High blood pressure in individuals with type2 diabetes mellitus increases the risk of cardiovascular events. The international management guidelines recommend starting pharmacological treatment with blood pressure values >140/90mmHg. However, there is no optimal cut-off point from which cardiovascular events can be reduced without causing adverse events. A blood pressure range of >130/80 to <140/90mmHg seems to be adequate. These values can be achieved through non-pharmacological (diet, exercise) and pharmacological interventions (using drugs that have been shown to reduce cardiovascular events). The choice of one or several drugs must be individualised, according to factors including, ethnicity, age, and associated comorbidities, among others.
La hipertensión arterial en individuos con diabetes mellitus tipo2 incrementa el riesgo de eventos cardiovasculares. Las guías internacionales de manejo recomiendan iniciar tratamiento farmacológico con valores de presión arterial >140/90mmHg. Sin embargo, no existe un punto de corte óptimo a partir del cual se logre reducir los eventos cardiovasculares sin originar eventos adversos; un rango de presión arterial >130/80 y <140/90mmHg parece ser el adecuado. Estos valores pueden alcanzarse mediante intervenciones no farmacológicas (dieta, ejercicio) y farmacológicas (por fármacos que hayan demostrado reducir eventos cardiovasculares). La elección de uno o varios fármacos debe ser individualizada, de acuerdo con factores como etnia, edad, comorbilidades asociadas, entre otros.
Cardiovascular disease (CVD) is still the leading cause of morbidity and mortality in adults, and, of all the major cardiovascular risk factors (CVRF), hypertension (HTN) is the most prevalent. However, diabetes mellitus (DM) is also considered an independent risk factor for the development of CVD. The co-existence of HTN and DM consequently represents a greater CVRF than the isolated presence of only one of these conditions, taking into account that the two diseases have certain metabolic aspects in common (e.g. dyslipidaemia, obesity, endothelial dysfunction, insulin resistance, atherosclerosis). It has been established that patients with HTN at the time of diagnosis with type2 DM (T2DM) have a high risk of all-cause mortality when compared with normotensive patients without T2DM.1 Blood pressure (BP) control plays a key role in patients with T2DM. Different studies have evaluated multiple BP targets and their association with cardiovascular (CV) outcomes, but some questions remain unanswered. Lowering BP using pharmacological (antihypertensives [anti-HTN]) and non-pharmacological interventions is the most effective strategy for reducing CV morbidity and mortality in individuals with T2DM. Evidence is available from randomised clinical trials, observational studies and meta-analyses in different clinical settings (e.g. elderly patients with isolated systolic hypertension, individuals with essential hypertension or high CVRF, or patients with coronary artery disease (CAD) or a history of stroke). International guidelines recommend starting or increasing anti-HTN treatment if BP>140/90mmHg, although the ideal cut-off point for patients with T2DM is not yet clear.2 BP targets for patients with T2DM have varied between the following values: <140/90, <140/85, <140/80, <130/85, <130/80mmHg. However, these targets were supported by poor-quality evidence.
Search criteriaA thorough search of the literature for epidemiology articles in English and Spanish was performed in various databases (PubMed, Cochrane Library, Embase, DARE, LILACS and SciELO) using the following terms (in English and Spanish): [“diabetes”, “arterial hypertension”, “cardiovascular outcomes”, “diet”, “exercise”, and “antihypertensives”], alone or in combination. The primary focus of the review was based on randomised clinical trials, meta-analyses, systematic reviews and longitudinal studies with cardiovascular outcomes from January 1960 to April 2018.
Global burden of diabetes mellitus and hypertensionDM is defined as a state of chronic hyperglycaemia, of heterogeneous aetiology, caused by abnormalities in carbohydrate, fat and protein metabolism. Specific DM-related complications can be both microvascular (retinopathy, nephropathy and neuropathy) and macrovascular (CVD, cerebrovascular disease and peripheral arterial disease). Chronic hyperglycaemia also refers to the so-called intermediate states between normal blood glucose levels and the presence of DM (prediabetes), which include impaired fasting glucose (IFG), defined as fasting blood glucose levels ≥100mg/dl and <126mg/dl, and impaired glucose tolerance (IGT), defined as blood glucose levels 2h after 75g oral glucose load ≥140mg/dl and <200mg/dl. Both IFG and IGT represent and define a high risk for the subsequent development of T2DM and CVD.1–3 The relationship between T2DM and CVD is complex and multifactorial since T2DM can coexist with other CVRF, such as HTN, dyslipidaemia, smoking and obesity, and with other associated metabolic states (increased oxidative stress, low-grade inflammation, insulin resistance, endothelial dysfunction, autonomic neuropathy and hypercoagulability), which together contribute directly to the development of CVD.4 Individuals with T2DM have a two- to four-fold higher risk of developing CVD than those without T2DM, and around 70% of patients with T2DM aged ≥65 die from CVD. Also, individuals with T2DM but no history of CVD are at the same risk as non-diabetic