This document is an update to the clinical practice recommendations for the management of cardiovascular risk factors (CVRF) in diabetes mellitus. The consensus has been developed by a multidisciplinary team made up of members of the Cardiovascular Risk Group of the Spanish Diabetes Society (SED). The work is a necessary update as, since the last review three years ago, there have been many clinical trials that have studied the cardiovascular outcomes of numerous drugs in the diabetic population.
We believe that this guideline update may be of interest to all clinicians treating patients with diabetes.
El presente documento es una actualización de las recomendaciones de práctica clínica para el manejo de los factores de riesgo cardiovascular (FRCV) en la diabetes mellitus. Este consenso ha sido elaborado por los miembros del Grupo de Riesgo Cardiovascular de la Sociedad Española de Diabetes (SED). El trabajo es una actualización necesaria, ya que desde la última revisión hace tres años, son numerosos los ensayos clínicos que han estudiado los resultados cardiovasculares de distintos fármacos en la población diabética. La presente actualización de la guía creemos que puede ser interés para todos aquellos clínicos que tratan a pacientes con diabetes.
Atherosclerotic cardiovascular disease (CVD) includes acute coronary syndrome (ACS), established chronic ischemic cardiomyopathy (myocardial infarction [MI], stable or unstable angina and coronary revascularization), ischemic cerebrovascular accidents (CVA), transitory ischemic accidents (TIA) and peripheral arterial disease (DAP). CVD is the main cause of morbidity and mortality in individuals with diabetes, and it is also the chief cause of the direct and indirect costs of diabetes.1,2 Cardiovascular risk factors (CVRF) are defined as biological characteristics, conditions and/or changes in lifestyle that increase the probability of suffering CVD or dying due to any cause over the medium to long term.3 Probability tables and equations have been prepared to evaluate this risk, based on prospective studies of populations. Age, sex, smoking, diabetes, total cholesterol and cholesterol linked to low density lipoproteins (LDL-c), cholesterol linked to high density lipoproteins (HDL-c) and arterial blood pressure (ABP) are all considered to be classic CVRF. Other CVRF defined as non-classical (family history, obesity, fat distribution, triglyceride levels, lipoprotein [a], stress and socioeconomic status) may all modulate the calculated risk. Medical consensus documents / recommendations /clinical guides play an important role in guiding preventive, diagnostic and treatment strategies for a range of diseases. They are used as reference sources by medical professionals and societies, and they have to be updated at regular intervals.2,4,5 Recommendations have the aim of making everyday care more uniform.
To evaluate the quality of evidence and power of recommendations we selected the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system.6 The GRADE system defines quality of evidence as the degree of confidence we have when estimating whether an effect will be appropriate when making a recommendation. Each key outcome or result is evaluated; therefore, the same comparison of a therapeutic or preventive intervention may be assigned different quality of evidence scores. The GRADE system classifies quality of evidence into four different categories: high, moderate, low and very low. A recommendation is categorised as high quality (++++), if it is considered unlikely that new studies will appreciably change the level of confidence in a treatment for the diabetic population. Recommendations are classified as moderate (+++) when it is possible that subsequent studies may lead to a major change in our confidence in the effect of a treatment, and recommendations classified as low (++) or very low (+) are those which are considered to have a more doubtful risk/benefit balance, or when they may change substantially based on future studies. GRADE recommendations may be strong1 or weak.2 Strong or grade 1 recommendations may be followed by all or almost all patients, while grade 2 or weak ones mean that although the alternative is suitable for the majority of patients, the decision must be taken on an individual basis, using a multidisciplinary approach.6,7
In this consensus document we review the most important aspects which have emerged in the past two years in connection with CVRF in diabetes. We also include our regular update2,8,9 of the recommendations of the Diabetes and Cardiovascular Risk Workgroup of the Spanish Diabetes Society (SED), fundamentally emphasising the new cardiovascular risk (CVR) classification for the population with diabetes, as well as the emergence of new treatments which help not only to control glycaemia, but also have a cardiovascular (CV) protector effect.5,8,9 We also examine the role of lipid-reducing treatment in the prevention of CVR, with target levels of LDL-c and triglycerides. The therapeutic aims and indications of drugs are described based on CVR, underlining recommendations depending on associated comorbidities (Table 1).
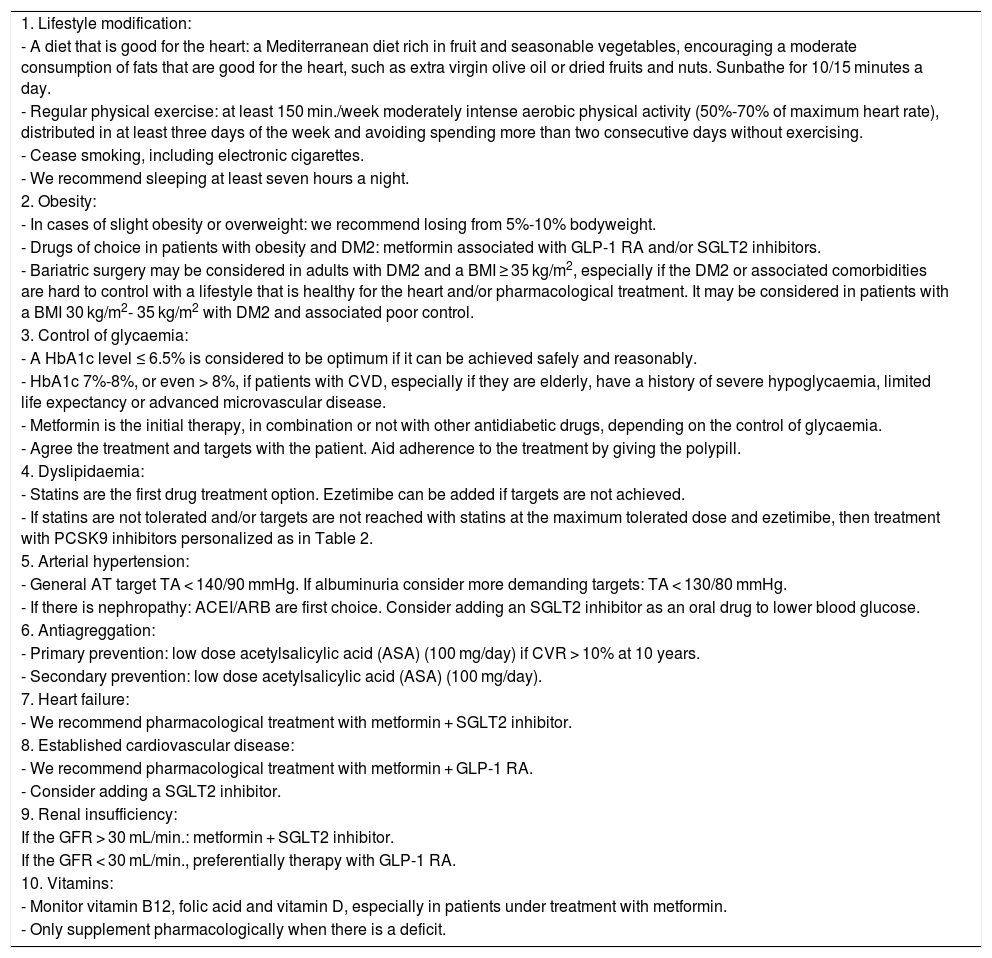
General recommendations by the Diabetes and Cardiovascular Disease Workgroup (SED, 2021).
