Atherogenic dyslipidaemia is characterized by an increase in the plasma levels of total triglycerides (TG) and a fall in the cholesterol of high density lipoproteins (HDLc). Together with both of these lipid alterations which define atherogenic dyslipidaemia, we find an increase in TG-rich lipoprotein and carriers of apolipoprotein B (apoB) usually with a moderate increase, sometime with close to normal values, of the concentration of low density lipoproteins (LDLc), with a predominance of small dense LDL particles.1
Atherogenic dyslipidaemia is highly important as it is associated with different diseases which are currently widespread in the general population. These diseases are accompanied by a high degree of cardiovascular risk (CVR), including being overweight (37%), obesity (17%), diabetes (14%) and metabolic syndrome (30%).2,3 Moreover, atherogenic dyslipidaemia is in itself an indicator of high CVR in subjects with diabetes; it is therefore associated with a higher risk of silent myocardial ischemia or angiographic coronary disease in patients with type 2 diabetes and LDLc levels <130mg/dL.4
The fact is that there is a high prevalence of atherogenic dyslipidaemia in the Spanish population: it is present in 34% of diabetics, 21% of high-risk patients with controlled LDLc, and from 21% to 34% of patients with a history of vascular disease in some location (coronary, cerebral or the peripheral arteries).5
DiagnosisAtherogenic dyslipidaemia is characterized by hypertriglyceridaemia, which results from the increase in all of the TG-rich lipoproteins and their residual particles, and by moderate elevation of LDL; i.e., the set of atherogenic lipoproteins which contain apoB. All of these atherogenic lipoproteins can be quantified in terms of non-HDL cholesterol (non-HDL-c) or apoB. On the other hand, there is a reduction in HDLc.
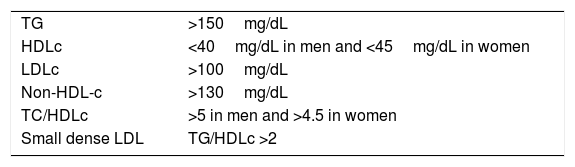
Together with these alterations, the presence of an increase in small dense LDL particles, calculated indirectly by the TG/HDLc index, and the increase in atherogenic coefficients, especially TC/HDLc, form the set of the findings in this dyslipidaemia,6 as shown in Table 1.
Atherogenic dyslipidaemia.
| TG | >150mg/dL |
| HDLc | <40mg/dL in men and <45mg/dL in women |
| LDLc | >100mg/dL |
| Non-HDL-c | >130mg/dL |
| TC/HDLc | >5 in men and >4.5 in women |
| Small dense LDL | TG/HDLc >2 |
HDLc: high density lipoprotein cholesterol; LDLc: low density lipoprotein cholesterol; LDL: low density lipoproteins; TG: triglycerides.