individuals with a previous history of myocardial infarction (MI). Although CVD-attributable mortality rates have decreased among individuals with and without T2DM, the global CVD burden among patients with T2DM is still unacceptably high. Furthermore, at the time of diagnosis, most patients with T2DM have at least one CVRF (associated or unassociated) for manifest atherosclerotic disease, which suggests that both diseases share genetic, epigenetic and environmental factors.5,6 In 2017, it was estimated that 451 million people worldwide had T2DM (with projections of 693 million cases by 2045). It was also estimated that 49.7% of those with T2DM were undiagnosed. However, it was established that 374 million people had IGT and around 5 million deaths worldwide were attributable to T2DM in the age range of 20–99 years. Finally, the estimated global healthcare expenditure for this condition in 2017 was 850,000 million US dollars.7–10
Meanwhile, HTN is a major CVRF and is considered one of the five main causes of death worldwide. HTN has a pattern of silent clinical presentation, which means that a large group of individuals are undiagnosed and untreated, leading to a marked increase in the risk of sudden and/or premature death and other microvascular and macrovascular complications. In 2010, it was established that worldwide 31.1% of the adult population ≥20 years-old had HTN, defined as systolic BP (SBP) ≥140mmHg and/or diastolic BP (DBP) ≥90mmHg. It was also estimated that 9–10 million deaths worldwide were caused by HTN.11–13 This is extremely important, especially if we consider that of all the individuals with HTN, only 57% are aware of their condition, 40.6% are taking some kind of anti-HTN and only 13.2% have adequate blood pressure control.14,15
HTN is common among individuals with T2DM and the frequent coexistence of these two conditions in the same patient is considered a strong determinant of endothelial dysfunction, atherosclerotic disease and vascular damage. It has been established that the prevalence of HTN is 1.5–2.0 times higher in patients with T2DM than in those without T2DM, and its frequency depends on certain associated factors, such as severity of obesity and atherosclerosis, insulin resistance, old age and glomerular filtration rate.16,17 Based on observational studies, the prevalence of HTN (defined as BP ≥140/90mmHg or BP under control with anti-HTN) in individuals with T2DM is currently >50%. This frequency may be influenced by the fact that both conditions, when present (separately) for a long time in the same individual, increase the probability of co-existence.18,19 Few studies have evaluated the association between co-existence of HTN and DM and the presence of CVD. However, the few studies conducted show that the risk of CVD in these types of patients is at least five times higher than in individuals without HTN and without T2DM, and is at least three times higher in men and 5–7 times higher in women. Furthermore, the presence of HTN is responsible for a 7.2-fold increase in death in individuals with T2DM.20,21 Global differences in the distribution of observed frequencies of HTN and T2DM seem to be associated with aspects such as worldwide economic development, and, therefore, with changes in lifestyle, environmental factors, alcohol consumption, smoking, obesity, low levels of physical activity, and nutritional, demographic and epidemiological transition.22
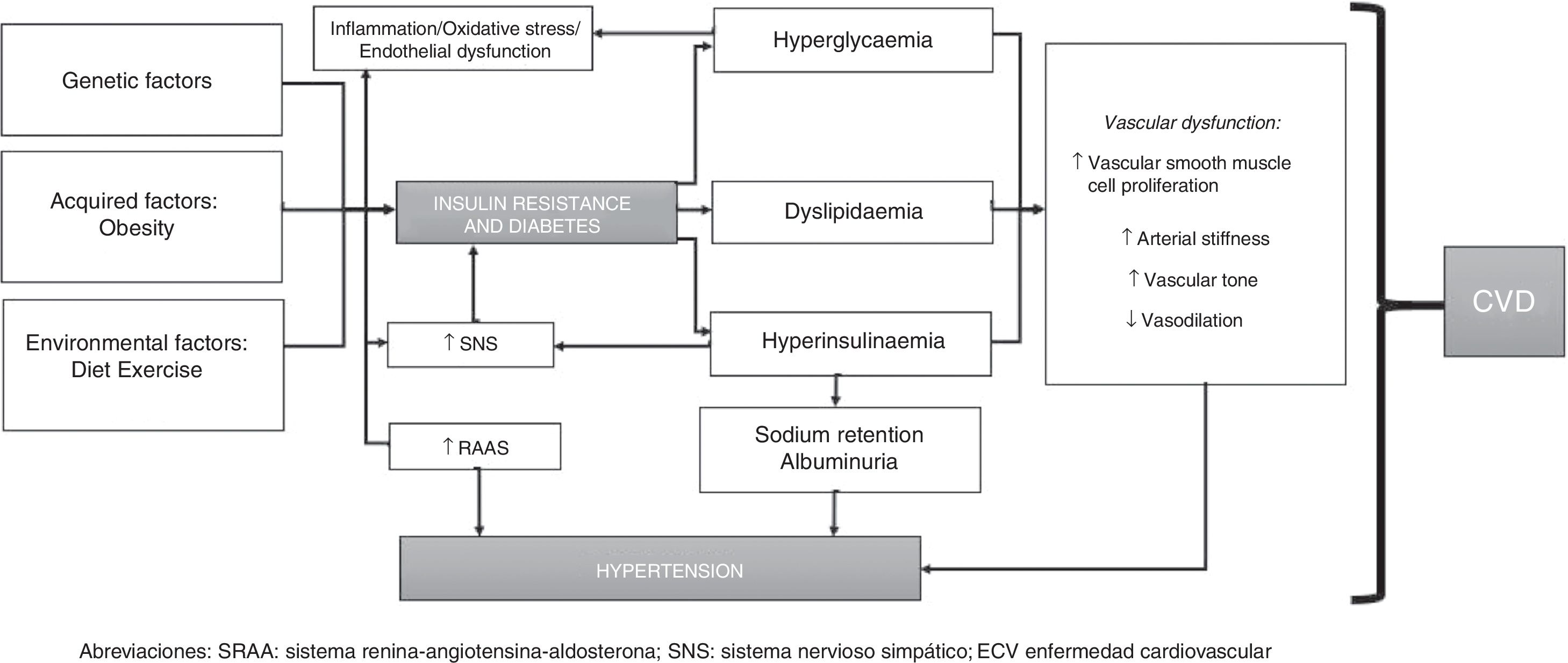
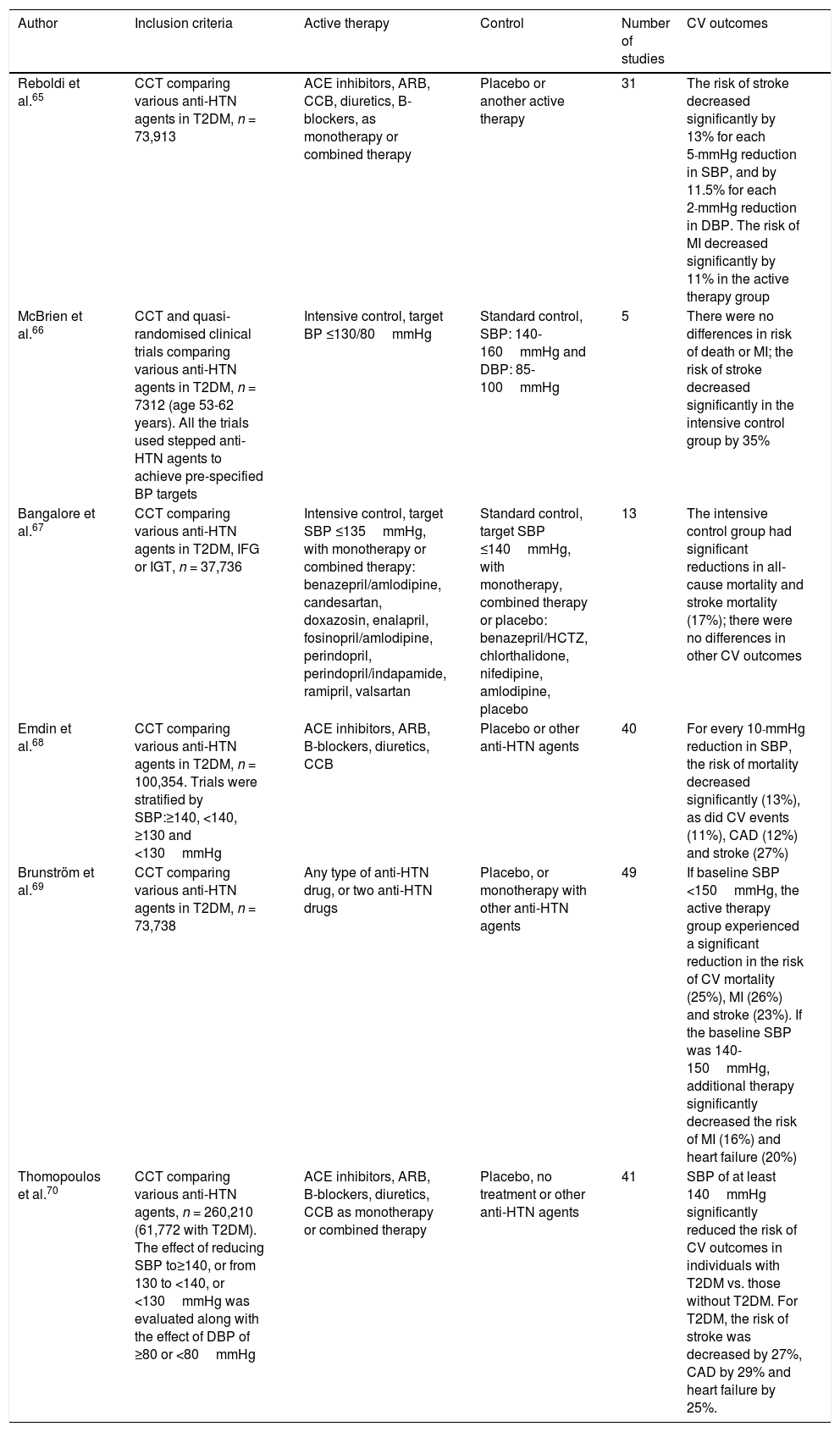
PathophysiologyThe pathophysiology of HTN and T2DM is highly interrelated and poorly understood. HTN in individuals with DM is generally considered to be volume dependent since hyperglycaemia increases extracellular fluid osmolality. Therefore, as water shifts from the intracellular space to the extracellular space (to maintain osmotic balance), the extracellular fluid volume expands causing intracellular dehydration, resulting in a state of volume overload (unless hyperglycaemia is sufficiently severe to cause osmotic diuresis, in which volume overload is less likely). Furthermore, endothelial cells play a critical role in maintaining vascular homeostasis and their dysfunction is associated with CVD and other metabolic disorders. Endothelial dysfunction is therefore considered to be the most important factor in the development of HTN in patients with T2DM. Such dysfunction is characterised by upset of the regulation of balance between vasodilation and vasoconstriction, together with increased oxidative stress, vascular inflammation, changes in fibrinolytic and prothrombotic pathways, abnormal smooth muscle cell proliferation and impaired repair mechanisms.23–25
In addition to impaired endothelial function, the renin-angiotensin-aldosterone system (RAAS) is also a key modulator of vascular function and RAAS hyperactivity is involved in endothelial dysfunction. The RAAS comprises several components: renin, angiotensinII (ATII), angiotensin-converting enzyme (ACE), angiotensinI (ATI) receptor and aldosterone. Interactions between these components promote vasoconstriction, proliferation and oxidative stress, vascular remodelling and injury, cell growth, inflammation and activation of some pro-inflammatory transcription factors (such as nuclear factor κB). Together, these molecules increase and promote hyperplasia/hypertrophy of vascular smooth muscle cells, sodium reabsorption and enhanced peripheral vascular resistance, with subsequent increases in BP.