| 1. Lifestyle modification: |
| - A diet that is good for the heart: a Mediterranean diet rich in fruit and seasonable vegetables, encouraging a moderate consumption of fats that are good for the heart, such as extra virgin olive oil or dried fruits and nuts. Sunbathe for 10/15 minutes a day. |
| - Regular physical exercise: at least 150 min./week moderately intense aerobic physical activity (50%-70% of maximum heart rate), distributed in at least three days of the week and avoiding spending more than two consecutive days without exercising. |
| - Cease smoking, including electronic cigarettes. |
| - We recommend sleeping at least seven hours a night. |
| 2. Obesity: |
| - In cases of slight obesity or overweight: we recommend losing from 5%-10% bodyweight. |
| - Drugs of choice in patients with obesity and DM2: metformin associated with GLP-1 RA and/or SGLT2 inhibitors. |
| - Bariatric surgery may be considered in adults with DM2 and a BMI ≥ 35 kg/m2, especially if the DM2 or associated comorbidities are hard to control with a lifestyle that is healthy for the heart and/or pharmacological treatment. It may be considered in patients with a BMI 30 kg/m2- 35 kg/m2 with DM2 and associated poor control. |
| 3. Control of glycaemia: |
| - A HbA1c level ≤ 6.5% is considered to be optimum if it can be achieved safely and reasonably. |
| - HbA1c 7%-8%, or even > 8%, if patients with CVD, especially if they are elderly, have a history of severe hypoglycaemia, limited life expectancy or advanced microvascular disease. |
| - Metformin is the initial therapy, in combination or not with other antidiabetic drugs, depending on the control of glycaemia. |
| - Agree the treatment and targets with the patient. Aid adherence to the treatment by giving the polypill. |
| 4. Dyslipidaemia: |
| - Statins are the first drug treatment option. Ezetimibe can be added if targets are not achieved. |
| - If statins are not tolerated and/or targets are not reached with statins at the maximum tolerated dose and ezetimibe, then treatment with PCSK9 inhibitors personalized as in Table 2. |
| 5. Arterial hypertension: |
| - General AT target TA < 140/90 mmHg. If albuminuria consider more demanding targets: TA < 130/80 mmHg. |
| - If there is nephropathy: ACEI/ARB are first choice. Consider adding an SGLT2 inhibitor as an oral drug to lower blood glucose. |
| 6. Antiagreggation: |
| - Primary prevention: low dose acetylsalicylic acid (ASA) (100 mg/day) if CVR > 10% at 10 years. |
| - Secondary prevention: low dose acetylsalicylic acid (ASA) (100 mg/day). |
| 7. Heart failure: |
| - We recommend pharmacological treatment with metformin + SGLT2 inhibitor. |
| 8. Established cardiovascular disease: |
| - We recommend pharmacological treatment with metformin + GLP-1 RA. |
| - Consider adding a SGLT2 inhibitor. |
| 9. Renal insufficiency: |
| If the GFR > 30 mL/min.: metformin + SGLT2 inhibitor. |
| If the GFR < 30 mL/min., preferentially therapy with GLP-1 RA. |
| 10. Vitamins: |
| - Monitor vitamin B12, folic acid and vitamin D, especially in patients under treatment with metformin. |
| - Only supplement pharmacologically when there is a deficit. |
It is essential to improve and modify the lifestyle of patients with diabetes to help control the disease and reduce their CV risk.2 Nevertheless, such efforts should not lead to any delay in pharmacological treatment, which should be commenced simultaneously and adjusted depending on the response to lifestyle changes. The fact that the latter are more cost effective than pharmacological therapies must always be borne in mind. One of the most important studies to advocate lifestyle interventions was Look AHEAD, due to its design, duration and population.10,11 Lifestyle changes are important, especially in those individuals with a higher body mass index (BMI) score, waist circumference and waist/hip index, because they have a beneficial effect on insulin resistance, among other factors.8 It is also essential to detect malnutrition and/or the risk of suffering this at an early stage, especially in geriatric patients with diabetes, due to their higher risk of sarcopenia and associated fragility.12 It is fundamental to know the calorie intake of patients and to recommend a diet which helps them to lose from 5% to 10% body weight. The Mediterranean diet should be considered the paradigmatic cardioprotector diet, so that it is especially recommended in our field.7,13 This diet is characterized by high consumption of fruit and vegetables, cereals and legumes, nuts and dried fruit (walnuts), and virgin olive oil, all of the highest quality, with a moderate consumption of fish, fowl and eggs and low intake of dairy products, red or processed meat and sweets, together with moderate consumption of wine with meals. We should evaluate how much dietary fibre is consumed (14 g / 1,000 kcal) as well as foods containing whole grains (half of grain intake), to ensure a fibre intake of from 30 to 45 g/day. We recommend reducing the consumption of sweetened drinks, that saturated fats should account for <7% of the total intake of calories and that no trans fats should be consumed.14 Some saturated fat should be replaced by polyunsaturated and monounsaturated fatty acids, especially those derived from virgin olive oil and walnuts, underlining that on condition that it does not account for more than 35% total calorie intake, the quality of fat consumed is more important than the quantity. The American Diabetes Association (ADA) recommends Dietary Approaches to Stop Hypertension (DASH) to reduce CV risk in the population with diabetes.2
Correct hydration is necessary, and the best sources for this are water and infusions. No systematic use of antioxidant dietary supplements is recommended (vitamins E or C, selenium, magnesium, chrome or carotene, among others) because no tests support their clinical efficacy or long-term safety.2 Meals should also be planned, selecting foods and portions/swaps to include the recommended daily amount of all macro- and micronutrients while preventing overweight or obesity and postprandial hyperglycaemia.
The dietary carbon hydrate glycaemic load and/or index should be taken into account, avoiding foods with a high glycaemic index.15,16
It is becoming increasingly necessary to evaluate and study different dietary products and functional foods and the degree to which they are processed. These products are growing exponentially in the market, due to commercial pressure and the increasing interest of the population in consuming them. They may interact with drugs or affect cholesterol concentration and CV risk. Some of these products, such as red yeast rice, contain monacolin K, that is, lovastatin, so that they may increase the side effects of statins.17
As phytosterols use the same transport mechanism as ezetimibe, the sterol transporter (Niemann-Pick C1-Like 1, NPC1L1), they will interact with ezetimibe and therefore reduce its efficacy.18
Lastly, it is important to determine vitamin B12 levels, especially in patients treated with metformin to prevent raised homocysteine levels secondary to vitamin B12 deficit.2,19
RecommendationsConsume a low carbon hydrate Mediterranean diet supplemented with extra virgin olive oil and walnuts (1/+++).
Encourage the consumption of extra virgin olive oil and dried fruit and nuts (1/++++).
Vitamin B12 and folic acid in case of deficiency (1/++).
Physical exerciseThe importance of physical exercise was pointed out in 1992 by the American Heart Association (AHA), which included a lack of physical activity as a CVR factor that was also associated with an increased risk of type 2 diabetes mellitus (DM2).9,20,21 A sedentary lifestyle is now considered to be one of the chief CVRF. Regular physical exercise increases glucose capture and reduces the risk of DM2, prevents arterial hypertension (AHT) and improves cognition. In fact, physical exercise is associated with an average fall of 6 to 7 mmHg in hypertensive patients in systolic and diastolic ABP. Another one of its benefits is that it increases HDL cholesterol.
It is important to assess the type, frequency, duration and intensity of physical exercise and activity, and this is also the case for elderly individuals.22,23 In apparently healthy adults the risk of CV complications during exercise is extremely low (with from 5 to 17 deaths /million/year, depending on the intensity of the activity). No previous cardiological study is therefore required in low-risk diabetic patients. A cardiological and ergometric study would be relevant for diabetics with a sedentary lifestyle who decide to commence a demanding training programme. Patients with diabetes are recommended to undertake at least 150 minutes per week of moderately intense aerobic physical activity (from 50% to 70% maximum heart rate), distributed over at least three days of the week, avoiding more than two consecutive days without activity.22–25 Patients with diabetes who are under treatment with insulin or oral sulfonylurea-type antidiabetic drugs with a history of hypoglycaemia or previous episodes of severe hypoglycaemia are recommended to check their capillary glycaemia not only before and during exercise but also afterwards, to prevent and detect late hypoglycaemia associated with physical exercise.
RecommendationsUndertake regular aerobic physical exercise (>150 min/week) (1/+++).
Moderate aerobic physical activity (50%-70% maximum heart rate distributed over at least 3 days per week (1/+++).
Avoid more than 2 consecutive days without physical exercise (2/++).
Stress and psychosocial factorsDiabetes has recently been associated with high rates of anxiety and depression, and this may adversely affect adherence to treatment as well as favouring the development of complications secondary to the disease.26 It is not only important to assess the mood, mental health and psychological well-being of patients, as their socio-cultural context should also be examined, as this is becoming more and more important in the overall evaluation of diabetic patients. Sociocultural level is now considered to be another CVRF that is increasingly considered to be relevant in the development of diabetes and CVR.
