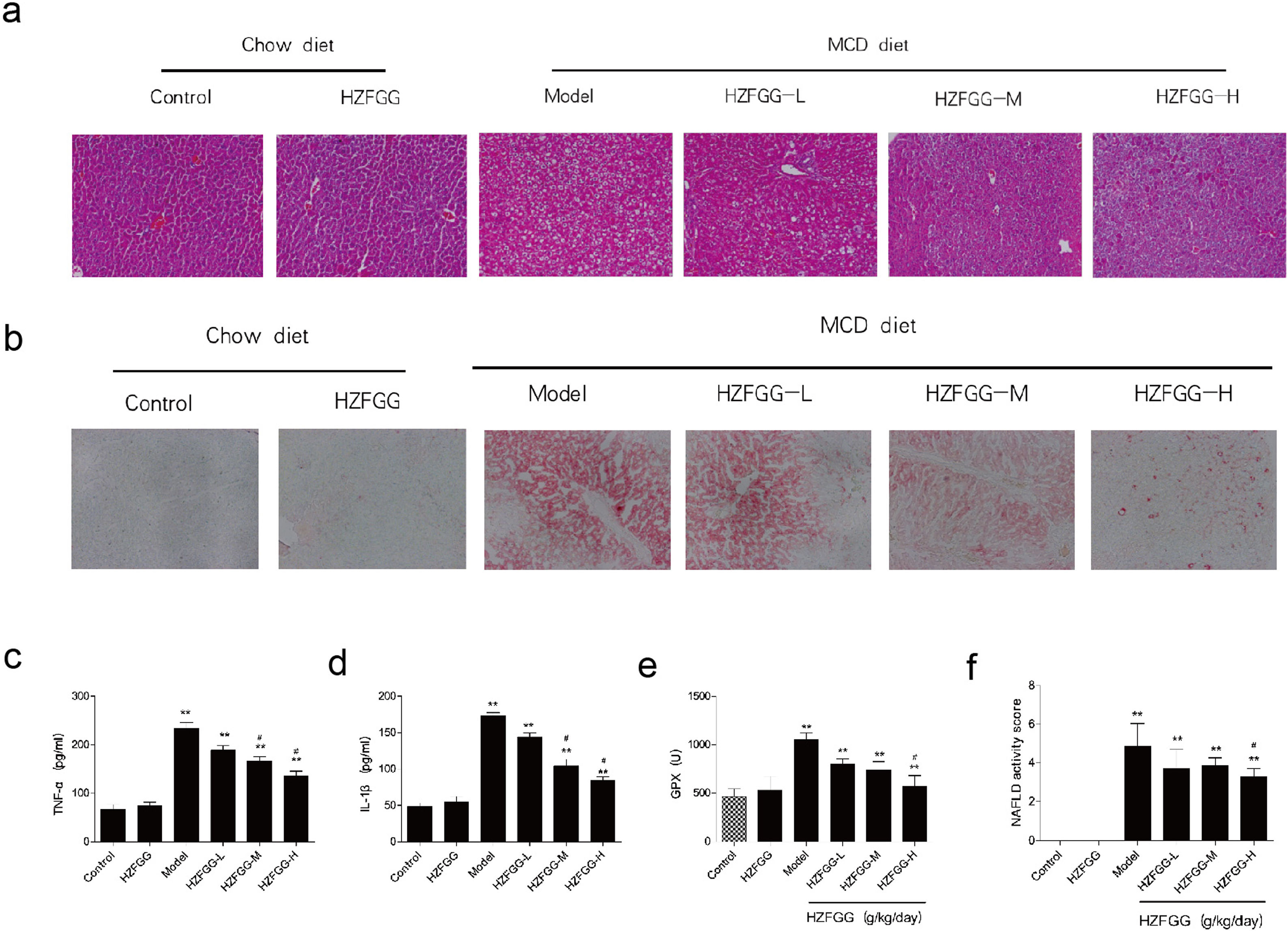
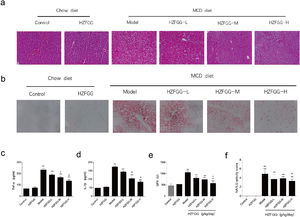
HZFGG attenuates MCD-induced liver steatosis and injury in miceWith 4-week MCD diet, hepatocytes were observed by histological H&E and oil red O staining. As shown in Fig. 3(a) and (b), MCD diet triggered obvious hepatic steatosis, a large number of lipid droplets appeared in mice liver tissue compared with the normal-diet mice. HZFGG treatment significantly reduced the number and size of lipid droplets. Inflammation involved in the pathogenesis of NAFLD, The serum levels of TNF-α and IL-1β were measured by an ELISA kit, as expected, MCD diets markedly increased these parameters, treatment with HZFGG significantly decreased MCD diet-induced elevation of TNF-α and IL-1β in a dose-dependent manner (Fig. 3(c) and (d)). These results were consistent with the observation from H&E, which showed decreased lobular inflammation. Oxidative stress which could causes the infiltration of Kupffer cells, cell death, and then liver damage,22,23 is an important role in the pathogenesis of NAFLD. Reactive oxygen species (ROS) can react with the accumulated lipids in the liver to cause lipid