Diabetic individuals with HTN generally have low or normal circulating levels of renin and these levels are even lower in patients with diabetic nephropathy. However, levels classed as “normal” are actually unsuitably high for observed volume expansion in T2DM, although it is clear that an absolute excess of renin production is an unlikely cause of HTN in T2DM.26–28
Arterial stiffness may occur in both T2DM per se and in association with HTN since both conditions cause endothelial dysfunction and inflammation, which can promote increased levels of adhesion molecules and inflammatory cytokines (mainly ICAM-1 and TNF-α). This arterial stiffness has been reproduced in several studies, which have shown that HTN contributes to arterial stiffness and endothelial dysfunction in patients with T2DM.29
Moreover, in individuals with T2DM, insulin resistance and hyperglycaemia produce inflammation and oxidative stress, and dyslipidaemia, which often affects diabetic patients with HTN, is also associated with arterial stiffness and vascular dysfunction. Hyperinsulinaemia also causes sodium retention and increased sympathetic nervous system (SNS) activity, with subsequent increases in circulating catecholamines, causing an increase in BP, which in turn activates the RAAS (also promoting inflammation) and converts this process into a vicious circle.30,31
However, advances in genomic studies offer a broader view of the pathogenesis of HTN and T2DM. Both HTN and T2DM are characterised by their complex nature and their high genetic variability, with gene-gene, gene-environment and epigenetic factor interactions. Different genes have been implicated in the development of HTN, including CACANA1H, IPO7, PMS1, SLC24A4, YWHAZ, GPR98, ARRDC3, C21orf91, SLC25A42, and HLA-B genes. Genes associated with T2DM include SLC44A3, F3, RBM43, RND3, GALNTL4, CPA6, LOC729013 and transcription factors such as TCF7L2, which is strongly associated with the development of T2DM in various ethnic groups.32–34
To conclude, the pathophysiology of HTN in individuals with T2DM can be summarised as follows: insulin resistance (and resulting hyperinsulinaemia) induces activation of the SNS and RAAS, promoting sodium and water retention. T2DM, however, leads to increased vascular reactivity and vascular smooth muscle cell proliferation, which play a significant role in the development of HTN. Hyperglycaemia and increased total body sodium can cause excess extracellular fluid and plasma volume expansion. Furthermore, individuals with T2DM have enhanced vascular sensitivity to vasoactive hormones (especially if dietary sodium intake is high), which, together with hyperinsulinaemia, helps keep BP levels high (since insulin promotes sodium retention and enhances SNS activity). This is mediated by genetic and epigenetic factors and interactions between such factors (Fig. 1).
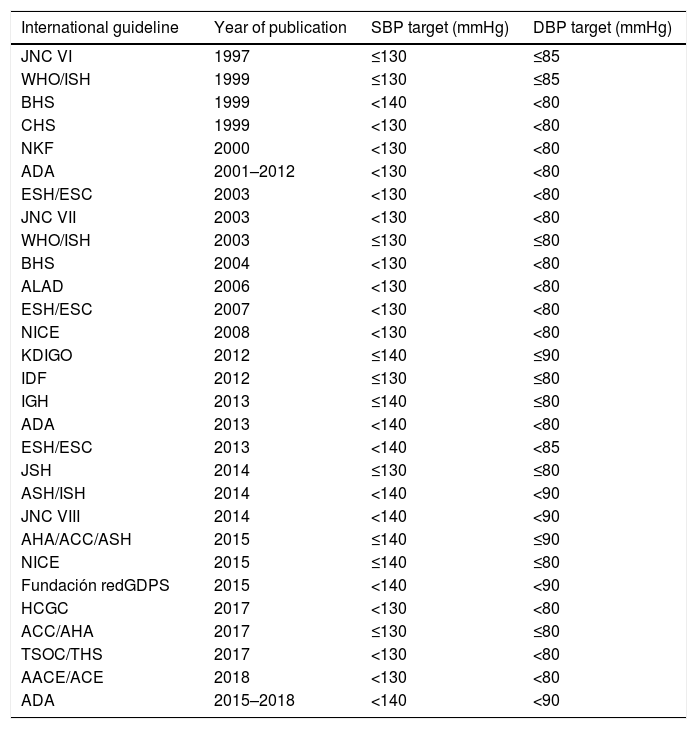
History of blood pressure control targets in type 2 diabetes mellitusThe various international management guidelines have focused on establishing BP thresholds for individuals with T2DM and have discussed one or more evidence-based management options. Thus, the following targets have been described over the years: BP <130/85, <130/80, <140/90, <140/80 and <140/85mmHg (Table 1). Initial guidelines and recommendations established an ideal target of BP <130/80mmHg. However, studies supporting this recommendation showed that the BP values achieved were >135/85mmHg, indicating that the recommendation to keep BP below 130/80mmHg was perhaps not the most appropriate. Moreover, some studies showed that strict BP control failed to reduce expected CV outcomes.35–37 This led to some queries and concerns. For example, would starting drug therapy to achieve an established SBP and DBP threshold change the results or clinical outcomes? Would drug therapy to achieve a specific SBP and DBP target change CV outcomes or mortality? Or were there any differences amongst the various anti-HTN drugs in terms of CV outcomes and mortality? Despite this, there was evidence that BP >115/75mmHg was associated with increased rates of micro/macrovascular complications and mortality, indicating that BP control in diabetic individuals was important for preventing fatal and non-fatal clinical outcomes. Furthermore, the existing relationship between BP and CV outcomes was also considered to be consistent, constant and independent from other CVRF since observational studies in adults with no history of vascular disease showed that the risk of death from vascular (CV and cerebrovascular) events increased linearly and progressively according to BP. It was also found that aggressively reducing BP did not necessarily result in substantial improvements in vascular injury. Moreover, trying to achieve stricter BP targets has been documented to require higher doses of anti-HTN drugs or a combination of several such drugs, thereby increasing the risk of adverse effects, including major CV events (known as the “J-curve phenomenon”). It has also been established that the relationship between BP and CV outcomes showed a “U-shaped” curve, indicating that the rate of vascular events was high with either very low or very high BP levels and that BP <110–120/60–70mmHg may predict an increased risk of vascular events.38–40
Blood pressure targets in type 2 diabetes mellitus, from various management guidelines.