As well as being associated with a higher CVR, psychosocial factors and a low socioeconomic level, social isolation, depression or hostility and work or family stress all worsen the prognosis of patients with established ischemic cardiac pathology and significantly hinder the control of classical CVRF.27–29 Lack of adherence to therapies is a major barrier against the secondary prevention of CVD. The result of poor therapeutic adherence would be an increase in severe CV complications, as therapeutic objectives would not be achieved and mortality would therefore increase. The quality of life for surviving patients would be lower, with a higher care burden and an increase in medical costs due to complications and hospitalizations. A reduction in co-payment, automatic reminders, mail order pharmacies, advice by medical professionals and combination fixed dose therapies all improve compliance with therapies.29 The polypill for the secondary prevention of CVD was the first combined fixed dose treatment approved in Europe as a replacement therapy in adult patients who were properly controlled with single component drugs administered separately at equivalent therapeutic doses.30,31
RecommendationsAnalysis of patient capacity to undertake everyday activities (1/+++).
Evaluation of their sociocultural context (1/+++).
Use of the polypill once treatment is established (1/++++).
SleepCircadian rhythm analysis is increasingly thought to be important, discovering patients’ sleep patterns, as well as sleep evaluation and/or analysis. Individuals are currently advised to sleep for at least 7 hours.2,9 We should rule out the existence of sleep pathologies such as sleep apnoea and, if they are present, to treat them to improve patient quality of life and make it possible to control their CVRF.32,33
RecommendationsThe advice is to sleep for at least 7 hours a day (1/+++).
SmokingSmoking is the modifiable CVRF which has the greatest impact on CV health. Patients with DM2 who smoke have a significantly higher total CV risk, as well as the risk of mortality, CVA and acute myocardial infarction (AMI) compared to non-smokers.34,35 In clinical practice we should advise all patients to cease smoking every time they visit. They are also recommended to avoid exposure to tobacco smoke (passive smoking). If patients accept that they should stop smoking, they should be referred to specific units for smoking cessation and/or be prescribed treatment for this, as a routine part of the care of diabetic individuals.5 Tobacco products with a lower level of risk are not themselves completely risk-free in this strategy.36 Although they are less harmful than conventional cigarettes, the vapour from electronic cigarettes contains potentially toxic substances that are heat-generated sub-products of the solvent used, together with the trace ingredients of their aromatic additives. Studies have already indicated that these have a harmful vascular effect.37
RecommendationsCease smoking (1/++++).
All types of conventional and electronic cigarette are unadvisable (1/++).
Refer patients to smoking cessation units if they find it hard to stop smoking (1/+++).
ObesityMany studies have shown that an increase in body fat leads to an increase in CVR.9 The relative risk of diabetes in males with a BMI of 35 kg/m2 is 40 times higher than it is for those with a BMI of 23 kg/m2. Small weight losses of from 5% to 10% correspond to better control of clinical and metabolic parameters, as well as psychological factors, without the need for pharmacological treatment, simply with lifestyle changes and dietary modifications.38,39 Patients with diabetes and CV complications would benefit more if we add pharmacological intervention and surgery.9 Pharmacological treatment added to lifestyle modifications leads to greater weight loss than lifestyle changes alone. Moreover, weight loss is greater over time in patients who receive pharmacological treatment.40,41
Bariatric surgery (BS) should be considered in adults with DM2 and a BMI ≥ 35 kg/m2, especially if their DM2 or associated comorbidities are hard to control with a lifestyle that is good for heart health and/or pharmacological treatment. When assessing costs it is fundamental to consider that individuals with DM2 who are subjected to BS need additional lifelong lifestyle changes and continuous strict monitoring.42 BS has been shown to normalize glycaemia and lead to remission of DM2 in 40% to 95% of patients, depending on the length of time over which their diabetes has evolved, the surgical procedure used, C-peptide levels and the remission criteria used.43,44 BS improves the CVRF and CV episodes too over the long term in DM2.44,45 In patients with DM2 and a BMI of from 30 to 35 kg/m2, BS still offers glycaemic benefits; metabolic surgery should therefore be considered in adults with DM2 and a BMI from 30.0 to 34.9 kg/m2 if their hyperglycaemia is not controlled properly by oral or injected antidiabetic drugs and insulin in mono- or combined therapy.2,7
The first line drugs for obese diabetic patients would be metformin associated with sodium-glucose cotransporter 2 (SGLT2) inhibitors and/or glucagon-like peptide-1 receptor agonists (GLP-1 RA),2 if they are not being treated with any of them, as the GLP-1 RA bring about the greatest weight loss.46,47 Obese diabetic patients with high or very high CVR should now consider SGLT2 or GLP-1 RA drugs to be first line treatments, individualizing for their degree of obesity and associated CV factors, in association or not with metformin. If a patient is overweight and at low risk, metformin will be the first line drug.
RecommendationsDiet and physical exercise are recommended for patients who are overweight or with grade 1 obesity, to achieve a weight loss of at least from 5% to 10% (1/+++).
The recommended drugs in patients with obesity and diabetes treated with metformin are SGLT2 inhibitors and/or GLP-1 RA (1/+++).
BS would be indicated in adults with DM2 and a BMI > 35 kg/m2 (1/+++).
BS should be considered in patients with type 1 obesity and those who are grade 2 overweight, together with poorly controlled DM2 and CVRF (2/++).
Control of glycaemia: glycosylated haemoglobin (HbA1c) and CV riskLevels of HbA1c ≥ 6.5% have been set as the diagnostic criterion for DM, on condition that they are measured in a laboratory which uses the standardized method approved by the National Glycohaemoglobin Standardization Program (NGSP), certified and standardized by the Diabetes Control and Complications Trial (DCCT), the International Diabetes Federation (IDF) and the European Association for the Study of Diabetes (EASD). Other necessary conditions are that the patient does not have anaemia or haemoglobinopathy, that they are not pregnant (after the third month), postpartum, or have glucose-6-phosphate-dehydrogenase deficiency, acquired immunodeficiency syndrome (AIDS), and are not in haemodialysis or therapy with erythropoietin, in which case only glycaemic criteria will be used as the results of HbA1c will be altered.48,49
Several studies have confirmed the importance of glycaemic control in DM.2,9 A 0.9% fall in HbA1c reduces CV episodes by about 10% to 15%.4,48 The HbA1c target should be set on an individual basis and factors such as age, life expectancy, comorbidities, duration of the diabetes, the risk of hypoglycaemia or adverse results of the same, patient motivation and adherence to treatment. A HbA1c level of ≤ 6.5% is considered optimum if it can be achieved safely and reasonably; a reasonable target would be 7% HbA1c. In young patients without other CVRF or complications stricter HbA1c targets should be considered, using stronger drugs to achieve low levels of HbA1c (∼6.5%), on condition that this can be achieved without significant hypoglycaemia or adverse effects. In patients with CVD, especially those who are elderly, a history of severe hypoglycaemia, limited life expectancy, advanced microvascular disease or macrovascular complications, with chronic diabetes, the HbA1c target should be less demanding, at 7% to 8% or even higher.2,50
Patients with type 1 DM (DM1) should be treated with intensive insulin therapy, either with multiple doses of insulin (three or four doses per day) or with continuous subcutaneous infusion of insulin, fundamentally with insulin analogues, which are associated with a lower risk of hypoglycaemia. In type 2 diabetics the cost-effectiveness of these treatments should be assessed, taking age into account together with factors in connection with the risk and severity of hypoglycaemia. The recently introduced delayed action insulin analogues such as glargine U300 and degludec U100/U200 have longer-lasting and more stable pharmacokinetic and pharmacodynamic characteristics than glargine U100 and detemir.51 The new prolonged action analogues (U-300 glargine or degludec) give rise to less risk of hypoglycaemia than glargine U-100 in patients with DM1, although it is important to bear in mind that the cost and/or intensification of the treatment is higher in economic terms.51–53 In patients with DM2 and at high risk of CV events, degludec was not inferior to glargine in terms of the incidence of major CV events.53
The fact that premixed insulins are less flexible in terms of dosage must be borne in mind, together with their association with more frequent hypoglycaemic events in comparison with basal and basal-bolus regimes.2 Treatment to lower blood glucose has to be optimized in patients with DM1 and DM2 at an early stage, to ensure CV safety over the medium to long term.
Metformin was the only drug used to lower blood glucose that had been shown to have a lower CVD risk. Several clinical trials (SAVOR-TMI-53 [saxagliptin], EXAMINE [alogliptin], TECOS [sitagliptin] and CARMELINA [linagliptin]) showed a neutral effect of dipeptidyl peptidase-4 (DPP4) inhibitors on CV events (references of each clinical trial).