Non-HDL-c essentially represents the sum of the cholesterol of the lipoproteins that contain apoB, i.e., the atherogenic lipoproteins which are able to form deposits on the arterial wall. Due to this, it has been recommended that for patients with atherogenic dyslipidaemia the most appropriate therapeutic parameter is non-HDL-c or apoB, as both parameters correlate better with CVR than LDLc.7
Non-HDL-c has been shown to be a solid factor in CVR, so that in habitual clinical practice is may substitute apoB, as it is more economical and easier to calculate. This is because it is only necessary to subtract HDLc from total cholesterol, and these analytical parameters are available in all of the clinical laboratories in hospitals. The current recommendation is to measure apoB when this is clinically possible, especially in cases with atherogenic dyslipidaemia and its associated alterations, hypertriglyceridaemia, obesity, metabolic syndrome, diabetes and clinical cardiovascular disease.8
The scientific evidence for the association between high levels of LDLc and increased risk of cardiovascular disease is strong and incontrovertible. Nevertheless, even with suitable control of LDLc, there remains a considerable percentage of subjects who maintain a high vascular risk that is attributable to other lipid alterations, such as hypertriglyceridaemia and a fall in HDLc. The highest CVR is found when alterations coexist in all 3 lipid fractions: raised LDLc and triglycerides, and lower HDLc.9
In their meta-analysis of 62,154 patients included in 8 studies, Anderson et al.9 showed that non-HDL-c has a better correlation with CVR than LDLc; additionally, the subjects who attained therapeutic objectives for LDLc but not for non-HDL-c had a 32% increase in risk compared with those who achieved both objectives. Similar data were supplied by Boekholdt et al.10
Likewise, a recent study showed that progression of the atheroma plaque was more closely associated with concentrations of non-HDL-c than it was with those of LDLc. The lowest levels of non-HDL-c and TG therefore showed a significant association with plaque regression through the different CVR categories.11
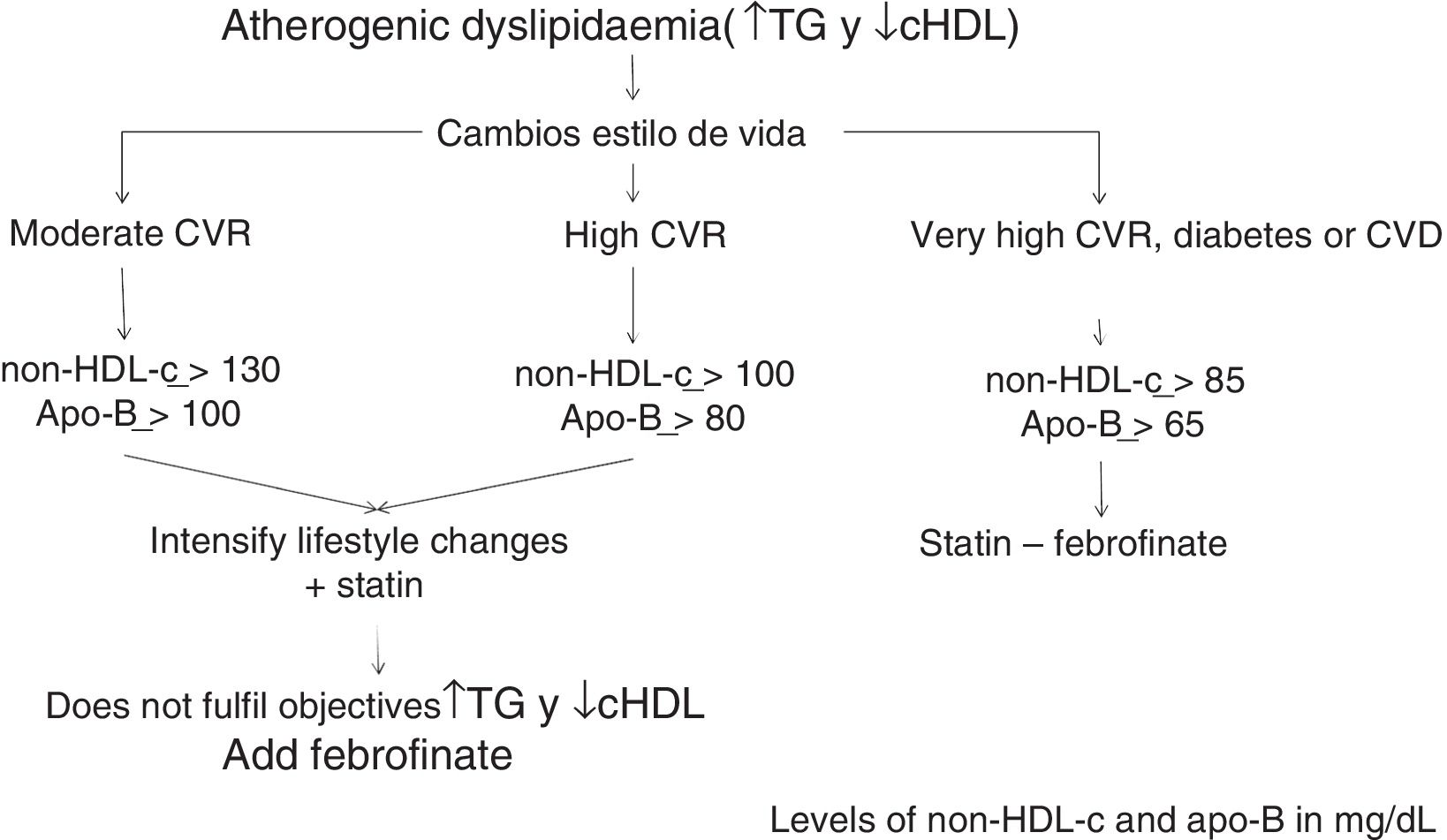
We therefore had to consider that in patients with atherogenic dyslipidaemia the main risk predictor and thus the main target for control is non-HDL-c.12 On the other hand, it has been established that the CVR in subjects with atherogenic dyslipidaemia is double or triple that of the general population and, in the majority of cases, they have a high CVR.13,14
Calculation of non-HDL-c based on total cholesterol minus HDLc is the CVR-dependent objective: its values have been set as those of the LDLc target plus 30mg/dL.