| International guideline | Year of publication | SBP target (mmHg) | DBP target (mmHg) |
|---|---|---|---|
| JNC VI | 1997 | ≤130 | ≤85 |
| WHO/ISH | 1999 | ≤130 | ≤85 |
| BHS | 1999 | <140 | <80 |
| CHS | 1999 | <130 | <80 |
| NKF | 2000 | <130 | <80 |
| ADA | 2001–2012 | <130 | <80 |
| ESH/ESC | 2003 | <130 | <80 |
| JNC VII | 2003 | <130 | <80 |
| WHO/ISH | 2003 | ≤130 | ≤80 |
| BHS | 2004 | <130 | <80 |
| ALAD | 2006 | <130 | <80 |
| ESH/ESC | 2007 | <130 | <80 |
| NICE | 2008 | <130 | <80 |
| KDIGO | 2012 | ≤140 | ≤90 |
| IDF | 2012 | ≤130 | ≤80 |
| IGH | 2013 | ≤140 | ≤80 |
| ADA | 2013 | <140 | <80 |
| ESH/ESC | 2013 | <140 | <85 |
| JSH | 2014 | ≤130 | ≤80 |
| ASH/ISH | 2014 | <140 | <90 |
| JNC VIII | 2014 | <140 | <90 |
| AHA/ACC/ASH | 2015 | ≤140 | ≤90 |
| NICE | 2015 | ≤140 | ≤80 |
| Fundación redGDPS | 2015 | <140 | <90 |
| HCGC | 2017 | <130 | <80 |
| ACC/AHA | 2017 | ≤130 | ≤80 |
| TSOC/THS | 2017 | <130 | <80 |
| AACE/ACE | 2018 | <130 | <80 |
| ADA | 2015–2018 | <140 | <90 |
AACE, American Association of Clinical Endocrinologists; ACC, American College of Cardiology; ACE, American College of Endocrinology; ADA, American Diabetes Association; AHA, American Heart Association; ALAD, Asociación Latinoamericana de diabetes (Latin American Association of Diabetes); ASH, American Society of Hypertension; BHS, British Hypertension Society; CHS, Canadian Hypertension Society; DBP, diastolic blood pressure; ESC, European Society of Cardiology; ESH, European Society of Hypertension; Fundación redGDPS, Fundación de red de Grupos de Estudio de la Diabetes en Atención Primaria de la Salud [Foundation of Study Groups Network on Diabetes in Primary Healthcare]; HCGC, The Hypertension Canada Guidelines Committee; IDF, International Diabetes Federation; IGH, Indian Guidelines on Hypertension; ISH, International Society of Hypertension; JNC, Joint National Committee; JSH, Japanese Society of Hypertension; KDIGO, Kidney Disease Improving Global Outcomes; NICE, National Institute for Health and Care Excellence; NKF, National Kidney Foundation; SBP, systolic blood pressure; THS, Taiwan Hypertension Society; TSOC, Taiwan Society of Cardiology.
Studies evaluating the impact of BP control in individuals with T2DM were designed primarily to evaluate the effect of glycaemic control in individuals with T2DM, or to evaluate the presence of CV outcomes in subgroups of subjects with T2DM (who were part of studies on anti-HTN therapy). These studies were characterised by their high heterogeneity and lack of uniformity (e.g. in terms of their definition of BP targets). Each of these studies evaluated several groups of anti-HTN drugs, including ACE inhibitors, angiotensin receptor blockers (ARB), calcium-channel blockers (CCB), dihydropyridines and thiazide diuretics (all considered first-line anti-HTN drugs), and, to a lesser extent, other anti-HTN drugs that may be useful according to the patient's baseline characteristics or conditions (vasodilators, alpha-blockers, beta-blockers and loop diuretics). The studies mentioned focused on evaluating the following aspects (Tables 2 and 3):
- 1.
Effect of anti-HTN therapy on population aged ≥60 years and CV outcomes.
- 2.
Effect of anti-HTN therapy, evaluating strict control vs. less strict (or moderate) BP control and CV outcomes.
- 3.
Effect of anti-HTN therapy, CV outcomes and mortality.
- 4.
Effect of anti-HTN therapy, comparing intensive control vs. less intensive or conventional BP control.
- 5.
Effect of anti-HTN therapy with regards to different DBP targets or categories and CV outcomes.
- 6.
Effect of anti-HTN therapy and CV outcomes in individuals at high CVR.
- 7.
Effect of anti-HTN therapy and CV outcomes in individuals with diabetic nephropathy.
- 8.
Effect of anti-HTN therapy and CV outcomes, beyond the effect attributable to a reduction in BP.
- 9.
Effect of anti-HTN therapy and stroke recurrence.
- 10.
Effect of intensive therapy (target-based, using a combination of drugs and lifestyle changes) vs. conventional multifactorial therapy, and CV outcomes.
- 11.
Effect of anti-HTN therapy and CV outcomes in individuals with established heart disease.
- 12.
Effect of anti-HTN therapy and atherosclerotic disease progression.41–70
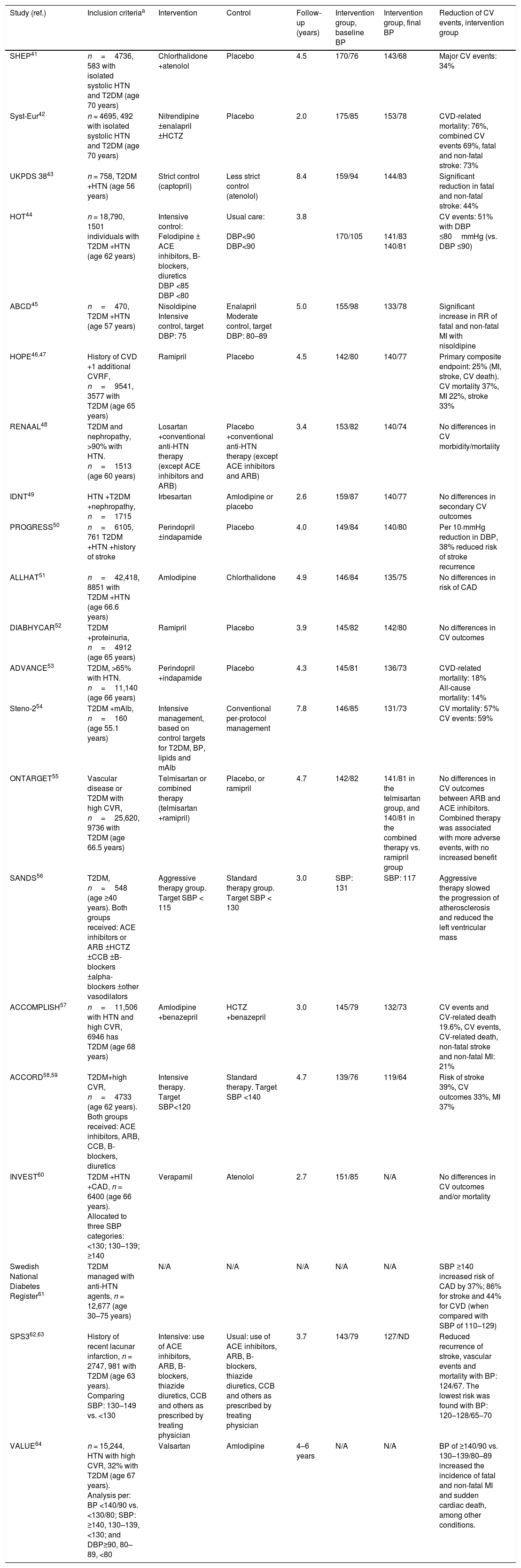
Observational studies and clinical trials that have evaluated the effect of blood pressure therapy in type 2 diabetes mellitus.