After 2015, four CV safety trials have been published, of SGLT2 (EMPA-REG [empagliflozin], CANVAS [canagliflozin], DECLARE TIMI [dapagliflozin] and VERTIS CV [ertugliflozin]). EMPA-REG and CANVAS confirmed the superiority of empagliflozin and canagliflozin for the combination of CV mortality, non-fatal myocardial infarction or non-fatal CVA (denominated “three point MACE” - major adverse CV events). DECLARE-TIMI showed a lower rate of CV death and hospitalization due to heart failure (HF) in patients treated with dapagliflozin, although the three point MACE did not. This difference between studies probably reflects the lower level of CV risk of the cohort studied in DECLARE TIMI54–61 and in VERTIS CV, which did show fewer hospitalizations due to HF.54–62
SGLT2 inhibitors are now first line drugs in patients with established macrovascular complications, together with the GLP-1 RA; the SGLT2 inhibitors are the drug of choice for diabetic patients with HF.63 Although SGLT2 inhibitors are the standard drugs for patients with HF and DM2, the mechanisms of their cardioprotector effect has yet to be elucidated, and we have to control their side effects, especially the risk of ketoacidosis, genital-urinary infections and their high cost. It is important to underline that SGLT-2 inhibitors must be suspended 3 to 4 days prior to surgery. Moreover, SGLT2 inhibitors have also given rise to a true paradigm shift in the treatment of renal diabetic disease, as will be described below, and they are fundamental in diabetes because of the role in protecting the kidneys and heart.64
GLP-1 RA are administered subcutaneously, with the exception of semaglutide which is also available in an oral version (that has yet to be commercialized in Spain). They are classified as having a short half-life and being for daily administration (immediate release exenatide and lixisenatide) and with a long half-life and weekly administration (extended release exenatide, liraglutide, dulaglutide and semaglutide).
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) study showed there is a benefit in patients with DM2 and high CVR of adding liraglutide to their usual treatment.65 The results are similar to those of empagliflozin in the EMPA-REG OUTCOME study, although in the latter the benefits were shown from the start of the study. Based on the CV results of the studies of GLP1 analogues, liraglutide in the LEADER study, semaglutide in the SUSTAIN-6 study, albiglutide in the HARMONY study and the REWIND study with dulaglutide, a CV benefit was proven, reducing the CV outcome in terms of 3 point MACE in comparison with a placebo in protection against CV episodes.66–69
The recommendation is now to use SGLT2 inhibitors or GLP-1 RA in patients at high or very high CVR, and the guides state that SGLT2 inhibitors or GLP-1 RA are the first line drugs of choice for patients with DM2 and high CVRF, as monotherapy or combined with metformin.63 We should underline that the CV benefits of both of these groups of drugs do not depend solely on reducing HbA1c, so that they may be considered for DM2 patients with CVD regardless of the HbA1c target level. To improve the CV benefit of drug treatment for CV risk it is appropriate to evaluate combining the usual treatment with an SGLT2 inhibitor or a GLP-1 RA, or even giving these drugs to patients with CVD although they have HbA1c levels within the therapeutic target level, to obtain a CV benefit independently of HBA1c levels.2,64 Lastly, it should be emphasised that not all of the guides have the same objectives nor make the same recommendations, so that the National Institute for Health and Care Excellence (NICE) guide has a more economic bias and is less up-to-date, and it does not express the same opinion.70
RecommendationsAn optimum HbA1c level should be the target (≤ 6.5%) only condition that this can be achieved reasonably and safely (1/+++).
HbA1c levels of 7% to 8% or even >8% may be considered in patients with CVD, especially when they are elderly or have a history of severe hypoglycaemia, limited life expectancy, advanced microvascular disease or macrovascular complications, with long-term diabetes (1/+++).
Metformin is the first drug to be considered for DM patients, a SGLT2 inhibitor can also be considered as the first therapeutic option, or a GLP-1 RA in diabetic individuals with established CV disease (1/++++).
The SGLT2 inhibitors and GLP-1 RA are drugs of choice in combination with metformin in patients with CVRF (1/+++).
SGLT2 inhibitors should be evaluated as the first option in patients with DM2 and HF (1/+++).
Arterial blood pressure: AHT and DMThe European Guides for Cardiovascular Management2,9,71 still contain no relevant new data. We recommend measuring the ABP of diabetic patients every time they visit. If high levels of systolic arterial pressure are detected (SAP) >140 mmHg and/or diastolic arterial pressure (DAP) > 90 mmHg in patients with diabetes, then the relevant screening should be performed using self-measurement of ABP (AMPA) or outpatient monitoring of ABP (MAPA). The target of an ABP below 140/90 mmHg is based on the results of the angiotensin-converting enzyme (ACE) and Action to Control Cardiovascular Risk in Diabetes- Blood Pressure (ACCORD-BP), the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation Blood Pressure (ADVANCE BP), Hypertension Optimal Treatment (HOT) studies, and even the Systolic Blood Pressure Intervention Trial (SPRINT),72–74 although the latter did not include patients with DM. Intensive treatment (SAP < 120 mmHg) does not reduce CV events, although it does reduce the risk of cerebrovascular accidents (CVA) at the cost of increasing adverse effects.2,74 The control target should be set on an individual basis, while advising a SAP < 140 mmHg in general. SAP levels lower than 130 mmHg may be appropriate in younger individuals and patients with microalbuminuria. Although the DAP target in patients with diabetes is < 90 mmHg, this limit is not firmly established and from 80 mmHg to 90 mmHg is acceptable, depending on patient age and associated comorbidities. For patients with a confirmed ABP ≥ 140/90 mmHg, as well as lifestyle changes and weight loss, when applicable, DASH75 recommends a reduction in sodium of at least 2.3 g/d (100 mmol/d) and an increase in potassium intake (∼4.7 g/d [120 mmol/d]) on condition there is no altered urinary excretion of potassium, and potassium intake should be restricted to less than 4.7 g/d (120 mmol/d) to prevent adverse cardiac effects (arrhythmia) due to hyperpotassaemia.76 It is important to reduce alcohol consumption and increase physical activity, and pharmacological therapy should commence if the APB targets set in the guides are not achieved. In cases of DM” the American College of Endocrinologists Guide recommends lifestyle modifications for all diabetic subjects, to keep ABP under 130/80 mmHg in the majority of subjects and pharmacological antihypertensive treatment when levels are ≥ 140/90 mmHg or ≥ 130/80 mmHg if the CVR > 10% in 10 years. When ABP is >160/100 mmHg treatment with 2 antihypertensive drugs should commence.3,9 Angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), beta-blockers, calcium channel antagonists and thiazide diuretics are the preferred options for first-line treatment.77 Medication selection should be based on factors such as the presence of albuminuria, CVD, HF or a state of post-myocardial infarction, as well as patient race/ethnicity, possible metabolic side effects, adherence to treatment and cost. Patients with diabetes and AHT should be treated pharmacologically with an ACEI or an ARB, due to their greater protective effect against the appearance or progression of nephropathy. The ACEI and ARB may delay the progression of diabetic nephropathy and retinopathy, so that they are especially indicated in patients with DM.9,78,79 If one of them is not tolerated, then one may be used to replace the other. To achieve ABP targets a combination of two or more drugs is usually required, at their maximum dose, and it is advisable to commence treatment with 2 drugs from the start if ABP is higher than 160/100.3 If there is a need to use 3 drugs and ABP is poorly controlled then referral of the case to the specialised hospital AHT department should be considered.80,3 We should consider administering one or more antihypertensive drugs before going to bed, to prevent nocturnal AHT, as nocturnal ABP is a stronger predictor of a CV episode than daytime ABP.80 We do not recommend the use of combined ACEI and ARB therapy, in particular in patients with diabetic nephropathy, due to the risk of aggravating kidney failure and facilitating hyperpotassaemia. The combination of aliskiren with an ACEI or ARB in patients with altered renal function or diabetes is contraindicated. Candesartan and Valsartan are still authorised for the treatment of HF in combination with an ACEI only in those patients who cannot use mineralocorticoid antagonists. If ACEI, ARB or diuretics are used, renal function has to be monitored together with the serum potassium level. When ABP is > 140/90 mmHg it is possible to add a diuretic to the ACEI or ARB and, if it remains high, to consider a calcium antagonist and, if poor control persists, to consider adding a beta-blocker. There is clearly room for improvement in control of ABP and the prevention of associated morbidity and mortality.2,81 However, there is still a lack of knowledge, treatment and control of AHT worldwide.82 All patients with DM2 and AHT are still advised to control and monitor their ABP using AMPA or MAPA, with the aim of unmasking white coat AHT and improving the diagnosis as well as adherence to medication. Therapeutic inertia must be avoided (leaving diabetic patients with APB levels of ≥ 140/90 mmHg), as this leads to an unacceptable cost in terms of human lives, sequelae and socioeconomic costs.81 The ADA recently stated that control targets should be set on an individual basis.2,3 For individuals with DM and AHT at high CVR (previous CVD or the risk of CVD over 10 years ≥ 15%), a target ABP below 130/80 mmHg may be appropriate if it can be achieved safely.2 For individuals with DM and AHT at low risk of CVD (a risk of CVD over 10 years < 15%), a target ABP below 140/90 mmHg is suitable.2,9
We should consider adding a SGLT2 inhibitor as an oral drug to lower blood glucose in patients with DM2, those who are overweight or have mild hypertension, as this not only aids weight loss, but also reduces the SAP (from 3 to 5 mmHg).