peroxidation. Detoxifying enzymes which scavenge ROS act as the first line of defense against ROS.24,25 HZFGG treatment markly elevated the expression of GPx levels in a dose-dependent manner compared with MCD-induced mice (Fig. 3(e)). In addition, the NAFLD activity score (NAS) was used to assess the development of NAFLD, it was calculated by steatosis, hepatocyte ballooning and lobular inflammation, as shown in Fig. 3(f), NAS significantly decreased by HZFGG supplementation. Our data indicate that HZFGG can attenuate hepatic injury including hepatic steatosis, inflammation and oxidative stress.
HZFGG attenuates MCD-induced liver steatosis and injury in mice. Representative photos of histological. (a) H&E and (b) oil red O staining in chow diet or MCD diet mice with or without HZFGG treatment. The levels of (c) TNF-α, (d) IL-1β and (e) GPx in chow diet or MCD diet mice with or without HZFGG treatment. (f) The NAFLD activity score (NAS) which was calculated by steatosis, hepatocyte ballooning and lobular inflammation in chow diet or MCD diet mice with or without HZFGG treatment. The data were presented as the mean±SD and **P<0.01 vs control group, #P<0.05 vs model group.
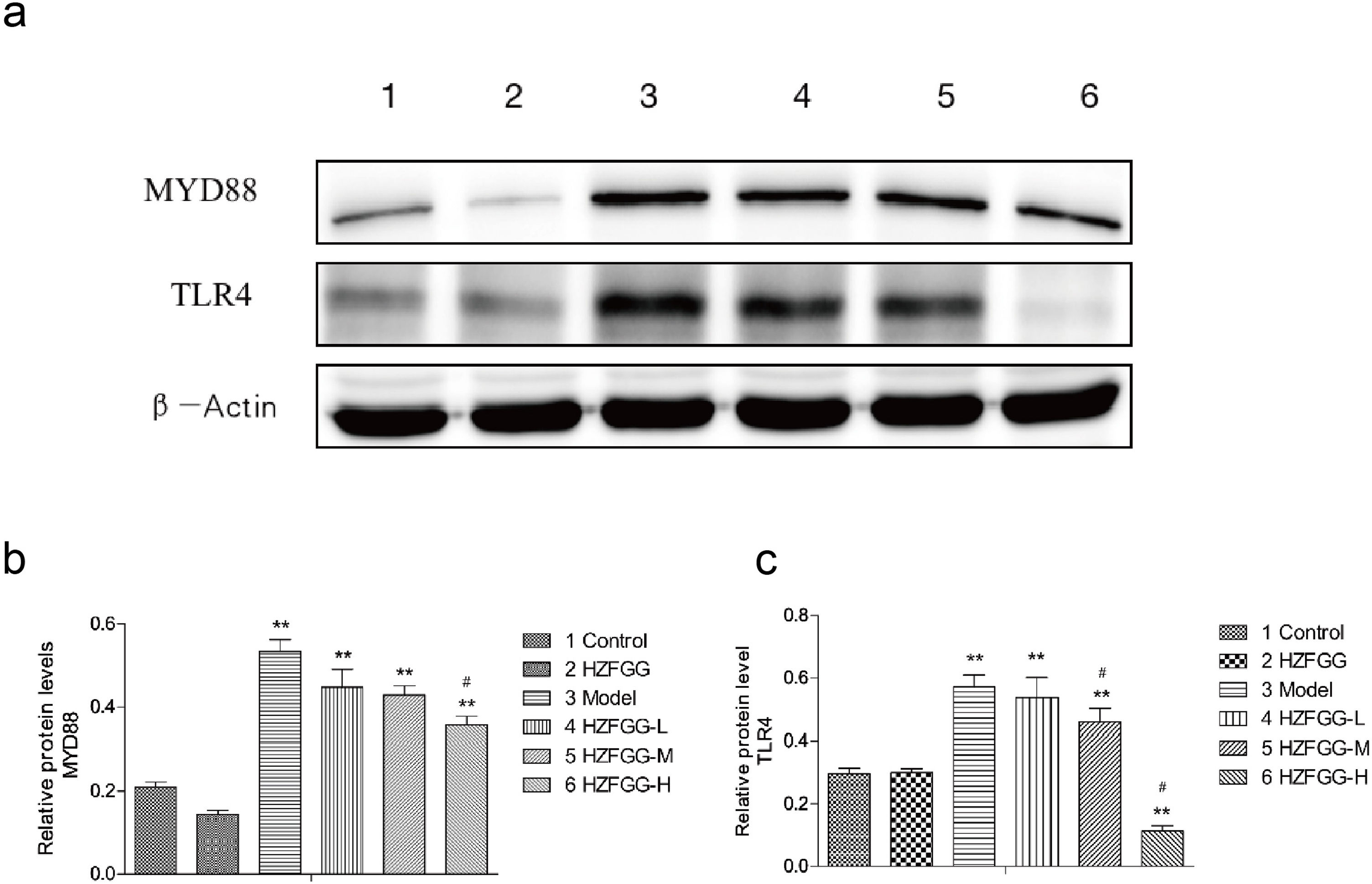
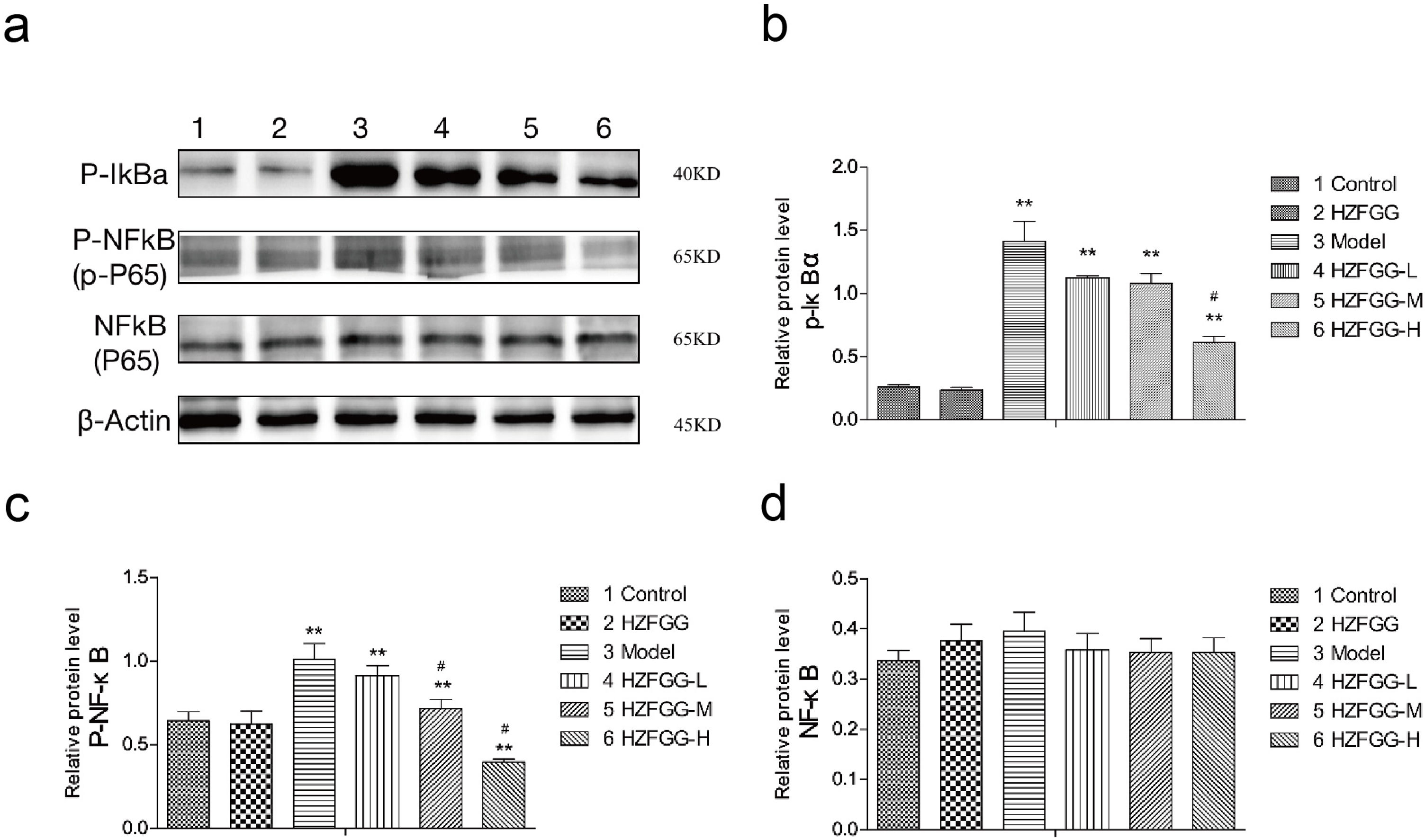
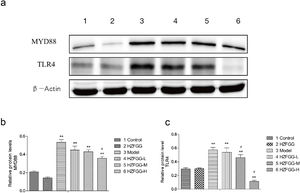
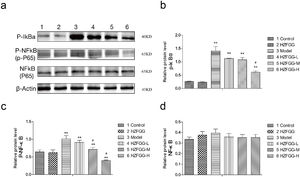
Subsequently, we explored the possible molecular mechanisms of the protective effects of HZFGG on MCD-induced NAFLD. It has been reported that TLR4/NF-κB signaling pathway is important in the pathogenesis development of NAFLD.26,27 We presumed that HZFGG might influence the TLR4/NF-κB pathway and attenuate the hepatic injury of MCD-induced mice. As shown in Fig. 4(a), the expression levels of the TLR4 and its downstream adaptor molecules MyD88 were increased evidently in MCD-diet group compared with control group, HZFGG treatment decreased the levels of the two proteins expression in a dose-dependent manner (Fig. 4(b) and (c)). NF-κB, phosphot-NF-κB and phosphot-IκBa were also detected, the western blotting shown that both expression levels of phosphot-NF-κB and phosphot-IκBa were elevated in MCD-diet group, the NF-kB was the same level in different group (Fig. 5(a)). HZFGG treatment could reduce the elevated expression levels of phosphot-NF-κB and phosphot-IκBa induced by MCD-diet (Fig. 5(b) and (c)), which indicated that HZFGG blocked the activation of the NF-kB. HZFGG has the function to inhibited the hepatic TLR4/NF-κB signaling pathway in MCD-induced NAFLD mice.
HZFGG inhibited the expression levels of the TLR4 and its downstream adaptor molecules MyD88. (a) Western blotting analysis results of TLR4 and MyD88 proteins in control group, HZFGG group, MCD diet group, MCD diet groups with HZFGG treatment, β-actin was used as the loading control. Results of quantification analysis of (b) MYD88 protein and (c) TLR4 protein in chow diet or MCD diet mice with or without HZFGG treatment. The data were presented as the mean±SD and **P<0.01 vs control group, #P<0.05 vs model group.
HZFGG blocked the activation of the NF-kB signaling pathway. (a) Western blotting analysis results of p-IκBa, p-NF-κB and NF-κB proteins in control group, HZFGG group, MCD diet group, MCD diet groups with HZFGG treatment, β-actin was used as the loading control. Results of quantification analysis of (b) p-IκBa, (c) p-NF-κB and (d) NF-kB proteins in chow diet or MCD diet mice with or without HZFGG treatment. The data were presented as the mean ± SD and **P<0.01 vs control group, #P<0.05 vs model group.
