To examine the frequency of severe hypercholesterolemia (HS) and its clinical profile, and the phenotype of familial hypercholesterolemia (FH), in the primary-care setting in a large health area of the Community of Madrid (CAM).
Material and methodsMulticenter study of subjects with a health card assigned to 69 health centers (Northwest/CAM area). HS was defined as cholesterol ≥ 300 mg/dL or LDL-cholesterol ≥ 220 mg/dL in any analysis performed (1-1-2018 to 12-30-2021); and FH phenotype as c-LDL ≥ 240 mg/dL (≥160 mg/dL if lipid-lowering treatment) with triglycerides < 200 mg/dL and TSH < 5 uIU/ml.
Results156,082 adults ≥ 18 years with an available lipid profile were analyzed. 6187 subjects had HS (3.96% of the laboratory tests studied, 95%CI 3.87%–4.06%). The mean evolution time of the diagnosis of hyperlipidemia in the computerized clinical record was 10.8 years; 36.5% had hypertension; 9.5% diabetes and 62.9% overweight/obesity. 83.7% were taking lipid-lowering drugs (65,7% low/moderate and 28.6% high/very high intensity). 6.1% had cardiovascular disease (94.2% treated with lipid-lowering agents), with LDL-cholesterol <55, <70 and <100 mg/dl of 1.8%, 5.8% and 20.2%, respectively. (vs 1%, 2.3% and 11.2% if no cardiovascular disease). 1600 subjects had FH phenotype (1.03%, 0.98%–1.08%).
ConclusionsFour out of 100 patients analyzed in primary care have HS, with high treatment level, but insufficient intensity, and poor achievement of treatment goals. One in 100 have the FH phenotype. The identification of both dyslipidemias by computerized records would allow their more precise and early detection and establish cardiovascular preventive strategies.
Estimar la frecuencia y perfil clínico de la hipercolesterolemia severa (HS) y del fenotipo de hipercolesterolemia familiar (HF) en el ámbito de atención primaria, en un área sanitaria de la comunidad de Madrid (CAM).
Material y métodosEstudio transversal, multicéntrico de sujetos con tarjeta sanitaria adscritos a 69 centros de salud (área NorOeste/CAM). Se definió HS como colesterol ≥ 300 mg/dL o colesterol-LDL ≥ 220 mg/dL en alguna analítica realizada (1-1-2018 a 30-12-2021); y fenotipo de HF como c-LDL ≥ 240 mg/dL (≥160 mg/dL si tratamiento hipolipemiante), con triglicéridos < 200 mg/dL y TSH < 5 uIU/ml.
ResultadosSe analizaron 156.082 adultos ≥ 18 años con perfil lipídico disponible. 6.187 sujetos tenían HS (3,96% de las analíticas estudiadas, IC95% 3,87%–4,06%). El tiempo medio de evolución del diagnóstico de hiperlipemia en la historia clínica informatizada fue 10,8 años; 36,5% tenían hipertensión; 9,5% diabetes y 62,9% sobrepeso/obesidad. El 83,7% tomaban hipolipemiantes (65,7% de baja/moderada y 28,6% de alta/muy-alta intensidad). El 6,1% tenían enfermedad cardiovascular (94,2% tratados con hipolipemiantes), con colesterol-LDL <55, <70 y <100 mg/dl de 1,8%, 5,8% y 20,2%, respectivamente (vs 1%, 2.3% y 11.2% si no había enfermedad cardiovascular). 1600 sujetos tenían fenotipo de HF (1,03%, 0,98%–1,08%).
ConclusionesCuatro de cada 100 pacientes analizados en atención primaria tienen HS. Hay un elevado nivel de tratamiento farmacológico, pero de insuficiente intensidad, y escaso logro de objetivos terapéuticos. Uno de cada 100 tiene fenotipo de HF. La identificación de ambas situaciones por registros informatizados permitiría su detección más precisa y precoz y establecer estrategias preventivas cardiovasculares.
Cardiovascular diseases (CVD) continue to be the leading cause of mortality in Western countries. In Spain, they account for 24.3% of all deaths.1 The 2017 Spanish Health Survey and the 2020 European Health Survey point out that high cholesterol is the second most common chronic health problem in the Spanish population aged ≥ 15 years, with a mere 15.4% of the subjects aware of the fact that they have high cholesterol.2,3
There is strong evidence that excess low-density lipoprotein cholesterol (LDL-C) is a major cause of atherosclerotic CVD and that appropriate treatment has yielded clear benefits in reducing cardiovascular morbidity and mortality.4–6 The European EUROASPIRE-V study reveals that only one third of patients with coronary events meet LDL-C targets,7 due to insufficient treatment compliance and poor prescription of lipid-lowering therapies. In primary care (PC) in Spain, for example, according to the IBERICAN [Identificación de la poBlación Española de RIesgo CArdiovascular y reNal] study (Identification of the Spanish population at risk for cardiovascular and renal disease), 50% of the 8000 individuals studied had dyslipidaemia,8 and only 25.8% had adequate control.
On the other hand, the prevalence of familial hypercholesterolaemia (FH) in the general population is approximately 1 in 200–300 subjects; nevertheless, the frequency [of FH] is unknown in 90% of all countries, and it is diagnosed late and carries a high burden of associated CVD.9–11 Spanish data with laboratory data records demonstrate a prevalence rate of the FH phenotype of 0.62%.11
Given the importance of the problem and the suboptimal control of risk factors,7,8 real world PC must necessarily be aware of the frequency and characteristics of subjects with severe hypercholesterolaemia (SH), who have a cardiovascular risk (CVR) profile that is at least high or very high if they have associated CVD. Some international studies have investigated the characteristics of people with SH with electronic records in PC,12,13 however, to the best of our knowledge, there are no current studies in Spain regarding this group of patients in the setting of PC.
The primary objective of this study is to estimate the frequency and identify the clinical profile (comorbidities, risk factors, drug prescription, and degree of control) of individuals with SH seen in PC, using computerised registry sources. This would make it possible to put forward strategies to prevent cardiovascular events in this population group with such a high CVR, as recommended in clinical practice guidelines.14,15 A secondary objective is to estimate the prevalence of people with a phenotype of possible FH. Such identification would enable warning mechanisms for professionals and patients to be developed, population screening to be conducted, and a more accurate diagnosis to be made, as well as subsequent referral to lipid units if necessary.