| Study (ref.) | Inclusion criteriaa | Intervention | Control | Follow-up (years) | Intervention group, baseline BP | Intervention group, final BP | Reduction of CV events, intervention group |
|---|---|---|---|---|---|---|---|
| SHEP41 | n=4736, 583 with isolated systolic HTN and T2DM (age 70 years) | Chlorthalidone +atenolol | Placebo | 4.5 | 170/76 | 143/68 | Major CV events: 34% |
| Syst-Eur42 | n = 4695, 492 with isolated systolic HTN and T2DM (age 70 years) | Nitrendipine ±enalapril ±HCTZ | Placebo | 2.0 | 175/85 | 153/78 | CVD-related mortality: 76%, combined CV events 69%, fatal and non-fatal stroke: 73% |
| UKPDS 3843 | n = 758, T2DM +HTN (age 56 years) | Strict control (captopril) | Less strict control (atenolol) | 8.4 | 159/94 | 144/83 | Significant reduction in fatal and non-fatal stroke: 44% |
| HOT44 | n = 18,790, 1501 individuals with T2DM +HTN (age 62 years) | Intensive control: Felodipine ± ACE inhibitors, B-blockers, diuretics DBP <85 DBP <80 | Usual care: DBP<90 DBP<90 | 3.8 | 170/105 | 141/83 140/81 | CV events: 51% with DBP ≤80mmHg (vs. DBP ≤90) |
| ABCD45 | n=470, T2DM +HTN (age 57 years) | Nisoldipine Intensive control, target DBP: 75 | Enalapril Moderate control, target DBP: 80–89 | 5.0 | 155/98 | 133/78 | Significant increase in RR of fatal and non-fatal MI with nisoldipine |
| HOPE46,47 | History of CVD +1 additional CVRF, n=9541, 3577 with T2DM (age 65 years) | Ramipril | Placebo | 4.5 | 142/80 | 140/77 | Primary composite endpoint: 25% (MI, stroke, CV death). CV mortality 37%, MI 22%, stroke 33% |
| RENAAL48 | T2DM and nephropathy, >90% with HTN. n=1513 (age 60 years) | Losartan +conventional anti-HTN therapy (except ACE inhibitors and ARB) | Placebo +conventional anti-HTN therapy (except ACE inhibitors and ARB) | 3.4 | 153/82 | 140/74 | No differences in CV morbidity/mortality |
| IDNT49 | HTN +T2DM +nephropathy, n=1715 | Irbesartan | Amlodipine or placebo | 2.6 | 159/87 | 140/77 | No differences in secondary CV outcomes |
| PROGRESS50 | n=6105, 761 T2DM +HTN +history of stroke | Perindopril ±indapamide | Placebo | 4.0 | 149/84 | 140/80 | Per 10-mmHg reduction in DBP, 38% reduced risk of stroke recurrence |
| ALLHAT51 | n=42,418, 8851 with T2DM +HTN (age 66.6 years) | Amlodipine | Chlorthalidone | 4.9 | 146/84 | 135/75 | No differences in risk of CAD |
| DIABHYCAR52 | T2DM +proteinuria, n=4912 (age 65 years) | Ramipril | Placebo | 3.9 | 145/82 | 142/80 | No differences in CV outcomes |
| ADVANCE53 | T2DM, >65% with HTN. n=11,140 (age 66 years) | Perindopril +indapamide | Placebo | 4.3 | 145/81 | 136/73 | CVD-related mortality: 18% All-cause mortality: 14% |
| Steno-254 | T2DM +mAlb, n=160 (age 55.1 years) | Intensive management, based on control targets for T2DM, BP, lipids and mAlb | Conventional per-protocol management | 7.8 | 146/85 | 131/73 | CV mortality: 57% CV events: 59% |
| ONTARGET55 | Vascular disease or T2DM with high CVR, n=25,620, 9736 with T2DM (age 66.5 years) | Telmisartan or combined therapy (telmisartan +ramipril) | Placebo, or ramipril | 4.7 | 142/82 | 141/81 in the telmisartan group, and 140/81 in the combined therapy vs. ramipril group | No differences in CV outcomes between ARB and ACE inhibitors. Combined therapy was associated with more adverse events, with no increased benefit |
| SANDS56 | T2DM, n=548 (age ≥40 years). Both groups received: ACE inhibitors or ARB ±HCTZ ±CCB ±B-blockers ±alpha-blockers ±other vasodilators | Aggressive therapy group. Target SBP < 115 | Standard therapy group. Target SBP < 130 | 3.0 | SBP: 131 | SBP: 117 | Aggressive therapy slowed the progression of atherosclerosis and reduced the left ventricular mass |
| ACCOMPLISH57 | n=11,506 with HTN and high CVR, 6946 has T2DM (age 68 years) | Amlodipine +benazepril | HCTZ +benazepril | 3.0 | 145/79 | 132/73 | CV events and CV-related death 19.6%, CV events, CV-related death, non-fatal stroke and non-fatal MI: 21% |
| ACCORD58,59 | T2DM+high CVR, n=4733 (age 62 years). Both groups received: ACE inhibitors, ARB, CCB, B-blockers, diuretics | Intensive therapy. Target SBP<120 | Standard therapy. Target SBP <140 | 4.7 | 139/76 | 119/64 | Risk of stroke 39%, CV outcomes 33%, MI 37% |
| INVEST60 | T2DM +HTN +CAD, n = 6400 (age 66 years). Allocated to three SBP categories: <130; 130–139; ≥140 | Verapamil | Atenolol | 2.7 | 151/85 | N/A | No differences in CV outcomes and/or mortality |
| Swedish National Diabetes Register61 | T2DM managed with anti-HTN agents, n = 12,677 (age 30–75 years) | N/A | N/A | N/A | N/A | N/A | SBP ≥140 increased risk of CAD by 37%; 86% for stroke and 44% for CVD (when compared with SBP of 110–129) |
| SPS362,63 | History of recent lacunar infarction, n = 2747, 981 with T2DM (age 63 years). Comparing SBP: 130–149 vs. <130 | Intensive: use of ACE inhibitors, ARB, B-blockers, thiazide diuretics, CCB and others as prescribed by treating physician | Usual: use of ACE inhibitors, ARB, B-blockers, thiazide diuretics, CCB and others as prescribed by treating physician | 3.7 | 143/79 | 127/ND | Reduced recurrence of stroke, vascular events and mortality with BP: 124/67. The lowest risk was found with BP: 120–128/65–70 |
| VALUE64 | n = 15,244, HTN with high CVR, 32% with T2DM (age 67 years). Analysis per: BP <140/90 vs. <130/80; SBP: ≥140, 130–139, <130; and DBP≥90, 80–89, <80 | Valsartan | Amlodipine | 4–6 years | N/A | N/A | BP of ≥140/90 vs. 130–139/80–89 increased the incidence of fatal and non-fatal MI and sudden cardiac death, among other conditions. |
ACE inhibitors, angiotensin-converting enzyme inhibitors; Anti-HTN, antihypertensives; ARB, angiotensin receptor blockers; B-blockers, beta-blockers; BP, blood pressure; CAD, coronary artery disease; CCB, calcium channel blockers; CV, cardiovascular; CVD, cardiovascular disease; CVR, cardiovascular risk; DBP, diastolic BP; HCTZ, hydrochlorothiazide; HTN, hypertension; mAlb,: microalbuminuria; MI, myocardial infarction; N/A, not applicable; SBP, systolic BP, T2DM: type2 diabetes mellitus.