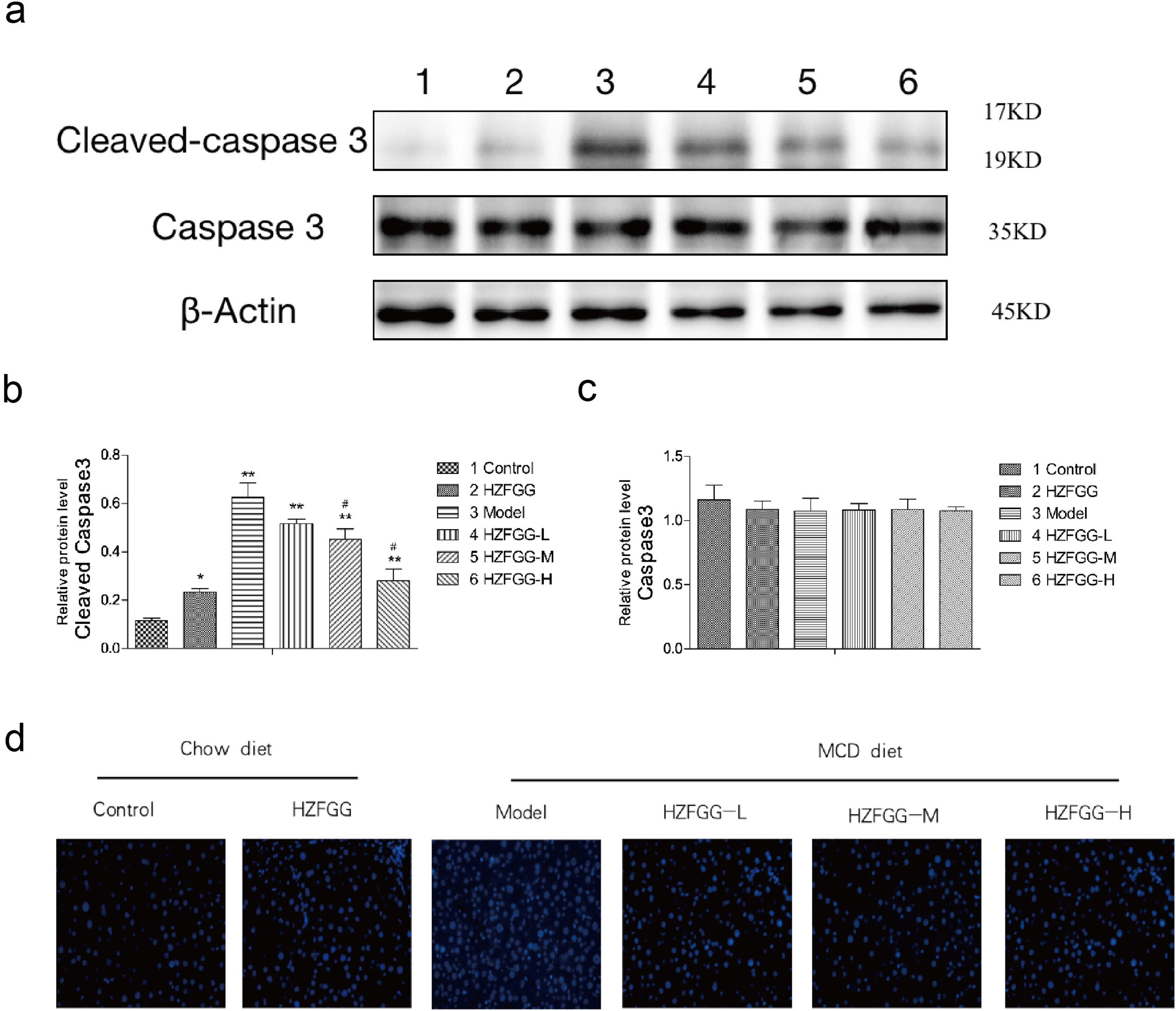
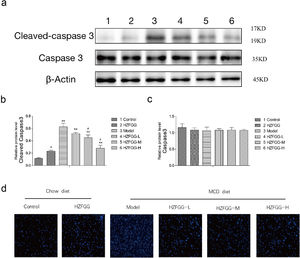
To detected the apoptosis of hepatocytes, we checked the expression level of cleaved-caspase-3, a major terminal shear enzyme in apoptosis, which was clearly increased with MCD diet and was decreased by HZFGG treatment in a dose-dependent manner (Fig. 6(a)). The quantitation results of the cleaved-caspase-3 and caspase-3 proteins expression levels were shown in Fig. 6(b) and (c). The liver sections were selected randomly in different groups to detected the apoptosis of hepatocytes through TUNEL assay, representative photos of TUNEL staining are shown in Fig. 6(d), the numbers of apoptotic cells were increased in MCD-induced group, which mean that the apoptosis of the hepatocyte were elevated and HZFGG treatment decreased the apoptosis index. These results suggested that HZFGG can alleviate the apoptosis induced by MCD-induced NAFLD mice.