MethodologyDesign and study populationObservational and multicentre study in 40 healthcare centres and 29 local clinics in the Autonomous Community of Madrid (CAM, for its acronym in Spanish) that provide care to all people with a health card (TSI, for its acronym in Spanish) (98.6% have a TSI issued by the CAM Department of Health)16 included in the Primary Care computer system for Madrid (AP-Madrid, for its acronym in Spanish) and belonging to the Northwest Care Directorate (DANO, for its acronym in Spanish). DANO covers the municipalities in the north-western area of CAM and comprises a total of 1,093,819 individuals.16 As per the above data, 48% and 52% of men and women with health cards use the SIP-CIBELES application, respectively; this proportion has remained constant over the last five years.
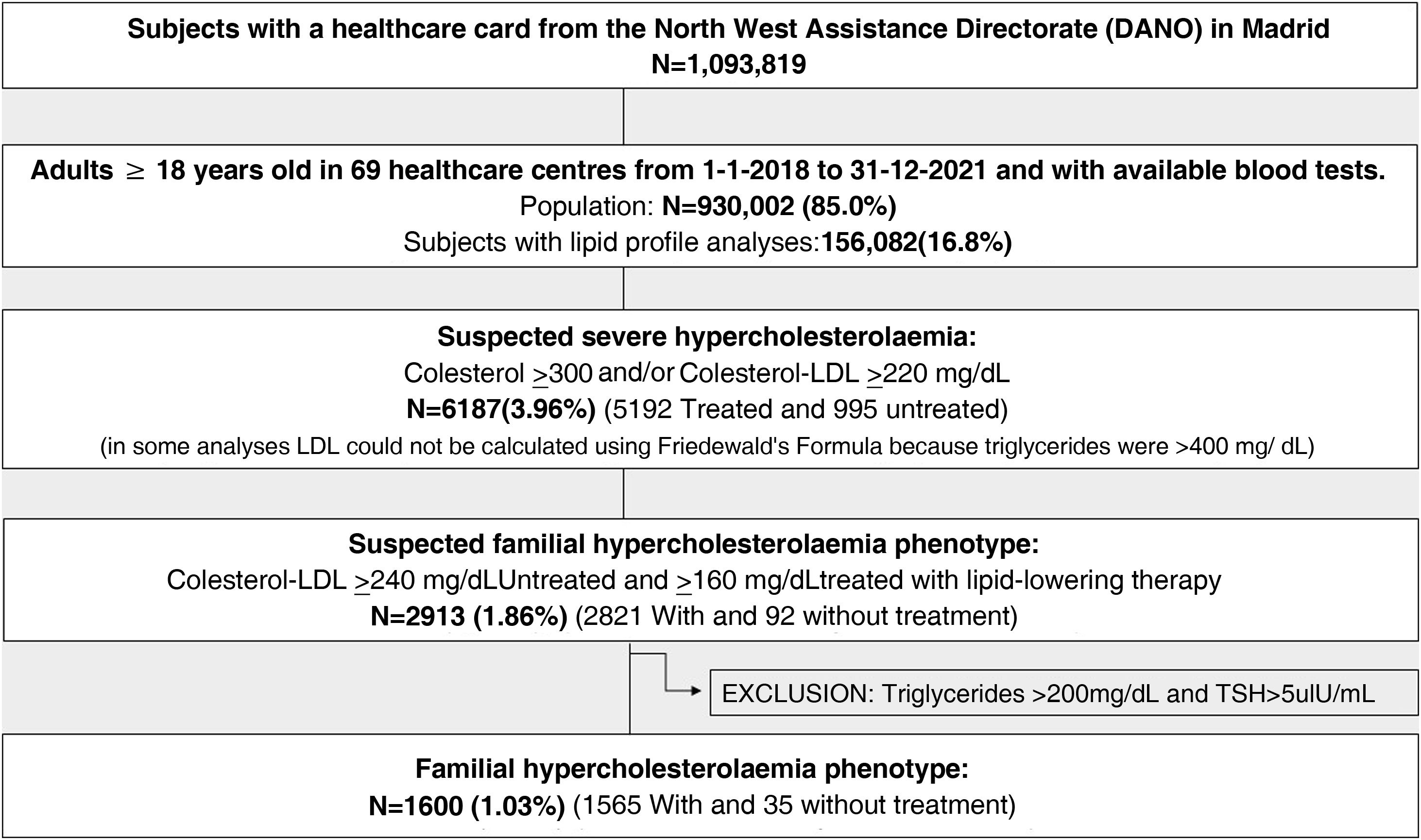
A total of 930,002 adults ≥ 18 years of age (85% of the population) were analysed; of these, 156,082 attended the healthcare centre and had blood tests with lipid profile data available during the study period (16.8%). The PC teams in the healthcare area are staffed by 541 physicians.17
Inclusion and exclusion criteriaPatients ≥ 18 years of age who consulted at healthcare centres within the healthcare area from 1 January 2018 to 1 January 2022 and who had a blood test. We selected those with total cholesterol ≥ 300 mg/dl or LDL-C ≥ 220 mg/dl on any of the blood tests performed during this period (which we refer to as severe hypercholesterolaemia [SH] according to standard clinical practice criteria, despite the fact that there is no consensus regarding the limits to be considered). We decided on this cut-off point because according to the 2019 European Societies of Cardiology and Atherosclerosis Dyslipidaemia Guidelines, patients with a markedly elevated risk factor, such as total cholesterol ≥ 310 or LDL-C ≥ 190 would be included in the high CVR category.14 We have chosen the most restrictive cut-off point for LDL-C (220 mg/dl), which would represent more than three times the target figure for high-risk patients for primary prevention, and almost four times the target figure for those in secondary prevention.14,15
Data sourceAnonymised data from the AP-Madrid database, which includes data on clinical activity of PC physicians in the DANO database, which provides access to socio-demographic data, coding of diagnoses based on the International Classification of Primary Care, second edition (ICPC-2),18 and to various general patient data (GPD), such as anthropometric measurements, laboratory data, and pharmacological prescriptions according to the ATC classification.19
VariablesSocio-demographic variables, health centre code, physician identification code, age, and sex were selected.