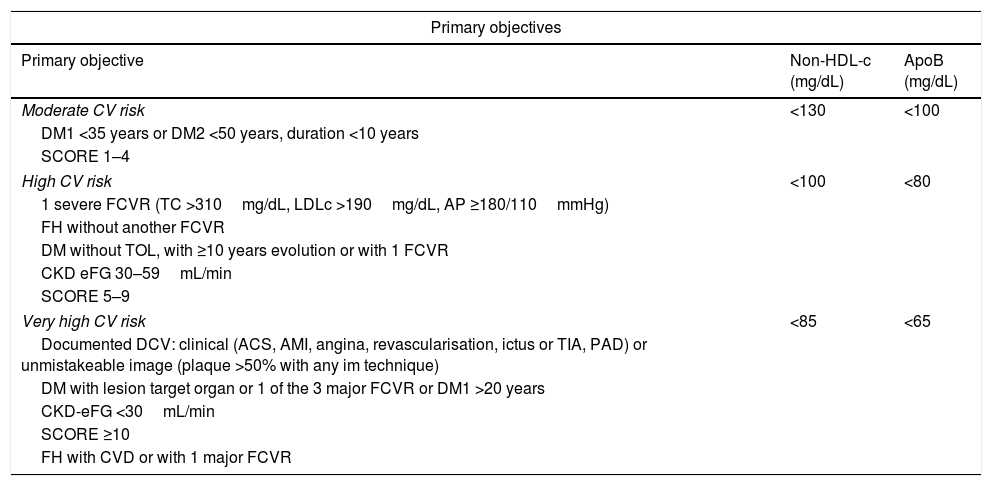
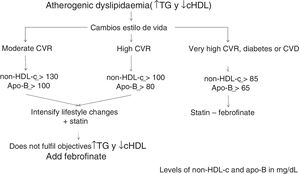
Consequently, the non-HDL-c and apoB goal will be: in moderate CVR <130mg/dL and <100mg/dL, respectively; in high CVR <100mg/dL and <80mg/dL, respectively, and in very high CVR <85mg/dL and <65mg/dL, respectively (Table 2).
Lipid objectives in atherogenic dyslipidaemia.
| Primary objectives | ||
|---|---|---|
| Primary objective | Non-HDL-c (mg/dL) | ApoB (mg/dL) |
| Moderate CV risk | <130 | <100 |
| DM1 <35 years or DM2 <50 years, duration <10 years | ||
| SCORE 1–4 | ||
| High CV risk | <100 | <80 |
| 1 severe FCVR (TC >310mg/dL, LDLc >190mg/dL, AP ≥180/110mmHg) | ||
| FH without another FCVR | ||
| DM without TOL, with ≥10 years evolution or with 1 FCVR | ||
| CKD eFG 30–59mL/min | ||
| SCORE 5–9 | ||
| Very high CV risk | <85 | <65 |
| Documented DCV: clinical (ACS, AMI, angina, revascularisation, ictus or TIA, PAD) or unmistakeable image (plaque >50% with any im technique) | ||
| DM with lesion target organ or 1 of the 3 major FCVR or DM1 >20 years | ||
| CKD-eFG <30mL/min | ||
| SCORE ≥10 | ||
| FH with CVD or with 1 major FCVR | ||
| Secondary objectives | TG (mg/dL) | HDLc (mg/dL) |
|---|---|---|
| After achieving the primary objective | <150 | >40H>45W |
ApoB: apolipoprotein B; non-HDL-c: cholesterol bound to atherogenic lipoproteins (total cholesterol minus high density lipoprotein cholesterol); HDLc: high density lipoprotein cholesterol; TC: total cholesterol; CV: cardiovascular; DM: diabetes mellitus; DM1: type 1 diabetes mellitus; PAD: peripheral arterial disease; CKD: chronic kidney disease; CVD: cardiovascular disease; eFG: estimated glomerular filtrate; FCVR: cardiovascular risk factor; W: men; FH: family hypercholesterolemia; AMI: acute myocardial infarct; TOL: target organ lesion; W: women; AP: arterial pressure; ACS: acute coronary syndrome; SCORE: European societies risk of cardiovascular death score; TG: triglycerides; TIA: transitory cerebral ischemia.