HZFGG ameliorates hepatocyte apoptosis induced by an MCD diet in mice. (a) Western blotting analysis results of cleaved-caspase-3 and caspase-3 proteins in control group, HZFGG group, MCD diet group, MCD diet groups with HZFGG treatment, β-actin was used as the loading control. Results of quantification analysis of (b) cleaved-caspase-3 and (c) caspase-3 proteins in chow diet or MCD diet mice with or without HZFGG treatment. (d) Representative photos of TUNEL staining in chow diet or MCD diet mice with or without HZFGG treatment. The data were presented as the mean±SD and **P<0.01 vs control group, #P<0.05 vs model group.
NAFLD is a common metabolic disease, and always occurrence with other risk factors such as obesity, diabetes, and hyperlipidemia. A part of NAFLD patients would progress to NASH, which is more possible to develop fibrosis, cirrhosis, and hepatocellular carcinoma and contribute the high liver- related mortality.9 Therefore it is important to management of NAFLD and NASH. Currently, there is still no effective drugs to treat the NAFLD, physical activity and nutritional intervention are the main therapeutic options, although a few compounds, such as pioglitazone and vitamin E, were reported having effects for patients with NAFLD.28 In the present study, the data demonstrate that HZFGG treatment prevents steatosis, liver injury, inflammation, oxidative stress, and apoptosis in the MCD diet-induced NAFLD model. Based on these data, HZFGG may have potential as a therapeutic formula for the alleviation of NAFLD.
Since the pathogenesis of NAFLD is complex, the Traditional Chinese medicine (TCM) have multiple bioactive components and may an alternative solution for treatment of NAFLD. The protective effect of HZFGG against NAFLD probably occurs through multiple anti-inflammatory and anti-oxidation candidate compounds present in HZFGG formula. Some studies shown that Alisol A 24-Acetate (AA), which is an active triterpenoid compound isolated from Rhizoma Alismatis, distinctly decreased the content of TG, the numbers of lipid droplets, and Oil Red O lipid content in a free fatty acids (FFAs) induced NAFLD HepG2 cell model.29 Salviae miltiorrhizae has been used to treat liver diseases for centuries, its aqueous extract had hepatoprotective effects both in alcoholic liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD), salviae miltiorrhizae could alleviate hepatic inflammation, fatty degeneration, and haptic fibrogenesis.30 Salvianolic acid B (SalB), one of water-soluble phenolic acid extracted from Danshen, can attenuated liver damage, hepatic steatosis, and inflammation in high-fat diet (HFD)-induced rat NAFLD mode,31 salvianolic acid A (SalA), an efficacious polyphenol compounds extracted from Danshen, markly attenuated HFD-induced obesity and liver injury, and distinctly decreased the accumulation of lipid in HFD-fed rat livers.32 The present study showed that HZFGG treatment significantly lowered some biochemical parameters such as TG, TC, ALT and AST, which demonstrated that HZFGG can decrease the lipid accumulation and alleviate hepatic damage. We also found that HZFGG decreased the number of lipid droplets and Oil Red O lipid content observed from the slices of H&E and oil red O staining. Oxidative stress and inflammation are important factors in the pathological process of NAFLD. According to reports that Danshen decreased the level of oxidative stress through reducting the MDA levels in NAFLD mice model.30 SalA ameliorated hepatic inflammation and oxidative stress both in high-fat diet (HFD)-induced mice NAFLD and palmitic acid (PA)-induced HepG2 cells, the pro-inflammatory cytokines, such as TNF-α and IL-6 were decreased in SalA treatment group, the levels of H2O2, methane dicarboxylic aldehyde (MDA) and superoxide dismutase (SOD) activity were markly inhibited by supplement SalA in HFD-diet rats.32 AA suppressed reactive oxygen species (ROS) and inflammation in a NASH mouse model and inhibited the expression of inflammatory cytokines and ROS in LX-2 cells33 in PA-induced AML-12 cells. Baicalin, an active flavonoid compound isolated from Scutellaria baicalensis Georgi shown protection by inhibiting ER stress, oxidative stress and apoptosis.34 Inflammatory cytokines TNF-α, IL-6 levels were markedly inhibited by AA in HepG2 Cells.29 In the present study, we found that HZFGG treatment increased the level of GPx which indicated reducing oxidative stress, additionally, the inflammatory cytokines including TNF-α, and IL-1β were decreased after HZFGG supplement. The apoptosis of liver tissue was detected by TUNEL assay, HZFGG treatment reduced the amount of TUNEL-positive cells, decreased the level of cleaved-caspase 3 and has no obvious influence the expression level of caspase 3. These data suggest that HZFGG might attenuant the MCD diet induced hepatic injury by decreasing the level of inflammatory cytokines, oxidative stress and hepatocellular apoptosis.