Systolic and diastolic blood pressure (BP) (mmHg), weight (kg), height (cm), body mass index (BMI) (kg/m2) (overweight if BMI ≥ 25 kg/m2 and obese if ≥30 kg/m2) were analysed in participants for whom these data were available. The time of evolution of the diagnosis of hyperlipaemia in the digitalised medical record was also considered. For the description of the control criteria, the most recent blood test within the study period was used, considering: basal glucose (mg/dl), total cholesterol (mg/dl), LDL-C (mg/dl), high-density lipoprotein cholesterol (HDL-C) (mg/dl), triglycerides (mg/dl), creatinine (mg/dl), thyroid stimulating hormone (TSH) (µIU/ml), the transaminases ALT (U/l) and AST (U/l), and GGT (U/l). Glomerular filtration rate (ml/min/1.73 m2) was calculated using the CKD-EPI20 formula. These analytical variables were extracted from samples obtained at the healthcare centres with an 8-h fasting period at baseline and sent to the two reference laboratories in the healthcare area. The Friedewald formula used in the reference laboratories was adopted to determine LDL-C; for triglycerides > 400 mg/dl, this formula is not used to calculate LDL-C.21 To examine the spectrum of lipid control taking into account LDL-C levels and since all patients have a high or very high CVR, different thresholds of LDL-C (<55, 70 or <100 mg/dl) were assessed (in individuals treated and those not treated with lipid-lowering agents and in subjects with available LDL due to triglycerides < 400 mg/dl) and depending on the presence or absence of associated CVD. Analysis of LDL-C concentrations was performed based on drug treatment by decades of age and the age of 55 years was regarded as an intermediate point of comparison between the groups. As for the degree of control according to non-HDL-cholesterol (in lipid-lowering treated and untreated and total subjects), control targets were considered if concentrations were <85, 100, or <130 mg/dl.14,15
The risk factors and cardiovascular comorbidities under consideration were identified from the electronic medical records of AP-Madrid, according to codes (CIAP-2).18 Diabetes (T90), hyperlipidaemia (T93), smoking (P22), hypertension (K85, K86, K87), stroke (K89, K90), renal failure (U99), peripheral arterial disease (K99), atrial fibrillation (K78), ischaemic heart disease (K74, K76), and heart failure (K77) were all analysed.
Drug prescription was organised according to the classification by therapeutic groups used in AP-Madrid, which incorporates the anatomical, therapeutic, and chemical classification (ATC), the European coding system for pharmaceutical substances and medicines.19
The lipid-lowering drugs group (C10, lipid-modifying agents) was examined with the following subgroups: C10AA01-C10AA08 (HMG CoA reductase inhibitors), C10AB (fibrates), C10AC (bile acid sequestrants), C10AX (other lipid-modifying agents: omega-3, ezetimibe), and the C10B group (lipid-modifying agents in combination). The presence of treatment with PCSK9 inhibitors could not be assessed as they were dispensed in hospital pharmacies and not available in PC registries. The intensity of lipid-lowering treatment was categorised as per the classification by Masana et al.22 into low, moderate, high, and very high intensity treatment, based on the statin used, dosage, and type of combination.22
A FH phenotype was defined based on cut-off points in adults suggestive of FH if LDL-C concentrations were ≥240 mg/dl (≥160 mg/dl if lipid-lowering treatment) according to the criteria defined by the Spanish Society of Arteriosclerosis (SEA)-ARIAN Project for automated detection of FH, provided that triglycerides were <200 mg/dl and TSH < 5 µIU/ml in the latest analysis during the period under study.11,23
Data analysisA descriptive study of all variables was conducted to detect outliers or other inconsistencies. Qualitative variables were presented with their frequency distribution, percentage, and 95% confidence interval (95% CI), and quantitative variables with their mean, standard deviation (SD), and 95% CI, if the variables conformed to a normal distribution; if they exhibited an asymmetrical distribution, they were expressed as median and interquartile range (P25−75). The association between qualitative variables was performed with the chi-square test or Fisher's exact test (if >25% of the expected cases were <5). Means were compared using Student's t-test, after performing Levene's test of homogeneity of variances, if the variables adhered to a normal distribution in the groups to be compared; for asymmetrical variables, the non-parametric Mann-Whitney U test was applied.
Statistical analyses were carried out with the Statistical Package for Social Sciences (SPSS) for Windows v.24 (IBM, Armonk, NewYork, USA).
Ethical aspectsData were requested from the Technical Support Unit of the Madrid Healthcare Service (SERMAS) from a single, centralised, and anonymised database. In doing so, international data protection standards and current Spanish legislation were respected. Identification data and clinical data are dissociated in the database, respecting patient autonomy and the rights and obligations of information and clinical documentation, and only researchers had access to the information.
The study received a favourable report from the Comisión Local de Investigación Noroeste de Madrid [Local Northwest Research Commission of Madrid] (code 04/2022).
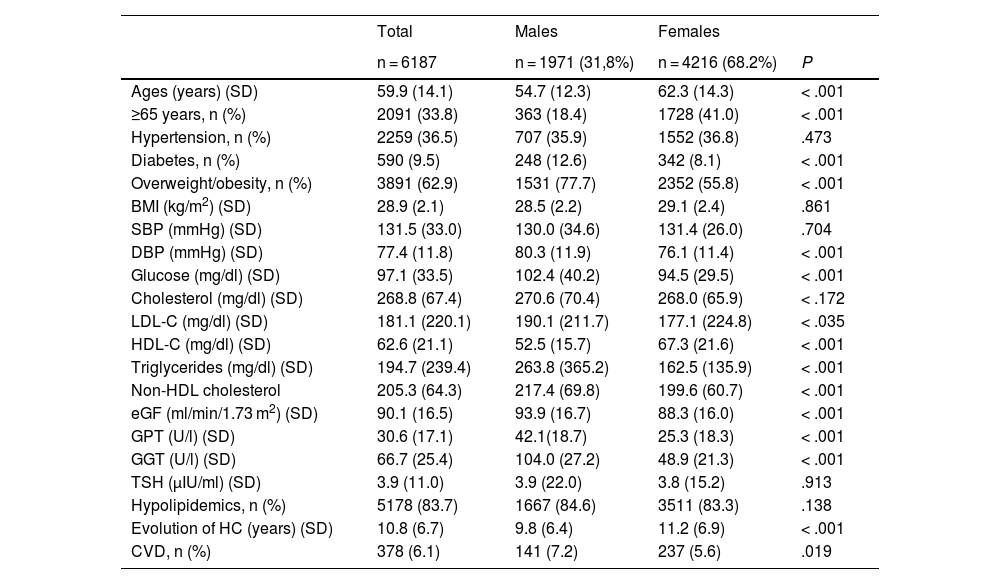
ResultsOf the 10,938,196 subjects in the study area, 930,002 were aged ≥ 18 years. A total of 156,082 individuals for whom a lipid profile was available were analysed and 6187 of them had SH. Fig. 1 illustrates the inclusion algorithm for the study. In the study population, females outnumbered males by more than twofold and were significantly older (62.3 vs 54.7 years) (P < .001) (Table 1). The most common comorbidities were: hypertension (36.5%) previously diagnosed in the clinical history, diabetes (9.5%), and overweight/obesity (62.9%). The proportion of diabetes and overweight/obesity was significantly greater in men. The mean time of evolution of hypercholesterolaemia was 10.8 years. A total of 83.7% were on lipid-lowering treatment. The blood pressure figures correspond to the mean of the 4300 individuals for whom this datum was available. The overweight and obesity data are from 2000 patients for whom weight and height data were available, and the mean TSH was calculated based on the figures that were available for 5390 participants.