Hypertriglyceridaemia is an independent CVR factor: concentrations of TG <150mg/dL are considered optimum.15 Thus although the therapeutic objective has not been clearly established, it may be considered to stand at <150mg/dL.
Plasma HDLc concentrations <40mg/dL in men and <45mg/dL in women are also considered to be independent CVR factors, so that it would be desirable to achieve figures higher than these.15,16
The PROVE IT-TIMI 22 study was a prospective study of 4162 patients hospitalised due to acute coronary syndrome and treated with statins (pravastatin or atorvastatin). Analysis of its results showed that patients whose LDLc remained under 70mg/dL obtained a 16% reduction in recurring cardiovascular episodes, while the group that reduced LDLc (<70mg/dL) and TG below 150mg/dL achieved a fall in cardiovascular episodes of 28%.17
Consensus points in the clinical evaluation of atherogenic dyslipidaemiaBased on the available data and most important clinical evidence, the key points in connection with atherogenic dyslipidaemia associated CVR are:
- –
Hypertriglyceridaemia is an independent CVR factor that is exacerbated in the presence of high levels of LDLc or low levels of HDLc. It is one of the key elements in the residual vascular risk caused by lipids.
- –
To evaluate overall CVR it is indispensible to determine TG and HDLc.
- –
The most useful markers when evaluating the risk attributable to atherogenic dyslipidaemia are non-HDL-c (at a level higher than LDLc) or apoB and TG. TG are a marker of residual lipoproteins that are rich in TG and, indirectly, of the cholesterol of these lipoproteins. Dividing the concentration of TG by 5 if levels are expressed in mg/dL or by 2.2 if they are expressed in mmol/L allows us to calculate TG-rich residual lipoprotein cholesterol. A level >30mg/dL (>0.3mmol/L) indicates that there is an excess of the said cholesterol.
- –
Non-HDL-c is the appropriate therapeutic target for controlling CVR in patients with atherogenic dyslipidaemia. Although apoB is the ideal marker as it is correlated well with non-HDL-c, it may not be generally available for routine use. Both parameters are highly stable for calculating CVR in cases of atherogenic dyslipidaemia and its associated alterations.
Atherogenic dyslipidaemia is an extremely important entity that clearly contributes to the residual risk after treatment with statins. This dyslipidaemia is under-diagnosed, under-treated and under-controlled. It is particularly relevant in patients at high CVR and those with abdominal obesity, metabolic syndrome and diabetes.18
It is necessary to consider the treatment of this entity based on the available scientific evidence, to improve how it is treated as well as patient adherence.
Lifestyle changesThe first measures to reduce CVR in all patients are a healthy diet, regular physical exercise and smoking cessation.
The Mediterranean diet, with a reduction in total calorie intake in case of weight gain or abdominal obesity, is accompanied by clear cardiovascular benefits and increased longevity. As well as its beneficial effects on the lipid profile, it also has positive effects on hypertension and hyperglycaemia. The consumption of alcohol must be moderated or avoided in cases of moderate to severe hypertriglyceridaemia.
Aerobic physical exercise too is primordial in atherogenic dyslipidaemia and in the prevention and treatment of metabolic syndrome, hyperglycaemia, diabetes and cardiovascular disease.
Pharmacological treatmentAs the majority of patients with atherogenic dyslipidaemia are at high or very high risk of cardiovascular disease, different lipid lowering drugs must be combined with lifestyle changes.