The mechanisms underlying the development of NAFLD is deserved further study. Inflammation is an adaptive response for maintaining homeostasis when encounter the harmful conditions or stimuli, additionally, it a common factor to drive the development from NAFLD to NASH and patients with NASH more proble get cirrhosis and, ultimately, hepatocellular carcinoma (HCC).35 Therefore, it is important to alleviated the inflammation of NAFLD. There are some pathways involving in the progress of inflammation, such as Jak-Stat pathway,36 NF-κB signaling,37 TLR4 pathway,38 and so on. Previous studies have suggested that Epigallocatechin Gallate alleviated inflammation through inhibiting the signaling of Toll-like Receptor 4 (TLR4) in HFD-induced NAFLD rats.38 Gegen Qinlian Decoction abated inflammatory response by suppressing toll-like receptor 4 signaling pathways.39 Lycium barbarum polysaccharides shown hepatoprotective effects through reducing nod-like receptor protein 3/6 (NLRP3/6) and NF-κB activity in MCD-diet NASH mice model.37 Additionally, TLR4/NF-kB pathway involved in NAFLD or NASH also been studied. In MCD-diet NASH mice and lipopolysaccharide (LPS)-induced RAW264.7cell, Scoparone treatment blocked TLR-4/NF-κB signaling in a dose-dependent manner.27 Patients with NAFLD showed high levels of TLR4 and NF-κB, in an in vivo NAFLD model, metformin and berberine (BBR) improved HFD-induced NAFLD rats through the inhibition of TLR4/NF-κB signaling.26 Our data showed that the proteins level of MyD88 and TLR4 were increased in MCD-diet mice and significantly decreased by HZFGG. The expression levels of phosphot-NF-κB and phosphot-IκBa were also been detected in the present study, they were both reduced after treatment with HZFGG. These results indicated that HZFGG might alleviated inflammatory response and liver damage through the TLR4/NF-κB signaling pathway in MCD induced NAFLD mice.
The Hepatic fibrosis is associated with high morbidity and mortality in humans, which deserved further study. besides, although MCD diet has been used to induced NAFLD model over 40 years, there are still some limitations in this mode.24 Such as, obesity and metabolic syndrome are the characterized of NAFLD, however, the MCD-diet induced weight loss of mice does not exhibit insulin resistance.40,41 It difficult to construct animal model that perfectly mimic the complexity pathogenesis of human NAFLD. Nevertheless, the genetic models as well as combination models of genetic and nutritional factors which have been used to verify the hypotheses of the progress of the NAFLD disease. Therefore, it deserved to further research the novel animal model to help people understand and therapeutic the NAFLD. In conclusion, our study demonstrated that HZFGG improved MCD-induced NAFLD by reducing the accumulation of lipid and alleviated Steatosis, oxidative stress, inflammation, apoptosis. HZFGG might through inhibited the TLR4/NF-κB signaling pathway to attenuating the inflammation and liver injury. Based on the data we studied, HZFGG may have potential as a therapeutic formula for the alleviation of NAFLD.
FundingThe authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Shaanxi Infectious Disease Clinical Medical Research Center (Integrated Chinese and Western Medicine) Construction Project (2020LCZX-02) and Shaanxi Provincial Science and Technology Department Key R&D Program Project (2017SF-339).
Conflict of interestsThe authors declare that there is no conflict of interest.