General characteristics of subjects with severe hypercholesterolemia, according to gender.
| Total | Males | Females | ||
|---|---|---|---|---|
| n = 6187 | n = 1971 (31,8%) | n = 4216 (68.2%) | P | |
| Ages (years) (SD) | 59.9 (14.1) | 54.7 (12.3) | 62.3 (14.3) | < .001 |
| ≥65 years, n (%) | 2091 (33.8) | 363 (18.4) | 1728 (41.0) | < .001 |
| Hypertension, n (%) | 2259 (36.5) | 707 (35.9) | 1552 (36.8) | .473 |
| Diabetes, n (%) | 590 (9.5) | 248 (12.6) | 342 (8.1) | < .001 |
| Overweight/obesity, n (%) | 3891 (62.9) | 1531 (77.7) | 2352 (55.8) | < .001 |
| BMI (kg/m2) (SD) | 28.9 (2.1) | 28.5 (2.2) | 29.1 (2.4) | .861 |
| SBP (mmHg) (SD) | 131.5 (33.0) | 130.0 (34.6) | 131.4 (26.0) | .704 |
| DBP (mmHg) (SD) | 77.4 (11.8) | 80.3 (11.9) | 76.1 (11.4) | < .001 |
| Glucose (mg/dl) (SD) | 97.1 (33.5) | 102.4 (40.2) | 94.5 (29.5) | < .001 |
| Cholesterol (mg/dl) (SD) | 268.8 (67.4) | 270.6 (70.4) | 268.0 (65.9) | < .172 |
| LDL-C (mg/dl) (SD) | 181.1 (220.1) | 190.1 (211.7) | 177.1 (224.8) | < .035 |
| HDL-C (mg/dl) (SD) | 62.6 (21.1) | 52.5 (15.7) | 67.3 (21.6) | < .001 |
| Triglycerides (mg/dl) (SD) | 194.7 (239.4) | 263.8 (365.2) | 162.5 (135.9) | < .001 |
| Non-HDL cholesterol | 205.3 (64.3) | 217.4 (69.8) | 199.6 (60.7) | < .001 |
| eGF (ml/min/1.73 m2) (SD) | 90.1 (16.5) | 93.9 (16.7) | 88.3 (16.0) | < .001 |
| GPT (U/l) (SD) | 30.6 (17.1) | 42.1(18.7) | 25.3 (18.3) | < .001 |
| GGT (U/l) (SD) | 66.7 (25.4) | 104.0 (27.2) | 48.9 (21.3) | < .001 |
| TSH (µIU/ml) (SD) | 3.9 (11.0) | 3.9 (22.0) | 3.8 (15.2) | .913 |
| Hypolipidemics, n (%) | 5178 (83.7) | 1667 (84.6) | 3511 (83.3) | .138 |
| Evolution of HC (years) (SD) | 10.8 (6.7) | 9.8 (6.4) | 11.2 (6.9) | < .001 |
| CVD, n (%) | 378 (6.1) | 141 (7.2) | 237 (5.6) | .019 |
BMI: body mass index; CVD: cardiovascular disease; DBP: diastolic blood pressure; eGF: estimated glomerular filtration; GGT: gamma glutamyl transpeptidase; GOT: glutamic-oxaloacetic transaminase; GPT: glutamic-pyruvic transaminase; HC: hypercholesterolaemia; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; SBP: systolic blood pressure; SD: standard deviation; TSH: thyroid-stimulating hormone.
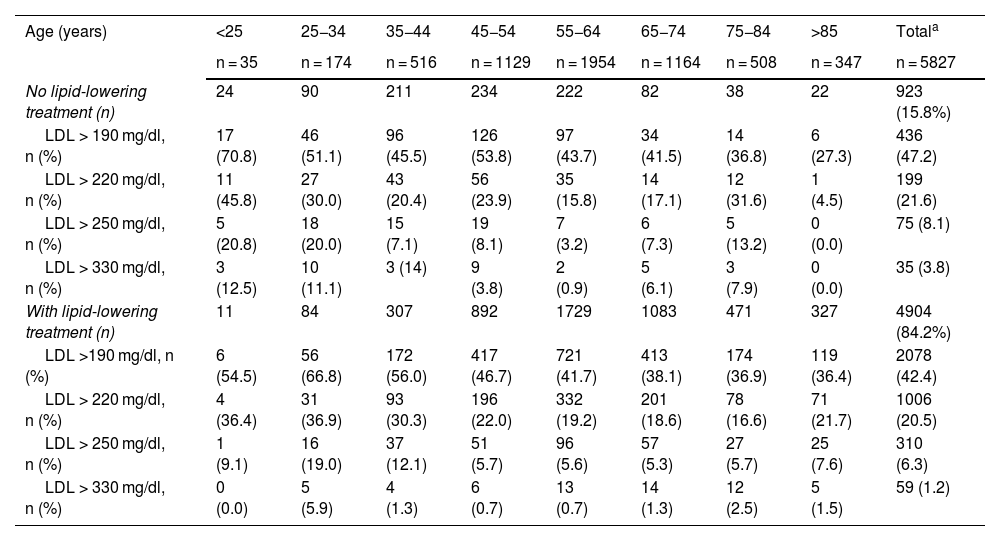
Table 2 illustrates the distribution of the LDL-C categories of the 5827 subjects with LDL-C data available due to having a triglyceride concentration of <400 mg/dl. Those not taking lipid-lowering treatment accounted for 15.8% of the sample. LDL-C concentrations at the various thresholds were worse for the younger age groups, with or without lipid-lowering treatment. For the individuals not receiving hypolipidemic drug treatment (923 patients), 51% of the people aged < 55 years (559 participants) had LDL-C values of >190 mg/dl vs. 41.5% of those aged ≥ 55 years (364 individuals) (P < .001). Among subjects receiving lipid-lowering medication (4904 subjects), 50.3% of them aged < 55 years (1294 patients) had LDL-C values of ≥190 mg/dl vs 39.5% among those aged >55 years (3610 individuals) (P < .001).