Meta-analyses that have evaluated the effect of blood pressure therapy in type 2 diabetes mellitus.
| Author | Inclusion criteria | Active therapy | Control | Number of studies | CV outcomes |
|---|---|---|---|---|---|
| Reboldi et al.65 | CCT comparing various anti-HTN agents in T2DM, n = 73,913 | ACE inhibitors, ARB, CCB, diuretics, B-blockers, as monotherapy or combined therapy | Placebo or another active therapy | 31 | The risk of stroke decreased significantly by 13% for each 5-mmHg reduction in SBP, and by 11.5% for each 2-mmHg reduction in DBP. The risk of MI decreased significantly by 11% in the active therapy group |
| McBrien et al.66 | CCT and quasi-randomised clinical trials comparing various anti-HTN agents in T2DM, n = 7312 (age 53-62 years). All the trials used stepped anti-HTN agents to achieve pre-specified BP targets | Intensive control, target BP ≤130/80mmHg | Standard control, SBP: 140-160mmHg and DBP: 85-100mmHg | 5 | There were no differences in risk of death or MI; the risk of stroke decreased significantly in the intensive control group by 35% |
| Bangalore et al.67 | CCT comparing various anti-HTN agents in T2DM, IFG or IGT, n = 37,736 | Intensive control, target SBP ≤135mmHg, with monotherapy or combined therapy: benazepril/amlodipine, candesartan, doxazosin, enalapril, fosinopril/amlodipine, perindopril, perindopril/indapamide, ramipril, valsartan | Standard control, target SBP ≤140mmHg, with monotherapy, combined therapy or placebo: benazepril/HCTZ, chlorthalidone, nifedipine, amlodipine, placebo | 13 | The intensive control group had significant reductions in all-cause mortality and stroke mortality (17%); there were no differences in other CV outcomes |
| Emdin et al.68 | CCT comparing various anti-HTN agents in T2DM, n = 100,354. Trials were stratified by SBP:≥140, <140, ≥130 and <130mmHg | ACE inhibitors, ARB, B-blockers, diuretics, CCB | Placebo or other anti-HTN agents | 40 | For every 10-mmHg reduction in SBP, the risk of mortality decreased significantly (13%), as did CV events (11%), CAD (12%) and stroke (27%) |
| Brunström et al.69 | CCT comparing various anti-HTN agents in T2DM, n = 73,738 | Any type of anti-HTN drug, or two anti-HTN drugs | Placebo, or monotherapy with other anti-HTN agents | 49 | If baseline SBP <150mmHg, the active therapy group experienced a significant reduction in the risk of CV mortality (25%), MI (26%) and stroke (23%). If the baseline SBP was 140-150mmHg, additional therapy significantly decreased the risk of MI (16%) and heart failure (20%) |
| Thomopoulos et al.70 | CCT comparing various anti-HTN agents, n = 260,210 (61,772 with T2DM). The effect of reducing SBP to≥140, or from 130 to <140, or <130mmHg was evaluated along with the effect of DBP of ≥80 or <80mmHg | ACE inhibitors, ARB, B-blockers, diuretics, CCB as monotherapy or combined therapy | Placebo, no treatment or other anti-HTN agents | 41 | SBP of at least 140mmHg significantly reduced the risk of CV outcomes in individuals with T2DM vs. those without T2DM. For T2DM, the risk of stroke was decreased by 27%, CAD by 29% and heart failure by 25%. |
ACE inhibitors, angiotensin-converting enzyme inhibitors; Anti-HTN, antihypertensives; ARB, angiotensin receptor blockers; B-blockers, beta-blockers; BP, blood pressure; CAD, coronary artery disease; CCB, calcium channel blockers; CCT, controlled clinical trials; CV, cardiovascular; CVD, cardiovascular disease; CVR, cardiovascular risk; DBP, diastolic blood pressure; HCTZ, hydrochlorothiazide; HTN, hypertension; IFG, impaired fasting glucose; IGT, impaired glucose intolerance; mAlb, microalbuminuria; MI, myocardial infarction; N/A, not applicable; SBP, systolic BP; T2DM, type2 diabetes mellitus.
Adequate management of BP in individuals with T2DM has proven to be a cost-effective measure since the benefits of such management on CV outcomes outweigh those achieved with glycaemic control per se. However, the initial recommendation for SBP <130mmHg only appeared to reduce the risk of ischaemic or haemorrhagic stroke (when compared with SBP >130 and <140mmHg). Nevertheless, on further analysis of the results of the ACCORD trial, individuals from the population with T2DM allocated to intensive therapy and who reached SBP levels of 119mmHg (vs. the standard group, which reached levels of 136mmHg), following the initial allocation to an intensive vs. standard decrease in HbA1c levels, who achieved an intensive decrease in SBP levels (and a standard decrease in HbA1c levels) were observed to experience a significant 39% reduction in the risk of stroke, a 33% reduction in the risk of combined CV outcomes and a 37% reduction in the risk of MI. This same trial found that individuals who were able to achieve SBP targets of <120mmHg experienced a significant increase in the risk of CV events compared with those who remained within the 120–139mmHg range.71,72 The results of the ACCORD trial weakened the conventional belief that “the lower the better”.
However, the established DBP targets discussed in the different management guidelines are <80, <85 and <90mmHg. In the HOT trial, for instance, a 51% decrease in the incidence of CV events was documented among individuals achieving DBP ≤80mmHg vs. those achieving DBP ≤85 and ≤90mmHg. In the UKPDS38 study, patients with T2DM and HTN who achieved DBP of 83mmHg with treatment had a 21% lower risk of MI and a 44% lower risk of stroke compared with those who achieved DBP of 87mmHg. Both trials (HOT and UKPDS38) were responsible for the recommendation established in many guidelines to achieve DBP ≤80 or ≤85mmHg.
Even with the results of earlier studies, it was not possible to ascertain whether SBP <130mmHg in diabetic individuals with HTN was better than SBP <140mmHg. It was evident, however, that SBP <120mmHg did not seem to benefit this population (in terms of CV outcomes). With regards to DBP, a DBP of <80mmHg was found to reduce the risk of vascular events in this population, although an overall reduction in CVR was achieved with DBP levels between 80 and 89mmHg. This also suggests that achieving SBP levels between 130 and 139mmHg almost always leads to DBP levels of <90 or <85mmHg, and even <80mmHg.73,74 Likewise, and despite the fact that achieving these DBP targets (between 70 and 79mmHg) has not been associated with undesirable effects in individuals achieving SBP control, there are concerns regarding the “J-curve phenomenon”, which may develop with DBP <80mmHg.75
Therefore, if it is considered that the constant perfusion of vital organs (heart, brain, kidneys) is compromised when BP falls progressively, anti-HTN therapy (and achieving very strict BP targets) may give rise to a turning point at which a very low BP may affect the perfusion and function of multiple organs. This has been shown in multiple studies in which SBP and DBP below certain levels have been associated with a higher incidence of CV events (“J-curve phenomenon”).76–78 Therefore, considering that coronary flow is a function of perfusion pressure and coronary artery resistance, the coronary perfusion pressure is the difference between the aortic diastolic pressure and left ventricular end-diastolic pressure. Therefore, low BP (particularly DBP) may compromise coronary perfusion, and since coronary perfusion occurs during diastole, diastolic hypotension could lead to coronary hypoperfusion, especially in people with a compromised coronary flow. This may explain why in experimental trials in which DBP levels <90mm/Hg (using sodium nitroprusside) are obtained, a marked reduction in coronary blood flow occurs in hypertensive individuals with left ventricular hypertrophy (LVH), while in those without LVH, no disruptions in coronary perfusion are found when reaching DBP close to 70mmHg. Therefore, hypertensive individuals with cardiovascular comorbidity (LVH, CAD) are more prone to presenting a “J-curve phenomenon”.79–81 Likewise, other studies have found an association between a DBP nadir <70, <80 or 85–89mmHg and the onset of vascular events in T2DM. The HOT trial found no differences in CV outcomes between treatment groups when allocated to a DBP target of ≤90, ≤85 or ≤80mmHg. Moreover, individuals with previous coronary heart disease who achieved a DBP of 80mmHg had a higher rate of MI than those with a DBP of 85mmHg. Such increased risk of MI with lower DBP was not shown in patients with no coronary heart disease. In the INVEST trial, individuals with established coronary artery disease and HTN had fewer CV events when DBP was <90mmHg, while the risk increased when DBP was <70mmHg. Moreover, in the ONTARGET study, the incidence of CV events decreased when BP fell from 145/82mmHg to 133/76mmHg, but increased again in individuals with lower BP targets (125/72 and 116/68mmHg).