StatinsTreatment with statins will start, selecting the type and dose necessary to achieve the therapeutic objective in terms of the required reduction in LDLc. The benefits of treatment with statins are well-known, and they have been clearly proven. A reduction of 1mmol/L (approximately 39mg/dL) in LDLc is associated with a 21% fall in the incidence of severe cardiovascular episodes and a 23% fall in coronary accidents.19
It is relevant that treatment with low power statins during 10 years in subjects at low CVR has been associated with a 23% reduction in non-mortal episodes of myocardial infarct. However, this reduction amounted to 53% when more powerful statins were used, including a significant reduction in cardiovascular events.20,21 Statins are the first pharmacological treatment to be used in patients with atherogenic dyslipidaemia, as a fall of 1mmol/L (39mg/dL) in residual cholesterol is also associated with an important reduction in cardiovascular events, similar to what has been observed with the reduction in LDLc.22
FibratesOnce treatment with statins has achieved the target LDLc level, there is still an unacceptably high risk of cardiovascular events due to the presence of the main components of atherogenic dyslipidaemia (hypertriglyceridaemia and reduced HDLc). Administering fibrates under these conditions corrects these lipid alterations and has additional cardiovascular benefits. Fibrates have been shown to be beneficial in primary and secondary prevention studies as well as in the diabetic population, above all in the subgroups with atherogenic dyslipidaemia or one of its components, with a fall of 28–30% in cardiovascular events.23,24
In the case of fibrate contraindication or intolerance and with raised TG, omega-3 fatty acids may be beneficial in these patients.25
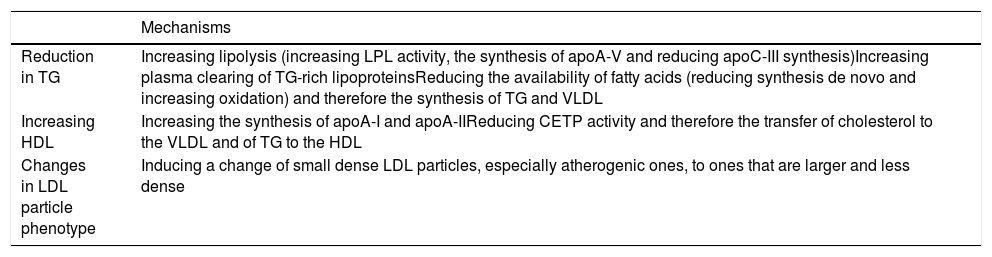
Fibrate-induced lipid changes are explained by modifications in the expressivity of several genes involved in lipid metabolism, through α receptors activated by peroxisome proliferation, with a fall in TG of 20–50%, in LDLc of 5–20% (with a reduction in small and dense LDL particles) and an increase in HDLc of from 5% to 20%. These effects depend on basal concentrations11 and are shown in Table 3.26
Lipid effects of fibrates.
| Mechanisms | |
|---|---|
| Reduction in TG | Increasing lipolysis (increasing LPL activity, the synthesis of apoA-V and reducing apoC-III synthesis)Increasing plasma clearing of TG-rich lipoproteinsReducing the availability of fatty acids (reducing synthesis de novo and increasing oxidation) and therefore the synthesis of TG and VLDL |
| Increasing HDL | Increasing the synthesis of apoA-I and apoA-IIReducing CETP activity and therefore the transfer of cholesterol to the VLDL and of TG to the HDL |
| Changes in LDL particle phenotype | Inducing a change of small dense LDL particles, especially atherogenic ones, to ones that are larger and less dense |
apoA-I: apolipoprotein A-I; apoA-II: apolipoprotein A-II; apoC-III: apolipoprotein C-III; apoA-V: apolipoprotein A-V; CETP: cholesterol esters transport protein; HDL: high density lipoproteins; LDL: low density lipoproteins; LPL: lipoprotein lipase; TG: triglycerides; VLDL: very low density lipoproteins.
Fenofibrate is the safest fibrate for association with a statin: adding it achieves a stronger cholesterol-lowering effect and control of non-HDL-c, TG and HDLc.
Unlike what occurs with gemfibrozil, the combination of fenofibrate and a statin has shown an excellent safety profile in all of the clinical studies, which include a large number of patients and prolonged treatment. Additionally, the effects of the fenofibrate–statin association on raised muscle or hepatic enzymes, or on the transitory increase in creatinine, do not differ from what has been observed in monotherapy regimes, so that the reversibility of its effects can be checked.