Distribution of subjects with severe hypercholesterolaemia (subjects with LDL-C values available for having triglycerides < 400 mg/dl) and according to LDL-C categories in the last analysis available; decades of age, and lipid-lowering drug treatment.
| Age (years) | <25 | 25−34 | 35−44 | 45−54 | 55−64 | 65−74 | 75−84 | >85 | Totala |
|---|---|---|---|---|---|---|---|---|---|
| n = 35 | n = 174 | n = 516 | n = 1129 | n = 1954 | n = 1164 | n = 508 | n = 347 | n = 5827 | |
| No lipid-lowering treatment (n) | 24 | 90 | 211 | 234 | 222 | 82 | 38 | 22 | 923 (15.8%) |
| LDL > 190 mg/dl, n (%) | 17 (70.8) | 46 (51.1) | 96 (45.5) | 126 (53.8) | 97 (43.7) | 34 (41.5) | 14 (36.8) | 6 (27.3) | 436 (47.2) |
| LDL > 220 mg/dl, n (%) | 11 (45.8) | 27 (30.0) | 43 (20.4) | 56 (23.9) | 35 (15.8) | 14 (17.1) | 12 (31.6) | 1 (4.5) | 199 (21.6) |
| LDL > 250 mg/dl, n (%) | 5 (20.8) | 18 (20.0) | 15 (7.1) | 19 (8.1) | 7 (3.2) | 6 (7.3) | 5 (13.2) | 0 (0.0) | 75 (8.1) |
| LDL > 330 mg/dl, n (%) | 3 (12.5) | 10 (11.1) | 3 (14) | 9 (3.8) | 2 (0.9) | 5 (6.1) | 3 (7.9) | 0 (0.0) | 35 (3.8) |
| With lipid-lowering treatment (n) | 11 | 84 | 307 | 892 | 1729 | 1083 | 471 | 327 | 4904 (84.2%) |
| LDL >190 mg/dl, n (%) | 6 (54.5) | 56 (66.8) | 172 (56.0) | 417 (46.7) | 721 (41.7) | 413 (38.1) | 174 (36.9) | 119 (36.4) | 2078 (42.4) |
| LDL > 220 mg/dl, n (%) | 4 (36.4) | 31 (36.9) | 93 (30.3) | 196 (22.0) | 332 (19.2) | 201 (18.6) | 78 (16.6) | 71 (21.7) | 1006 (20.5) |
| LDL > 250 mg/dl, n (%) | 1 (9.1) | 16 (19.0) | 37 (12.1) | 51 (5.7) | 96 (5.6) | 57 (5.3) | 27 (5.7) | 25 (7.6) | 310 (6.3) |
| LDL > 330 mg/dl, n (%) | 0 (0.0) | 5 (5.9) | 4 (1.3) | 6 (0.7) | 13 (0.7) | 14 (1.3) | 12 (2.5) | 5 (1.5) | 59 (1.2) |
LDL-C: low-density lipoprotein cholesterol.
Subjects with LDL concentrations available due to having triglycerides < 400 mg/dl; therefore, 84.2% are treated instead of the 83.7% reflected in Table 1 (all subjects).
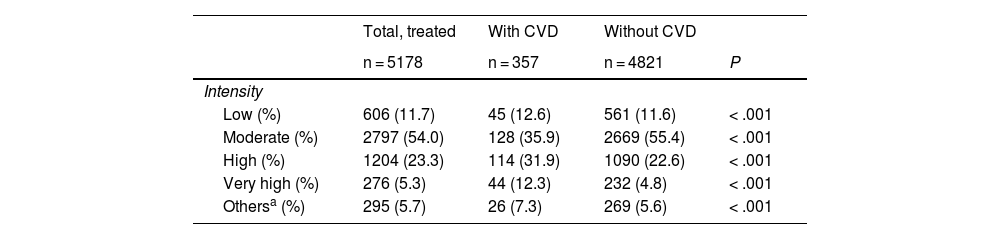
Of those patients on statins in monotherapy, 75.8% were taking statins alone; 12.6% were on statins together with ezetimibe in combination therapy; 4.3% were taking fibrates, and 7.3% were taking bile acid sequestrants, ezetimibe, and omega-3 in combination therapy. Table 3 depicts the proportion of subjects receiving pharmacological treatment by intensity of lipid-lowering therapy. Fifty-four per cent of the treated individuals were on moderate-intensity lipid-lowering therapy and 28.6% on high/very-high-intensity hypolipidemic therapy. There was a significantly higher proportion of individuals on high- and very-high-intensity treatment in the presence of CVD (P < .001). A total of 7.1% of subjects with CVD and 5.6% of those without CVD were not taking statin therapy.
Lipid-lowering drug treatment by intensity and presence of cardiovascular disease.
| Total, treated | With CVD | Without CVD | ||
|---|---|---|---|---|
| n = 5178 | n = 357 | n = 4821 | P | |
| Intensity | ||||
| Low (%) | 606 (11.7) | 45 (12.6) | 561 (11.6) | < .001 |
| Moderate (%) | 2797 (54.0) | 128 (35.9) | 2669 (55.4) | < .001 |
| High (%) | 1204 (23.3) | 114 (31.9) | 1090 (22.6) | < .001 |
| Very high (%) | 276 (5.3) | 44 (12.3) | 232 (4.8) | < .001 |
| Othersa (%) | 295 (5.7) | 26 (7.3) | 269 (5.6) | < .001 |
Intensity of lipid-lowering treatment (Ref.22):
Low: simvastatin 10 mg, pravastatin 10−20 mg, lovastatin 10−20 mg, fluvastatin 40 mg, pitavastatin 1 mg, ezetimibe 10 mg in monotherapy.
Moderate: atorvastatin 10−20 mg, rosuvastatin 5−10 mg, simvastatin 20−40 mg, pravastatin 40 mg, lovastatin 40 mg, fluvastatin 80 mg in monotherapy and combinations of ezetimibe 10 mg with pitavastatin 2−4 mg, simvastatin 10 mg, pravastatin 20 mg, lovastatin 20 mg, fluvastatin 40 mg, pitavastatin 1 mg.
High: atorvastatin 40−80 mg, rosuvastatin 20−40 mg in monotherapy and combinations of ezetimibe 10 mg with atorvastatin 10−20 mg, rosuvastatin 5−10 mg, simvastatin 20−40 mg, pravastatin 40 mg, lovastatin 40 mg, fluvastatin 80 mg, pitavastatin 2−4 mg.
Very high: atorvastatin 40−80 + ezetimibe 10 rosuvastatin 20−40 + ezetimibe 10.
IPCSK9 data not available.