If control is not achieved with 3 hypotensive drugs, including a diuretic, it would be possible to consider adding a mineralocorticoid receptor antagonist.3 If a patient has an albumin/creatinine-CAC- coefficient of ≥ 300 mg/g (A) or 30 to 299 mg/g (B) then ACE inhibitors or angiotensin receptor blockers should be used as the first line drug in AHT.2,3 Suspension of ACE inhibitors or ARB has been suggested for all patients to prevent or limit the spread of the SARS-CoV-2 virus, although this is not based on clinical evidence.83,84 On the contrary, experimental studies suggest that the ARB may be useful in these patients, limiting damage to the lungs by inhibiting type 1 angiotensin receptors.85
Patients with HF may benefit from using beta-blockers, as diabetics with benign prostate hypertrophy may benefit from alpha-blockers or diabetics with ischemic cardiomyopathy may benefit from beta-blockers,9 without forgetting that ACE inhibitors or ARB are also recommended as first-line therapy for hypertension in individuals with diabetes and ischemic cardiomyopathy. Lastly, remember that it is possible to use the polypill to control ABP, as this aids adherence and also seems to be more effective.
RecommendationsABP control targets should be set on an individual basis (1/++++).
The ABP target in hypertensive diabetic individuals with low CV risk (less than 15% risk of CVD in 10 years) is that it should be below 140/90 mmHg (1/++++).
The ABP target in hypertensive diabetic individuals at high CV risk (previous CVD or 15% or higher risk of CVD in 10 years) is that it should be below 130/80 mmHg (1/++).
If there is nephropathy (microalbuminuria): ACEI/ARB are the first choice (1/++++).
ACE inhibitors or ARB are recommended as first line therapy for hypertension in individuals with diabetes and ischemic cardiomyopathy (1/+++).
It is better to select combined antihypertensive drugs (polypill) if this is possible (1/+++).
Hyperlipidaemia/dyslipidaemiaDM2 is a known risk factor for CVD, and it is the main cause of death in the diabetic population. Hypercholesterolaemia is a key pathogenic factor in the development and progression of the vascular lesion.2 The population with DM has a high risk of CVD. It is therefore fundamental to know the lipid profile of patients with DM2 after diagnosis and to monitor it annually, with the aim of achieving therapeutic targets more precisely.86,87 A delay in the proper control of cholesterol levels is also known to be associated with increased CV risk. The different lipid-lowering drugs now available such as statins, ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, make it a priori possible to attain the therapeutic target levels of LDL-c.9,88
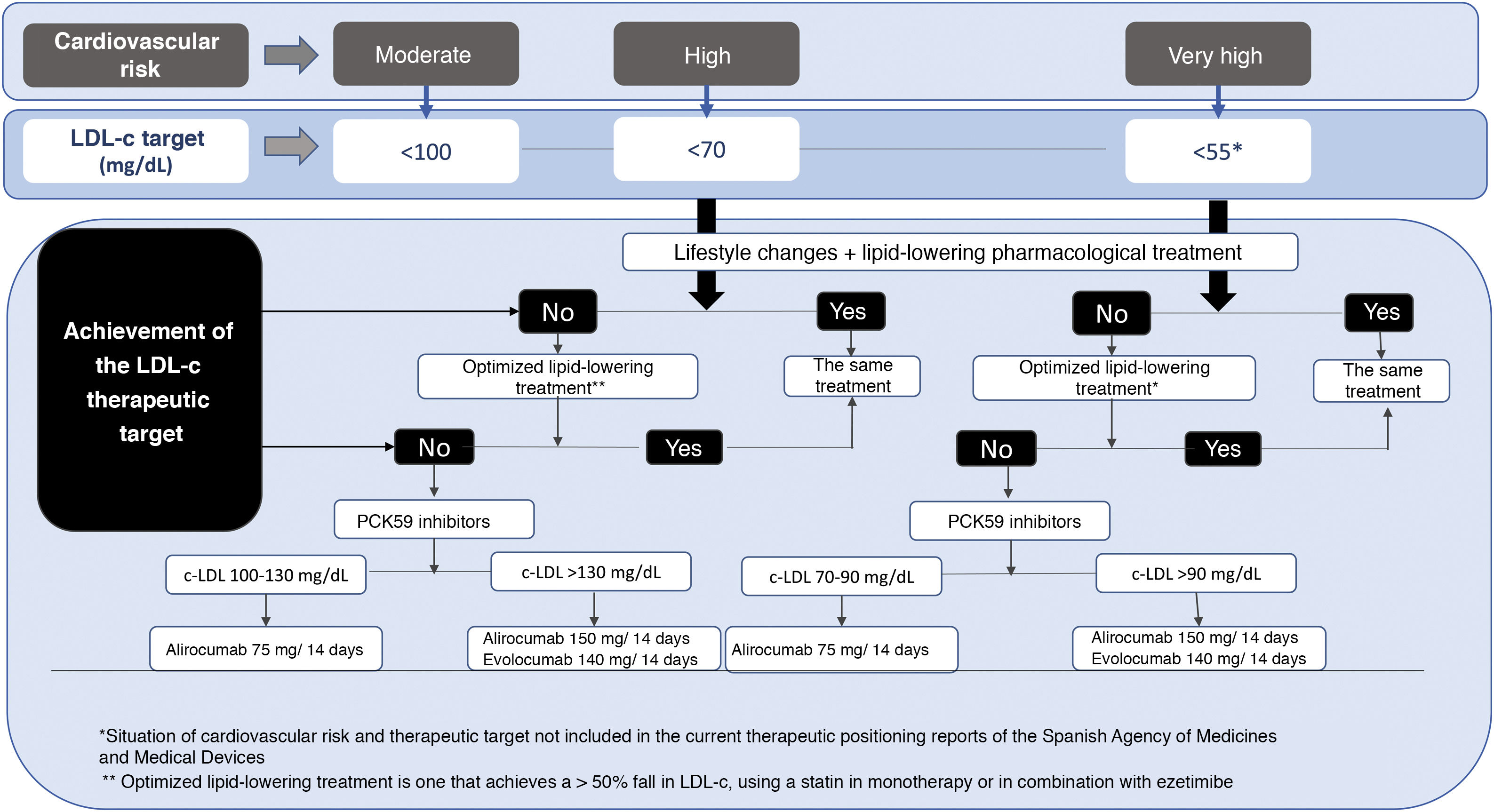
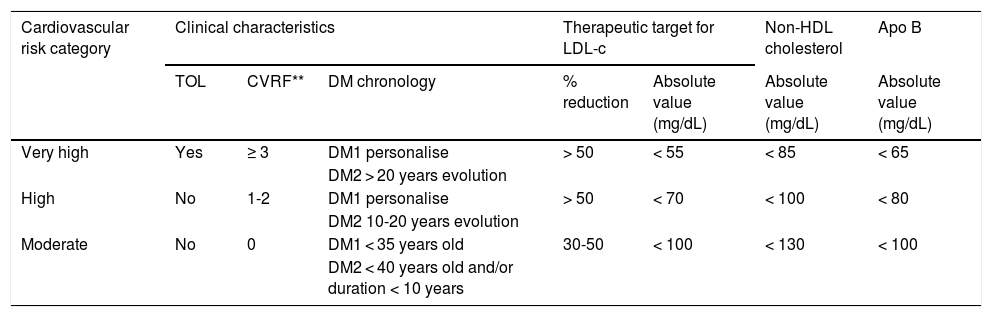
The recommendations of the American Association of Clinical Endocrinologists (AACE) and the American College of Endocrinology (ACE), the 2018 recommendations of the Cardiovascular Risk Workgroup of the SED5, the European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) 201987 and the American College of Cardiology (ACC)/American Heart Association (AHA) for the primary prevention of CVD in 2019,87 and more recently the recommendations of the American Diabetes Association (ADA, 2021)3 all underline the awareness of these scientific societies of the need to reduce CV risk in DM2 by means of the appropriate use of the available therapeutic strategies. Table 2 shows the stratification of CV risk and the therapeutic targets for the diabetic population according to risk category, chronology and the type of DM. It is crucial to emphasise the importance of determining lipoprotein (a) (Lp[a]) in diabetic individuals, as this not only helps us to quantify the risk, but also allows us to assess therapeutic options, favouring the use of PCKS9 inhibitor which is now the only drug that reduces Lp(a) levels by about 20%.