The above indicates that the “J-curve phenomenon” should be taken into account when establishing BP targets for the population with T2DM. Moreover, the nadir may be even higher than that established for non-diabetics. Therefore, if T2DM is considered an “equivalent” of CVR, this population should hypothetically have at least the same BP nadir to cause coronary hypoperfusion as the population without T2DM (but with LVH or established CAD). Finally, there is probably not just one nadir for individuals with T2DM but rather several nadirs, depending on the baseline characteristics of patients (functional class, ejection fraction, diastolic dysfunction, aortic insufficiency, obesity, smoking, associated comorbidities and the presence of other CVRF, and use of multiple anti-HTN drugs).82,83
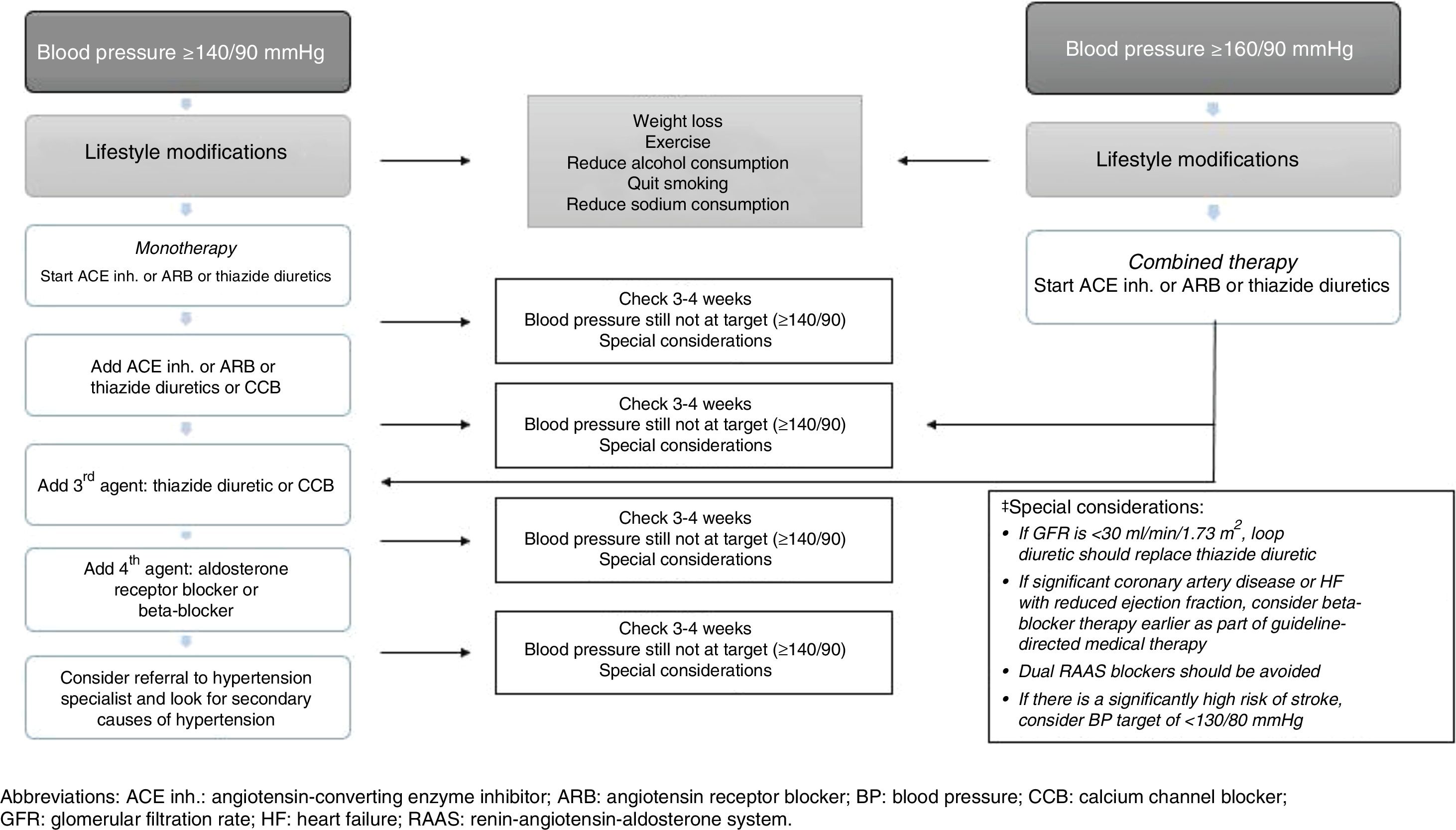
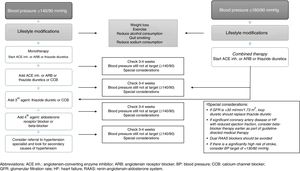
Recommended drug therapy for hypertension in individuals with type 2 diabetes mellitusThe various management guidelines that provide recommendations for the pharmacological management of HTN in patients with T2DM agree on several aspects,84–89 which are summarised below:
- 1.
Initial therapy should include anti-HTN drugs that have been proven to reduce CV outcomes. The BP threshold considered at the start of treatment (in monotherapy) is ≥140/90mmHg. The use of anti-HTN drugs such as ACE inhibitors, ARB or thiazide diuretics is recommended for monotherapy, provided that BP is ≥140/90 and <160/90, since BP ≥160/90 is an indication for combined anti-HTN therapy (Fig. 2).
- 2.
All major anti-HTN drug classes (e.g. ACE inhibitors, ARB, CCB, dihydropyridines and thiazide diuretics) should be recommended as first-line agents.
- 3.
In individuals with albuminuria (≥30mg/g creatinine), the first-line anti-HTN drugs are ACE inhibitors and ARBs.
- 4.
ACE inhibitors and ARBs can slow down the progression of nephropathy and retinopathy, and should therefore be considered as first-line anti-HTN therapy for these patients.
- 5.
In patients who do not have albuminuria, initial monotherapy can consist of ACE inhibitors, ARBs, thiazide diuretics, CCBs or dihydropyridines since, in the absence of albuminuria, the risk of kidney disease progression is low and ACE inhibitors and ARBs have not been found to offer superior cardioprotection when compared with other anti-HTN drugs.
- 6.
In patients with established CVD or heart failure, the use of beta-blockers may be recommended, taking into account that this type of drug has not been shown to reduce mortality in the absence of this condition. Moreover, loop diuretics may be useful in individuals with kidney disease or heart failure who are more prone to fluid retention.
- 7.
BP is harder to control in patients with T2DM than in those without T2DM so the use of combination therapy with anti-HTN drugs may be required in many patients.
- 8.
There is very limited evidence on the efficacy, safety and CV outcomes of drugs such as alpha-blockers (although they may be useful in patients with associated prostate disease) and drugs that affect the metabolism of aldosterone (spironolactone or eplerenone). However, such drugs may be considered in individuals with treatment-resistant HTN (while monitoring kidney function and serum potassium levels, especially when combined with ACE inhibitors, ARBs or other diuretics).
- 9.
Combination therapy should be considered with BP ≥150/100mmHg, with even stronger recommendations if BP is ≥160/100mmHg.
- 10.
Deciding which of the major anti-HTN drugs to use is the foundation of drug therapy for HTN in T2DM. However, this decision must be made on a personalised basis, considering factors such as: ethnicity, risk of adverse effects, blood glucose control, costs, drug interactions, CVD, heart failure and kidney function (which may have an impact on other aspects, such as treatment compliance, efficacy, outcomes and safety).