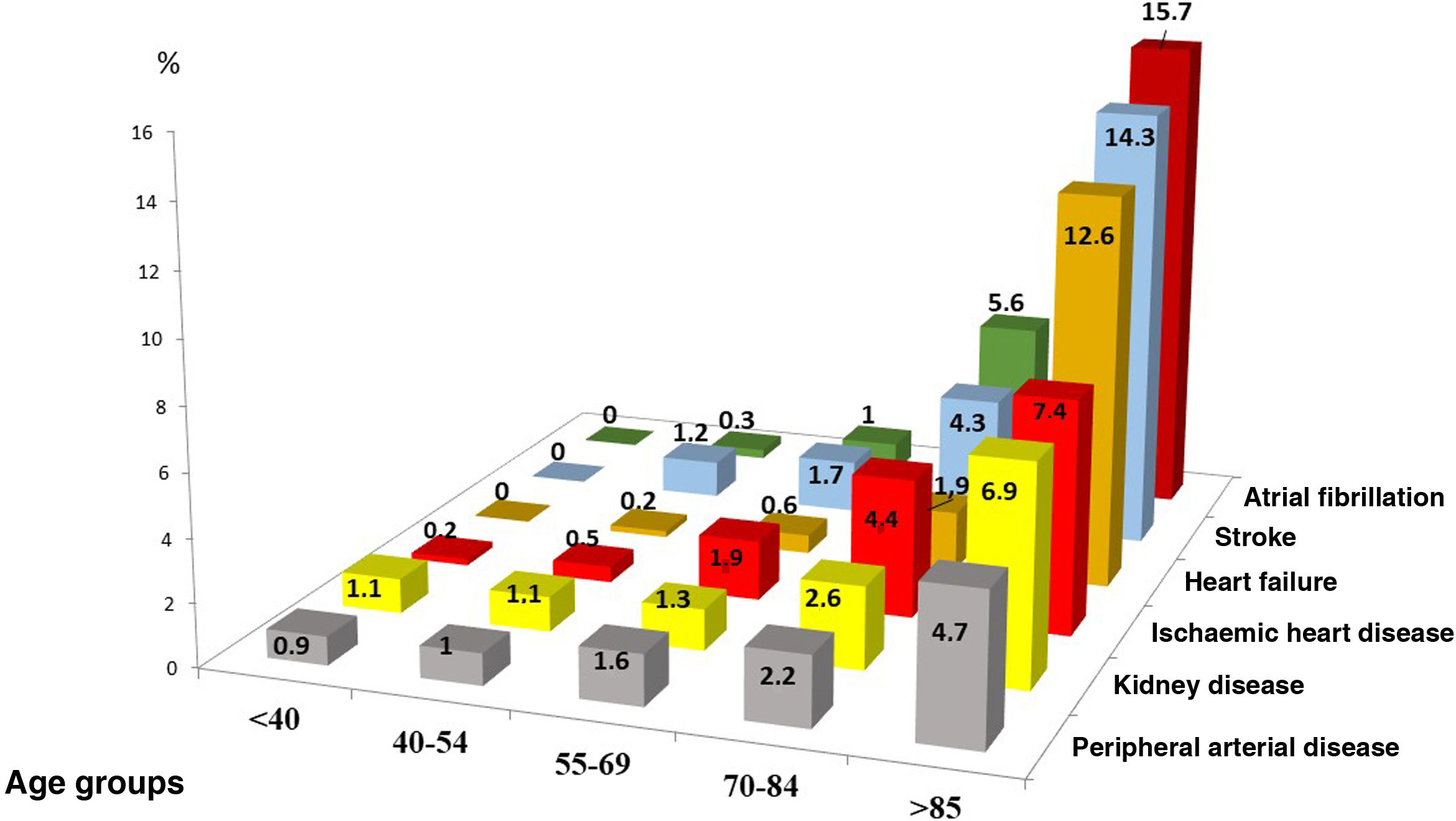
Of the subjects with SH, 6.1% had associated CVD. Among those aged 55–69 years, the most frequent comorbidity was ischaemic heart disease and stroke, and of those with ages > 85 years, atrial fibrillation (AF), stroke, and heart failure were the most common comorbidities (Fig. 2).
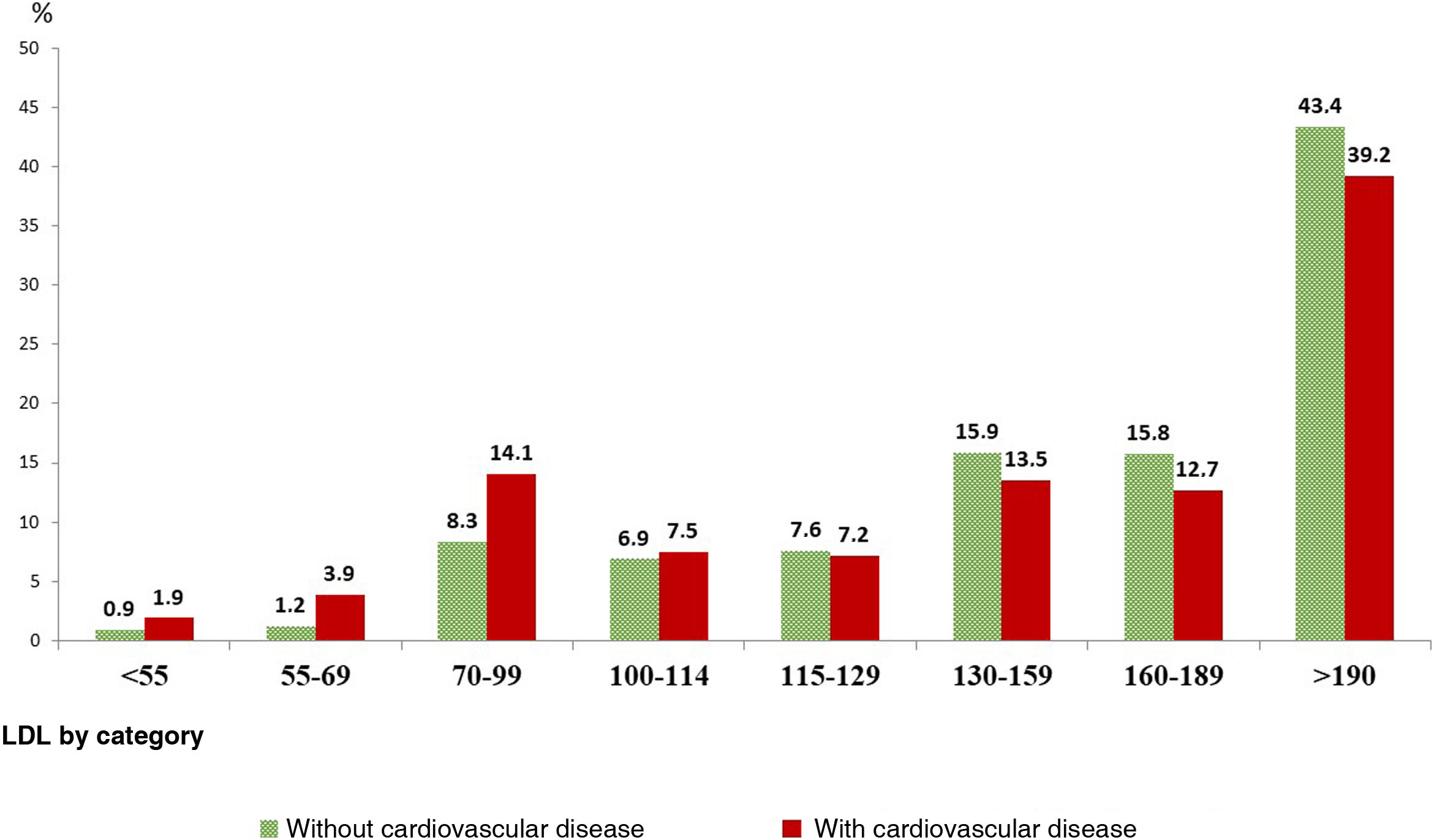
Fig. 3 illustrates the distribution of the different LDL-C thresholds (n = 5827 subjects with available LDL-C figures when triglycerides < 400 mg/dl), in both treated and untreated participants. Among the individuals with CVD (6.1%), 94.2% were taking lipid-lowering therapy. Overall, more than one third of the patients with CVD had LDL-C levels of ≥190 mg/dl, and almost 60% of those without CVD had LDL-C concentrations of ≥160 mg/dl.