Cardiovascular risk stratification and therapeutic targets in the population with diabetes*.
| Cardiovascular risk category | Clinical characteristics | Therapeutic target for LDL-c | Non-HDL cholesterol | Apo B | |||
|---|---|---|---|---|---|---|---|
| TOL | CVRF** | DM chronology | % reduction | Absolute value (mg/dL) | Absolute value (mg/dL) | Absolute value (mg/dL) | |
| Very high | Yes | ≥ 3 | DM1 personalise | > 50 | < 55 | < 85 | < 65 |
| DM2 > 20 years evolution | |||||||
| High | No | 1-2 | DM1 personalise | > 50 | < 70 | < 100 | < 80 |
| DM2 10-20 years evolution | |||||||
| Moderate | No | 0 | DM1 < 35 years old | 30-50 | < 100 | < 130 | < 100 |
| DM2 < 40 years old and/or duration < 10 years | |||||||
Apo: Apolipoprotein; CVRF: Cardiovascular risk factors; DM: Diabetes Mellitus; LDL-c: Cholesterol bound to low density lipoproteins; HDL: High density lipoproteins; TOL: Target organ lesion.
CVRF: age, sex, family history, arterial hypertension, smoking, hypercholesterolaemia, overweight/obesity (particularly abdominal obesity) and a sedentary lifestyle.
Adapted from the ESC/EAS 20198 guide. No subject with diabetes is considered to be at low cardiovascular risk.
Statins are still the keystone of lipid-lowering therapy in diabetic patients, with the aim of reducing CV episodes.1,5,9,82,89,90 They are contraindicated during pregnancy. Once statins have been given at the maximum tolerated dose, if the primary LDL target has not been achieved then ezetimibe may be considered. Although recent studies have pointed out that statins except pitavastatin confer a risk of developing diabetes,91 the slight increase in the risk of DM2 would not justify not using them.92 High dose statin therapy may be associated with a higher risk of developing DM than therapy at low or moderate doses. The primary prevention JUPITER study showed CV benefits, with a reduction in mortality after treatment with statins, although the majority of statins at high doses are associated with a higher incidence of DM, and they may slightly worsen the control of glycaemia. Nevertheless, the preventive effects on CV episodes and CV mortality were greater than the risk of developing DM. On the other hand, some studies found no association between statin use and a higher risk of developing diabetes.93,94 However, other studies found no high risk of diabetes associated with the use of statins.95
In all diabetic patients with CVD, if LDL-c target levels are not achieved then ezetimibe should be added. The results of the IMPROVE-IT study support the indication of ezetimibe in combination with statins when the latter do not achieve the target LDL-c level.96,97
If LDL-c levels are very high under treatment with statins and we believe that the addition of ezetimibe will not achieve appropriate lipid control, then a combined treatment with a PCSK9 inhibitor may be considered to attain the target levels of LDL-c. This may also be used in case of statin intolerance. PCSK9 inhibitors are administered subcutaneously every 14 or 28 days, and two versions are currently available: evolocumab and alirocumab.98 PCSK9 inhibitors have a powerful LDL cholesterol reduction action, so that it can be reduced by from 50% to more than 70%, regardless of whether it is used as a monotherapy or in combined treatment with statins or other lipid-lowering drugs.99,100 They reduce triglycerides and levels of Lp (a), and they raise HDL cholesterol levels, with a very low incidence of adverse effects. As well as reducing CVR, they reverse atheroma plaque.101,102 The FOURIER study also shows an additional benefit in the absolute reduction in CV events in diabetic patients (2.7% vs. 1.6% at three years).101 However, this benefit was restricted to a lower need for revascularizations, and there were differences in the combined variable of AMI, ictus or CV death (reduction of the absolute risk [RAR] 2%). Likewise, the results of the ODYSSEY102 study show that treatment with alirocumab in diabetic patients benefits them more than it does the population in general, given their higher basal risk (RAR in diabetics of 2.3%, vs. 1.6% in the study population as a whole). Thus together both of these studies, together with the accumulated evidence for risk, support more intensive treatment for LDL-c targets, in type 1 and type 2 diabetic populations.
The use of PCSK9 inhibitors is currently only financed for patients with diabetes who have established CVD (ischemic cardiomyopathy, ischemic AVC and DAP), when their LDL-c level is above 100 mg/dL in spite of the maximum tolerated dose of statins and ezetimibe, and this indication should now be updated and individualized (Fig. 1). We believe that financing should be broadened, as patients with DM2 are at very high or extreme risk, so that LDL-c levels higher than 55 mg/dL are associated with a very high CV risk.103
The remaining cholesterol (total cholesterol total – [HDL cholesterol + LDL cholesterol]) is considered to be one of the chief risk factors for atherosclerosis and CV episodes, and it is also an indirect marker of hypertriglyceridaemia. Non-HDL cholesterol (non-HDL-c) is thought to be a better CVR predictor in patients with high triglyceride levels.104 Fibrates may help to improve high levels of triglycerides, and they have been said to play a protective role in diabetic retinopathy and other microangiopathic complications.103 Nevertheless, we must not forget that the prime CV prevention objective is LDL-c, and that the evidence for a potential CV benefit of treatment with fibrates after statins emerged from the post-hoc analysis of randomized studies.105 When a patient with DM2 requires combination treatment using a statin associated with a fibrate to reduce the residual risk attributable to atherogenic dyslipidaemia, fenofibrate is the only one that can be recommended, and gemfibrozil is contraindicated.106,107
Patients with triglyceride concentrations ≥ 200 mg/dL, with HDL-c < 40 mg/dL and any concentration of LDL-c, even if they are under treatment with statins, should receive treatment not only to reduce their triglyceride concentrations, but also to correct the potentially atherogenic alterations of the other lipoproteins, i.e., treatments with fibrates and omega-3.
Omega-3 consists of eicosapentaenoic acid (EPA) esters and docosahexaenoic acid (DHA) esters. These act on lipids in plasma, reducing the level of triglycerides as a result of lowering the level of very low density lipoprotein (VLDL cholesterol) and also acting on homeostasis and ABP. They reduce triglyceride synthesis in the liver and increase the beta-oxidation of fatty acids, and this too contributes to the fall in triglyceride levels. Although the true benefit of omega-3 acids has yet to be established, they are of use in the treatment of hypertriglyceridaemia and secondary prevention after MI. The Reduction of Cardiovascular Events With Icosapent Ethyl-Intervention Trial (REDUCE-IT) clinical trial recently found that the ethylic ester of EPA at high doses confers a 25% fall in the relative risk of major CV events in comparison with a placebo. These events include CV death, non-fatal MI, non-fatal CVA, coronary revascularization and hospitalization due to unstable angina.108 REDUCE-IT found a fall in relative risk in high risk patients with high triglyceride levels who were under treatment with statins, but using 2 g twice a day. The beneficial effect of an omega-3 acid on morbimortality in patients with CV disease or at high CV risk are promising.109 Further contemporary studies are necessary with patients selected less rigorously and with more optimized treatment if omega-3 is to come into more general use, Thus in patients with atherosclerotic CVD or other CVRF who take a statin, whose LDL-c is controlled but with high levels of triglycerides (135 to 499 mg/dL), the addition of icosapent ethyl may be considered to reduce CV risk.2
Lastly, it is important to point out that microangiopathy is a complication in all patients with long-term DM 1, so that strict CV risk targets should be set in chronic DM1.9 These targets should be set on an individual basis for each case, depending on age at disease diagnosis, age, sex and CVRF.3
Recommendations for dyslipidaemiaFirst therapeutic option: statins (1/+++)If no control with statins, then add ezetimibe (1/++).