The FIRST study, which compared the effects of fibrates in subjects treated with atorvastatin on intima-media carotid artery thickness, found a significant reduction in the group treated with fibrates, especially the subjects with triglycerides >170mg/dL and in those with greater arterial intima-media thickness in the basal situation.27
Another work that supports the benefit of treatment with fenofibrate together with statins in subjects with metabolic syndrome is a prospective 5-year study with a large number of patients. This showed a reduction in the combined objective (ischemic coronary disease, cerebral ischemic disease and cardiovascular death) in 36% of the group with added fenofibrate in comparison with the placebo.28
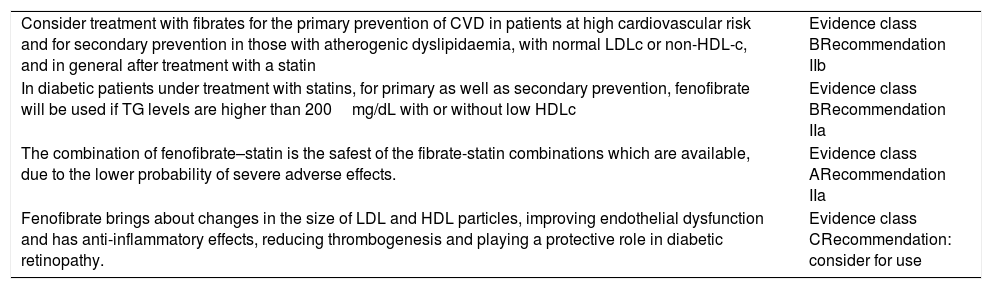
The main clinical evidence for treatment with fenofibrate is shown in Table 4.16,29
Evidence for treatment with fibrates.
| Consider treatment with fibrates for the primary prevention of CVD in patients at high cardiovascular risk and for secondary prevention in those with atherogenic dyslipidaemia, with normal LDLc or non-HDL-c, and in general after treatment with a statin | Evidence class BRecommendation IIb |
| In diabetic patients under treatment with statins, for primary as well as secondary prevention, fenofibrate will be used if TG levels are higher than 200mg/dL with or without low HDLc | Evidence class BRecommendation IIa |
| The combination of fenofibrate–statin is the safest of the fibrate-statin combinations which are available, due to the lower probability of severe adverse effects. | Evidence class ARecommendation IIa |
| Fenofibrate brings about changes in the size of LDL and HDL particles, improving endothelial dysfunction and has anti-inflammatory effects, reducing thrombogenesis and playing a protective role in diabetic retinopathy. | Evidence class CRecommendation: consider for use |
Non-HDL-c: cholesterol bound to atherogenic lipoproteins (total cholesterol minus high density lipoprotein cholesterol); HDLc: high density lipoprotein cholesterol; LDLc: low density lipoprotein cholesterol; CVD: cardiovascular disease; TG: triglycerides.
The results of clinical studies with fenofibrate indicate that as well as changes in the lipid profile, it has other protective vascular effects such as increasing the expression of nitric oxide and reducing oxidative stress. Fibrates have an anti-inflammatory action as they attenuate the production of inflammatory cytokines. Fenofibrate complements this anti-inflammatory action by significantly reducing reactive C protein, the CD40 ligand, monocyte-1 chemotactic protein and the macrophage stimulant factor. It also reduces the plasma concentrations of fibrinogen by up to 20%, thrombin-antithrombin complexes and plasminogen-1 activator inhibitor. Another important extralipid effect is the slowing down of the progression of diabetic retinopathy, independently of glycemic and lipid control. Additionally, these studies indicate that fenofibrate plays a protective role in nephropathy and diabetic neuropathy.29
The algorithm shown in Fig. 1 is based on these facts, for the treatment of atherogenic dyslipidaemia and the control of its associated CVR.
Consensus points in the treatment of atherogenic dyslipidaemia- –
Changes in lifestyle, a lipid reducing diet with appropriate calories for weight control, physical exercise and smoking cessation are highly effective in all patients and are the first step in managing atherogenic dyslipidaemia.
- –
Following changes in lifestyle, statins are the effective and safe initial treatment for the prevention of cardiovascular risk.
- –
Patients with hypertriglyceridaemia and low HDLc, that is, with atherogenic dyslipidaemia, would benefit from combined statin–fenofibrate therapy.
- –
Clinical practice guides and the European Medicines Agency indicate fenofibrate for the treatment of mixed hyperlipidaemia together with a statin when TG and non-HDLc are not properly controlled.
- –
The evidence for the clinical benefit and safety of the statin–fenofibrate association is solid. The combination of both drugs in a single tablet simplifies the dosage and may aid adherence over the long term.
This is a Spanish Arteriosclerosis Society workgroup and it receives financial support from Mylan.
Please cite this article as: Ascaso JF, Millán J, Hernández-Mijares A, Blasco M, Brea Á, Díaz Á, et al. Dislipidemia aterogénica 2019. Documento de consenso del Grupo de Dislipidemia Aterogénica de la Sociedad Española de Arteriosclerosis. Clin Investig Arterioscler. 2020;32:120–125.