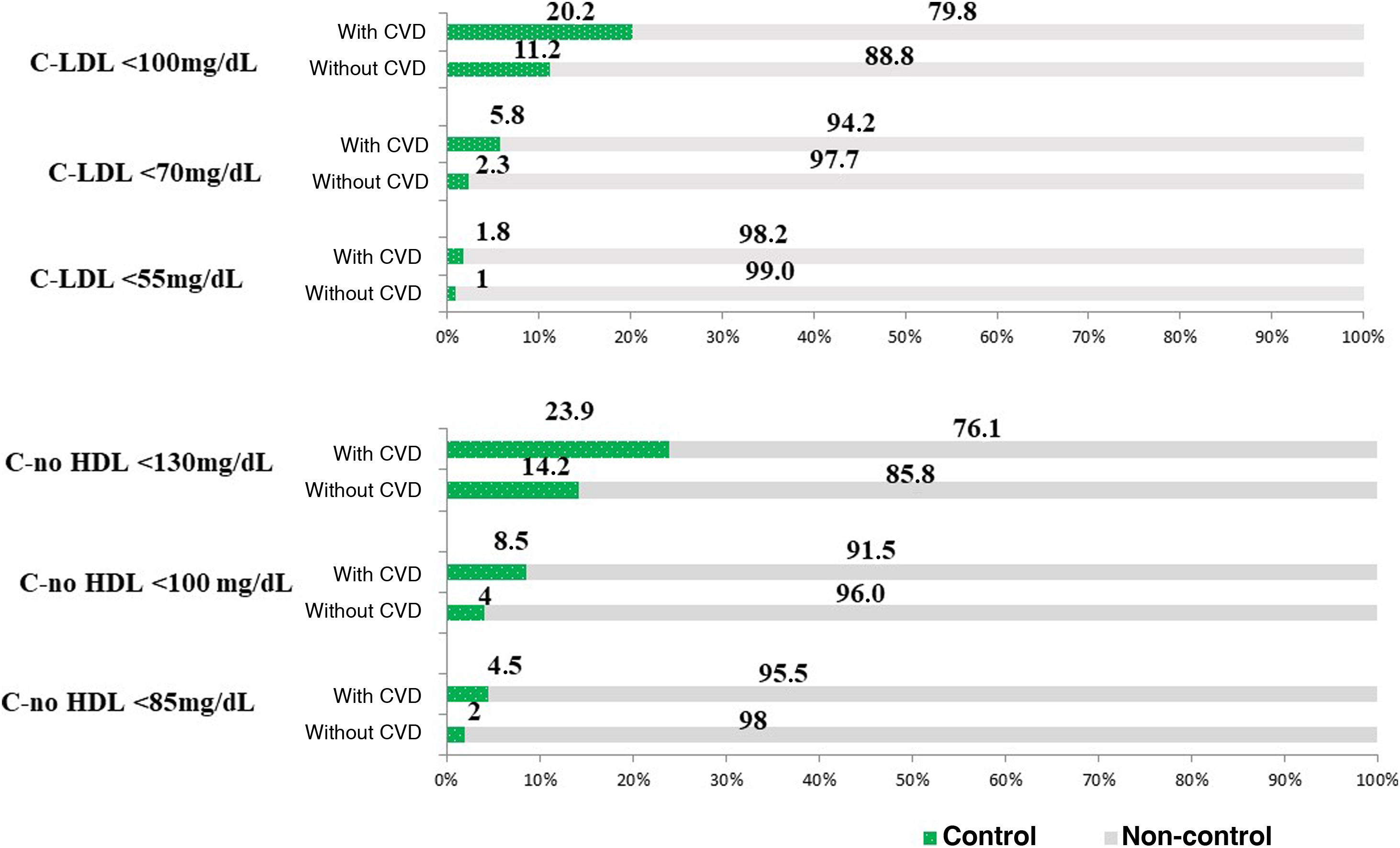
Fig. 4 displays the different LDL-C thresholds in subjects receiving hypolipidemic treatment (5827 participants with figures available for having triglycerides < 400) (Fig. 4A) and non-HDL cholesterol in the total number of subjects (Fig. 4B). Among the individuals with CVD and those treated (94.2%), target LDL-C figures of <55, 70, and 100 mg/dl were achieved in 1.8%, 5.8%, and 20.2%, respectively. The 83.8% of the CVD-free participants who were taking lipid-lowering drugs, and considering those for whom LDL-C data were available due to triglyceride concentrations being <400 mg/dl and LDL-C thresholds of <70 and <100 mg/dl, the control ratio was 2.3% and 11.2%, respectively. The proportions of control considering non-HDL cholesterol thresholds was somewhat better than when considering LDL-C figures.
Degree of control in subjects treated with hypolipidemic agents according to LDL-C (n = 5827) and non-HDL-cholesterol (n = 6187) categories based on the presence of cardiovascular disease.
4A: Categories as per LDL-C levels; 4B: Categories according to non-HDL-cholesterol levels.
LDL-C: cholesterol bound to low-density lipoprotein; non HDL-C: cholesterol not bound to high-density lipoprotein; CVD: cardiovascular disease.