When there is statin intolerance and/or incapacity to achieve the therapeutic targets with statins at maximum tolerated dose and ezetimibe, commence treatment with a PCSK9 inhibitor (1 /+++).
LDL-c targets for all diabetics are below 100 mg/dL (1/+++).
DM LDL-c targets not under 30 (2/++).
In patients with DM the LDL-c target level in primary prevention is from 100 to 70 mg/dL depending on CVRF, and in secondary prevention < 55 (1/+++).
Recommendations for statins depending on age or CV risk (2/+++).
For patients with atherosclerotic CV disease or other CVRF with LDL-c within target levels but high levels of triglycerides (135 to 499 mg/dL), treatment with ecosapent ethyl should be considered to reduce CV risk (1/+++).
Platelet aggregation inhibitors and other drugs in CV pathology: the indication for platelet antiaggregant drugs, beta-blocker and surgery in CVD and diabetes
Platelet aggregation inhibitors and other drugs in CV pathology: the indication for platelet antiaggregant drugs, beta-blocker and surgery in CVD and diabetesIn DM1 and DM2 with increased CVR (>10% at 10 years), the benefit of treatment with aspirin (75 to 162 mg/day) as a primary prevention strategy has been called into question in the last ten years due to the publication of several trials with neutral results (ASCEND, ARRIVE and ASPREE). These studies weighed the benefit (a fall in the number of CV events) as opposed to the adverse events (fundamentally bleeding) in several contexts, such as the general population with moderate CV risk, diabetic individuals and the elderly. Although the benefit of aspirin in secondary prevention (patients with a history of cerebral-cardiovascular disease) has now been clearly proven, overcoming possible risks, this is not the case in the context of primary prevention, where studies show that the risk of bleeding is higher, compared with the insignificant benefit it offers.108–111 Aspirin is recommended for diabetic men > 50 years old and women > 60 years old who have at least one major CVRF, such as a family history of CVD, AHT, smoking, dyslipidaemia or microalbuminuria. Aspirin should not be recommended for the primary prevention of CVD in adults with diabetes and a low risk of CVD (risk at 10 years < 5%), men < 50 years old and women < 60 years old without other CVRF, as the potential effects of bleeding probably outweigh the benefit.112,113 We recommend using aspirin as secondary prevention (75 to 162 mg/day).2,5,9 We should point out that recent meta-analyses raise the hypothesis that the efficacy of low-dose aspirin falls in patients who weigh more than 70 kg.114
Patients with CVD who are known to be allergic to acetylsalicylic acid (ASA) should be given clopidogrel (75 mg/day). Clinical practice guides specify treatment with two antiaggregant drugs during one year for patients with ACS who have suffered an AMI.115 After this period of time the patient continues treatment with a single antiaggregant drug, usually ASA. It is reasonable to give combined treatment during up to one year after an ACS with ASA (75-162 mg/day) and clopidogrel (75 mg/day). We recommend using a P2Y12 receptor inhibitor (define) for patients with DM and ACS who have been subjected to a percutaneous coronary intervention (PCI), and the duration will depend on the type of stent. Patients who have received a PCI due to ACS should preferably be given prasugrel or ticagrelor.115–118 The net clinical benefit (the ischemic benefit versus the risk of bleeding) has been said to improve with treatment using ticagrelor in the large pre-specified subgroup of patients with a history of PCI, while no net benefits were found for patients without a previous PCI.119
For patients with known CVD treatment with ACEI or statins (if they are not contraindicated) should be considered to reduce the risk of CV, together with ASA. For patients with a previous MI, beta-blockers should be continued for at least two years after the acute episode. It is very important to use beta-blockers after infarction to prevent sudden death.119 We recommend using ACEI and beta-blockers during at least three years after an AMI. Cardiac surgery should now be the therapeutic choice for the majority of DM2 patients with known multiple vessel disease.120,121
RecommendationsASA in primary prevention: low dose (100 mg/day) if CVR >10% at 10 years (1/++).
ASA in secondary prevention: low dose (100 mg/day) (1/++++).
ASA and clopidogrel after AMI with the emplacement of a stent should be administered for one year (1/+++).
We recommend the use of an ACEI and beta-blocker during at least two years following acute myocardial infarction (AMI) (1/+++).
SGLT2 inhibitors/GLP-1 RA should be used in combination with metformin due to their beneficial effects on CVR and CV mortality, selecting them according to which drugs is the most indicated for each patient (1/++++).
Cardiorenal diseaseDiabetic nephropathy is renal involvement resulting from chronic poor control of the disease. Microalbuminuria (an albumin/creatinine coefficient > 30 mg/g) is the first clinical sign of this (incipient nephropathy). The stage of nephropathy is associated with patient CV risk. Early detection and treatment is therefore important in patients with microalbuminuria, to reduce CV morbidity and the speed with which renal disease progresses, thereby reducing costs for the healthcare system.122–124
Metformin is considered to be reasonably safe in patients with a glomerular filtration rate (GFR) higher than 30 mL/min/1.73 m.9,22,124 It is still contraindicated in patients with a GFR < 30 mL/min.2 The GFR should always be measured prior to commencing treatment with metformin, and at least once a year if the GFR is higher than 60 mL/min. When combinations of fixed dose drugs that contain metformin are used in patients with reduced renal function, the restriction and efficacy of the other active ingredient used in the combination should also be considered. In general it is not recommended for patients with moderate kidney failure (GFR < 30 mL/min). Patients should be told to temporarily suspend metformin under conditions associated with dehydration or when iodized contrasts are used in studies, or when the risk of acute kidney failure increases.
In connection with the glinide drugs, repaglinide is the secretagogue that is classically indicated in chronic renal disease (CRD), and it may be used in dialysis. Although DPP-4 inhibitors have been shown to have beneficial and safe effects in the control of glycaemia in diabetic patients with CRD, without causing any additional adverse effect, the dose has to be adjusted depending on the degree of CRD, except for linagliptine.124,125 Pioglitazone too may be used in patients with CRD if it is not contraindicated for them, and attention must be paid to possible hydrosaline retention. GLP-1 RA may be used without altering the dose in category G2, G3a or G3b patients with CRD, especially in diabetic patients with CVD or established renal disease and obesity, given the proven benefit of weight and CVD reduction. However, they are contraindicated in G4 and G5 patients (with an estimated GFR below 30 mL/minute/1.73 m2).2,66 SGLT2 inhibitors may only be prescribed if the GFR is >60, and they are currently indicated in patients with CVD or established renal disease, with a proven benefit in CVD.2 Patients with CRD may be prescribed an SGLT2 inhibitor when estimated glomerular filtration (eGFR) ≥ 30 mL/min, particularly in those patients with a creatinine - albumin concentration (CAC) above 300 mg/g, to slow the progression of their CRD, cardiovascular disease (CVD) or both.2,124,126–128 Although the effect of SGLT1 inhibitors in lowering blood glucose falls at filtration rates < 60 mL/min, they remain effective down to a filtration rate of 30 mL/mn. Canagliflozine, dapagliflozine and empagliflozine have been shown to be capable of slowing the progression of renal disease.9 The SGLT2 inhibitor dose must be determined depending on the GFR. It is important to underline the cardiac and renal protector role of SGLT2 inhibitors, and this is greater in patients whose renal function is preserved (falls in the combined target of 33%, 44% and 56%, respectively, for patients with a GFR < 60 mL/min., 60 to 90 mL/min. and > 90 mL/min.). On the contrary, the fall in hospital admissions due to HF was greater in patients with a poorer basal renal function (40%, 31% and 12%, respectively, for filtration rates < 60 mL/min., 60 to 90 mL/min. and > 90 mL/min., respectively).127,128 Cardiovascular safety studies in patients with compromised renal function have yet to be completed, except for the CREDENCE study with canagliflozine. This study included patients with a GFR ≥ 30 mL/min., and it was halted early due to its findings in favour of canagliflozine. The risk of hospitalization due to HF was reduced in this study by 39%. Two studies are ongoing: EMPA-KIDNEY with empagliflozine, which includes patients with a GFR ≥ 20 mL/min., and DAPA-CKD with dapagliflozine, which includes patients with a GFR ≥ 25 mL/min.127,128
In general, we recommend treating patients with CRD (stages G3-G5 not in dialysis) with statins or a statin plus ezetimibe. The pharmacological reduction of hyperlipidaemia has been shown to safely reduce CV episodes in CRD.2,5,9,129–131 When deciding on treatment on an individual basis, it is important to remember that the dosage of some statins does not have to be adjusted at any stage of renal insufficiency (RI), such as atorvastatin. In RI stages 4 to 5 the dose of fluvastatin can be adjusted from 20 mg to 40 mg, lovastatin from 10 mg 20 mg, pravastatin from 10 mg to 20 mg, simvastatin 5 mg to 20 mg and pitavastatin from 1 mg to 2 mg. With rosuvastatin in stage 3 the dose will be from 5 mg to 20 mg per day, and in stages 4 to 5 the maximum dose of rosuvastatin is 10 mg/day.7,129–131
The dose of ezetimibe does not require adjustment, and its therapeutic efficacy has been proven. If there is intolerance of statins and/or if targets are not achieved with the maximum tolerated dose, then PCKS9 inhibitors that do not require adjustment for moderate to severe chronic renal insufficiency (CRI) can be used to reach the therapeutic target. If cyclosporine replaces tacrolimus in patients with renal transplant, the dose of statins should be reduced.