New approaches range from neutral effects of drug groups such as sulfonylureas, metformin, meglitinides and insulin on BP, drugs that have a neutral effect (or that may have a mild BP-lowering effect) such as α-glucosidase inhibitors and DPP-IV inhibitors, drugs that cause a moderate reduction in BP (thiazolidinediones, which reduce SBP but have a neutral or mildly lowering effect on DBP), to drugs that in addition to lowering BP also reduce micro- and macrovascular outcomes in T2DM (SGLT2 inhibitors [SGLT2-inh] and GLP-1 receptor agonists [GLP1-RA]).90,91
By definition, blood glucose lowering drugs (parenteral and non-parenteral) are not anti-HTN drugs. However, SGLT2-inh (empagliflozin and canagliflozin) and GLP1-RA (liraglutide and semaglutide) have been shown to reduce CV outcomes. The reduction in BP seen with SGLT2-inh is probably due to osmotic diuresis and the accompanying natriuretic effect since inhibition of SGLT2 induces increased water and glucose excretion (caused by the presence of non-reabsorbed glucose in the proximal renal tubule). Increased urine output (110–470ml/day) and natriuresis helps reduce plasma volume and lower BP. Furthermore, weight loss caused by SGLT2-inh (2–3kg) (either due to volume contraction or loss of fat and/or lean tissue) also helps lower BP. Finally, these drugs could also lower BP as a result of local inhibition of the RAAS (secondary to increased delivery of sodium to the juxtaglomerular apparatus since this reduces proximal tubular reabsorption of sodium secondary to increased sodium in the macula densa). Other mechanisms have also been described, such as indirect effects on nitric oxide release (secondary to reduced oxidative stress and improved blood glucose levels) or possible regulation of the autonomic nervous system (probably inducing activation of the parasympathetic nervous system). SBP is generally reduced by between 2.9 and 5.73mmHg while DBP is reduced by 1.09–2.62mmHg (significant reduction compared to placebo or other blood glucose lowering drugs).92–97
Moreover, GLP-1RA have been shown to have a mild BP-lowering effect. The mechanisms by which this type of drug lowers BP are not very clear and the most likely metabolic pathway is the increased release of atrial natriuretic peptide (ANP), mediated by GLP-1R (which is expressed in the atria). Therefore, GLP-1RA, on stimulating GLP-1R, can induce ANP-mediated natriuresis. Furthermore, GLP-1R regulates the sensation of fullness and water intake and this mechanism seems to explain (at least partly) the weight loss found in the different studies and affect, to a certain extent, BP levels. Finally, a GLP-1RA-mediated vasodilatory effect (independent of nitric oxide) has been described. From a clinical point of view, GLP-1RAs have the following effects: lixisenatide reduces SBP by 0.8mmHg, liraglutide reduces SBP by 1.2mmHg (but increases DBP by 0.6mmHg), semaglutide reduces SBP by 1.3-2.6mmHg (dependent on the dose used) with no changes in DBP, and exenatide (once weekly) reduces SBP by 1.6mmHg. The mean weight loss induced by this type of drug is 3–5kg, with a higher weight loss observed with high-dose liraglutide (3.0mg).97–100
Non-pharmacological treatmentNon-pharmacological recommendations for managing patients with HTN and T2DM are generally extrapolated from studies designed for patients with HTN but without DM. Recommendations that have been shown to have a significant effect on such individuals are weight loss, dietary sodium restriction and physical exercise.
In overweight or obese patients, the goal is to achieve a body mass index within the normal range (18.5–24.9kg/m2) and to achieve a waist circumference of <102 for men and <88cm for women. With regards to diet, the Dietary Approaches to Stop Hypertension (DASH) trial concluded that this diet reduces BP in patients without T2DM, but it was later demonstrated that, in addition to lowering BP in overweight individuals, this diet may improve insulin sensitivity as part of a comprehensive lifestyle modification programme (including weight loss, behavioural changes and frequent follow-up, at least every 4–6 months). The DASH diet is based primarily on a high intake of fruit and non-starchy vegetables, moderate intake of non-fat (or low-fat) dairy products, whole grains, lean meats, poultry, beans, soy products and eggs, fish, nuts, olive (or rapeseed) oil and limited consumption of refined grains such as white bread, cakes and desserts. One fundamental aspect is reduced sodium consumption (<2g/day, or <87mmol/day, or <5g/day of salt) and increased potassium consumption (from fruit and vegetables). Moreover, there is no convincing evidence on the risks or benefits of chronic caffeine consumption, but alcohol consumption has been shown to increase the frequency of HTN in different populations. Therefore, alcohol should be avoided or limited to a maximum of 30g/day for men and 15g/day for women.101–103
With regards to exercise, a sedentary lifestyle and lack of physical activity are clearly strong predictors of CV mortality (regardless of the presence of HTN and other CVRF). Dynamic exercise of moderate intensity (walking, jogging, swimming, etc.), 4–7 times a week (30–60min sessions), is recommended together with everyday routine activities. High-intensity exercise is even more effective and, together with dietary modifications, has been associated with more significant reductions in BP. Carrying out these recommendations may help reduce SBP by 5–10mmHg and DBP by 1–6mmHg, but the long-term effect on CV outcomes in patients with HTN and T2DM is not clear. Exercise intensity should be personalised based on the patient's clinical condition.104,105
Final considerationsThe results obtained in the studies outlined above have led to the consolidation of several BP-related recommendations for individuals with T2DM. Nevertheless, it is hard to establish an optimal BP for this specific population. Current evidence indicates that a target SBP of 130–139mmHg protects individuals from CV and kidney complications, while a SBP that is as close to 130mmHg as possible gives an additional and significant benefit (compared to higher values). Furthermore, a target of <130mmHg may be beneficial for individuals at high risk of cerebrovascular events (such as patients with a previous history of stroke) and patients with nephropathy and significant proteinuria. Finally, an SBP of <120mmHg should not be considered for this population since there is no evidence that such levels significantly reduce the frequency of CV outcomes or mortality, but may in fact be associated with the “J-curve phenomenon”. A DBP of 80–89mmHg may be established as the initial target for these patients. However, lowering DBP to 70–79mmHg may have additional benefits (provided that the rate of adverse effects does not increase). It must also be considered that target BP may vary among patients with T2DM (for example, due to factors such as age, target organ damage, newly diagnosed T2DM, ethnicity, presence of adverse effects, comorbidities, etc.), and, consequently, the target BP should be personalised. It must be remembered that a very strict target makes the use of multiple anti-HTN drugs practically obligatory (thereby increasing the risk of adverse events and probably poor compliance). Few studies have been conducted to establish target BP levels in individuals with T2DM >80 years of age. A target of <150/90mm/Hg may be reasonable, although the risk-benefit ratio must be considered when establishing stricter targets in such individuals.
ConclusionsIn individuals with T2DM and HTN, a target SBP of 130-139mmHg and a target DBP of 80–89mmHg has proven to be safe and reduces the risk of CV outcomes. SBP <130mmHg should be reserved for patients at high risk of cerebrovascular events. A DBP of 70–79mmHg is ideal, provided that the risk of adverse events or the “J-curve phenomenon” is not increased (especially in individuals with established CAD or LVH). These targets must be personalised according to the patients’ baseline characteristics and their comorbidities. Finally, an SBP of <120mmHg or a DBP of <70mmHg have failed to show reductions in CV outcomes and may even increase the onset of such events. First-line treatment includes ACE inhibitors and ARBs. Other anti-HTN drugs that can be used are CCBs, thiazide diuretics and beta-blockers, followed to a lesser extent by alpha-blockers and drugs that act on aldosterone metabolism. Based on current results on cardiovascular safety, reduced CV outcomes and moderate BP-lowering effects, the use of SGLT2-inh and GLP-1RA will surely appear in the various management guidelines for treating HTN in patients with T2DM.
Conflicts of interestH. V.-U. has served on speakers’ bureaus and advisory boards for the following pharmaceutical companies: Sanofi, Novo, Novartis, MSD, Boehringer Ingelheim and AstraZeneca. He has no conflicts of interest associated with this publication to declare.
M.F. C.-A. has no conflicts of interest to declare.
Please cite this article as: Vargas-Uricoechea H, Cáceres-Acosta MF. Metas de control de la presión arterial e impacto sobre desenlaces cardiovasculares en pacientes con diabetes mellitus tipo 2: un análisis crítico de la literatura. Clin Invest Arterioscler. 2019;31:31–47.