Finally, 1600 individuals had a FH phenotype, which accounts for 1.03% (95% CI: 0.98%–1.08%) in relation to the number of individuals with available blood tests and lipid profile.
DiscussionThis study, carried out in actual clinical practice, provides an up-to-date representation of the magnitude and clinical characteristics of SH and the estimation of the FH phenotype in a large sample of nearly one million adults seen in PC in the northwest of the community of Madrid. SH comprises 3.96% of the total lipid profile analyses available during the study period, which is higher than in earlier studies conducted in our setting, which consider more restrictive LDL-C limits (250 mg/dl),12 although lower than other studies that apply more lenient LDL-C limits (190 mg/dl).13 There is a high prevalence of associated CVR factors, in particular hypertension (36.5%) and excess weight (62.9%), moderate undertreatment (16.3%), low prescription of potent hypolipidemic agents in this high CVR group (used in as few as 1/3), and very low achievement of therapeutic targets among individuals on lipid-lowering treatment, in both primary prevention (2.3%–11.2%) and secondary prevention (1.8%–20.2%).
Subjects with a FH phenotype comprise 1.03% of the total number of participants for whom a lipid profile is available. This is presented merely as an illustration and should be taken with due caution, as the study sample is not representative of the population. Be that as it may, it could represent some 9703 individuals with a FH phenotype with respect to the entire population in Madrid with blood tests available during the study period (based on the population of Madrid with a TSI in 2021 = 6,794,867; 85% > 18 years = 5,775,636, 16.8% with available blood tests). The identification of these subjects could contribute to the design of strategies to establish an accurate diagnosis of FH.11–13
In particular, the proportion of subjects without lipid-lowering treatment or with low- or moderate-intensity treatment is worth noting, despite having SH, LDL-C values that are far from achieving the targets recommended by ESC/EAS guidelines and national and international consensus, both in primary prevention (2.3% at high risk, with LDL-C < 70 mg/dl) and secondary prevention (1.8% with LDL-C < 55 mg/dl).14,15,24–27 Nevertheless, these data are consistent with earlier studies in which the degree of control is clearly suboptimal, notably in patients with very high CVR, in whom absolute risk reduction is more important.28–30 This situation may be due, in part, to physicians' and patients' lack of awareness of the importance of treatment and of achieving treatment goals which can then lead to therapeutic inertia with the lack of initiating or intensifying treatment, without losing sight of treatment compliance issues by patients.7,31
The absence of studies, specifically in the PC setting in Spain, of this group of SH patients with high CVR is also noteworthy. Results obtained by means of a computerised LDL-C query of the different laboratory information systems12 have recently been published in the hospital setting, demonstrating a proportion of SH (LDL-C > 250 mg/dl) of 0.14%, much lower than that found in our study (0.69%−0.71%), possibly because the inclusion criteria are different and we have considered LDL-C figures > 220 or total cholesterol > 300 mg/dl. Similarly, the period studied is also different (a single year 2018 in that study versus 4 years in ours, from 2018 to 2021). We chose that period to expand the number of subjects likely to meet the inclusion criteria, given that this period includes the COVID-19 pandemic, during which there were presumably fewer analytical determinations.
The proportion of subjects with the FH phenotype is somewhat higher than that found in earlier studies,10,23 although in our study there could be a selection bias, since by including the period of the COVID-19 pandemic, there may have been fewer tests than usual and/or tests were performed on more severe subjects or those with who failed to comply with treatment due to the pandemic, which could not be assessed. Despite the existence of national and international consensus on the diagnosis and treatment of FH,10,32,33 there are a number of hurdles to proper diagnosis and follow-up.34
The identification of subjects with such high CVR due to SH could make it possible to inform patients of their risk status. Likewise, PC professionals could be informed of this situation, so that measures could be taken such as the intensification of drug therapy measures and lifestyle modification; all of this to reduce the CVR burden and with the ultimate aim of preventing CDV from developing or its repercussions on patients’ quantity and quality of life and to adapt the dispensing of lipid-lowering drugs in accordance with current recommendations and clinical practice guidelines.
Strengths and limitationsThis work presents the limitations inherent to cross-sectional and registry studies, preventing us from obtaining causal relationships. In our study, compared to other studies using random samples of physicians or patients, selection bias is kept to a minimum by analysing the entire population in a registry (with available data), which better represents the reality of clinical practice.
It is worth emphasising the possible bias caused by diagnostic coding, since while this is done according to an internationally accepted classification and the diagnoses are generally documented by hospital admission reports or complementary testing, there may be biases regarding those cases that did not require admission, inasmuch as they were not in acute situations, which could particularly affect renal failure, peripheral artery disease, and heart failure in its less symptomatic forms. Nonetheless, we have provided all the information available, both in PC and in the hospital, and, given the significant number of patients, this series can provide an approach to the reality of care for patients with severe hypercholesterolaemia in the Community of Madrid, where the vast majority of subjects have a health card.
Furthermore, the registries allow for reasonable analysis, comparisons, and evolutionary controls over time. It should also be noted that there is a bias due to the selection of patients for whom laboratory tests are available; nevertheless, the table presented here is as close as possible to real clinical practice.
There may be an underestimation of the frequency of SH due to having considered the more restrictive criterion of LDL value of 220 mg/dl. There may also be biases in the estimation of FH by considering different LDL values in subjects who were treated and those who were not treated with hypolipidemic agents. Nevertheless, a priori LDL-C concentrations were considered to be around 35% lower in individuals receiving treatment based on criteria proposed by the SEA, although this criterion is arbitrary.
Finally, in the absence of data concerning adherence to treatment, it is not possible to quantify the extent to which the lack of control is due to this or to therapeutic inertia.
ConclusionsThe frequency of SH in PC is not negligible: four out of every 100 patients in a large sample have SH, with moderate undertreatment but with insufficient intensity, and scant success in achieving therapeutic objectives in both primary and secondary prevention; furthermore, one out of every 100 patients has a FH phenotype. The identification of both situations by computerised registries would allow preventive cardiovascular strategies to be devised and early and more accurate detection to be made. This analysis can serve as a foundation for comparison with other studies carried out in Spain and other countries, and in other care settings, as well as to establish a methodology by which to identify and monitor these patients who have a high or very high CVR. It would be important to standardise diagnostic criteria to facilitate comparisons. Overall, studies are needed to examine whether automated screening for both SH and HF in the primary care setting can improve the prognosis of these patients.
FundingNone.
Conflict of interestsNone.
We are grateful to the Madrid Health Service Technical Support Unit (SERMAS, its acronym in Spanish).