The treatment of hypertriglyceridaemia in CRD should be based on lifestyle changes. We do not recommend the use of fibrates to reduce CVR and they should not be used if the GFR < 15 mL/min. Fibrates and omega-3 fatty acids may be considered for patients with markedly high fasting levels of triglycerides (>500 mg/dL) to prevent the risk of pancreatitis.132 There is evidence that while omega-3 fatty acids reduce triglycerides, this is not the case for CV episodes or mortality. Although omega-3 acids are generally safe, they may increase bleeding in subjects treated with ASA/clopidogrel. If triglycerides are not brought under control by statins or fibrates then omega-3 can be added, as this combination is safe and well-tolerated with a high dose of omega-3. Lastly, remember that the KDIGO guide recommends the use of antiaggregant drugs in CRD while keeping haemoglobin levels of 11 g/dL or higher, using erythropoietin to control anaemia.124,133
We suggest using metformin as the first line treatment with an SGLT2 inhibitor in diabetic patients with RI. We should not commence SGLT2 inhibitor therapy if the GFR < 30, considering adjusting the dose of metformin if the GFR is below 45 mL/min. If the GFR is lower than 30 mL/min. we should consider a GLP-1 RA as the first line treatment, adding a thiazolidinedione (TZD), a glinide (SU) or insulin for metabolic control. If GLP-1 RA are contraindicated then a DPP4 enzyme inhibitor may be added (DPP4i) as the first choice in RI combined with metformin.124
RecommendationsMetformin is considered to be reasonably safe in patients with a GFR higher than 30 mL/min./1.73 m2 (1/+++).
In primary prevention in general we recommend treating patients with CRD (stages G3-G5, not in dialysis) with statins or a statin plus ezetimibe (1/+++).
When ABP is below 140/90 mmHg, we suggest that ABP should be lower than 130/80 mmHg when there is albuminuria (1/++).
Fibrates are not recommended when the GFR < 15 mL/min. (1/+++).
For patients with DM2 and established renal disease we recommend a SGLT2 inhibitor or a GLP-1 RA with proven benefits for CV diseases (1/+++).
We recommend a SGLT2 inhibitor in patients with DM2 and diabetic renal disease, with proven CV benefits to reduce the risk of severe CV events and/or hospitalization due to HF (1/+++).
Diabetes and heart failureDM and ischemic cardiomyopathy are the most important risk factors for HF. The most common risk factors for developing HF in patients with DM are ischemic cardiomyopathy and AHT. The incidence of HF is 2.5 times higher in diabetic patients than it is in the general population. HF is the diabetic population has a poorer prognosis and doubles the risk of hospitalization or death due to HF in comparison with the general population. In spite of its importance HF is under-diagnosed in patients with DM2, and this increases its mortality.133 Diabetic patients without symptomatic HF may have subclinical anomalies in heart function and structure. These include left ventricular systolic dysfunction, diastolic dysfunction, an increase in left ventricular mass and relative thickening of the ventricular wall and increased left atrial appendage size. This anomalies are associated with a higher risk of symptomatic HF and death, which implies that recommendations for the treatment of patients with DM2 and HF may also be of use in diabetic patients without known HF and with risk factors for developing it in the future. The GLP1 RA liraglutide, semaglutide, albiglutide and dulaglutide66,67,134–137 have been shown to have CV benefit as they reduce the combined MACE 3 point CV outcome (CV death, MI or non-fatal CVA), although they do not change the hospitalization rate due to HF. The Dapagliflozine Heart Failure (DAPA-HF) study was published in 2019, showing that in a clinically relevant way dapaglifozine significantly reduces the incidence of aggravated HF or CV death in stable patients with heart failure with reduced systolic function (CIRSF) in patients with DM.138 The EMPA-RESPONSE-AHF study did not find any reduction in the improvement of the visual dyspnoea scale, the duration of hospitalization, N-terminal brain natriuretic propeptide (NT-proBNP) or the diuretic response to furosemide any more than a placebo. Nevertheless, it underlined the safety of the drug in acute HF in a population in which only 1/3 were diabetics. It also showed interesting data in connection with event reduction with empagliflozine in the vulnerable phase after hospitalization due to HF. However, there is a certain degree of controversy about the safety of these drugs in acute HF.
It is increasingly clear that the CV, renal and metabolic systems are all interconnected, sharing many of the same risk factors and pathological routes along the disease continuum. Dysfunction in one system may accelerate the appearance of dysfunctions in the others, leading to the progression of interconnected diseases such as DM2, CVD, heart failure and renal disease, which in turn lead to a higher risk of CV death.
It has been pointed out that for every 1% fall in HbA1c there is a 16% reduction in the risk of HF and CV events. To prevent and/or delay HF, as well as to improve survival in DM patients with HF, we recommend treatment of AHT, the use of statins in patients at high risk of coronary disease, the use of ACEI in patients with asymptomatic LV dysfunction and beta-blockers when there is LV dysfunction and a history of MI,137 and now the SLTG1 inhibitors. HF treatment classically consisted of lifestyle changes and pharmacological therapy for HF with a reduced ejection fraction (HF-REF), with ACEI or ARA-II. If the first group are not tolerated, beta-blockers and mineralocorticoid receptor antagonists (MRA) can also be used if they are not contraindicated. If the patient is still symptomatic then it is recommended that the ACEI be replaced by sacubitril/valsartan.137 Diuretics are used to improve symptoms and improve the capacity for exercise, and also if there are signs and/or symptoms of HF. SLGT2 inhibitors are now the first type of agents to improve CV mortality as well as the prognosis in HF in diabetic individuals with and without established HF,139 and their use has also been suggested for non-diabetic patients with HF. SGLT2 inhibitors have reduced the relative risk of hospitalization due to HF by 30% in primary as well as secondary prevention, regardless of whether or not HF existed beforehand. The GLP-1 RA are considered to be effective in reducing the combined 3 P-MACE CV objective, although they do not reduce the number of hospitalizations due to HF. The EMPAREG-OUTCOME study showed that daily doses of 10 mg and 25 mg empagliflozine reduced the rate of hospitalizations by 35%, with a clear differentiation of the curves in favour of the empagliflozine arm from the first weeks of treatment. CANVAS programme studies also showed a 33% fall in the hospitalization rate due to HF in patients who received canagliflozine in comparison with those who were given a placebo. Subsequent analyses of CANVAS suggest that greater benefits in terms of morbimortality are obtained in diabetic patients with HF. The DECLARE-TIMI 58 study, which included a far higher percentage of high risk diabetic patients who did not have established CV disease (59.4% in DECLARE vs. 34.4% in CANVAS vs. 0% in EMPAREG-OUTCOME), found that patients treated with dapagliflozine at 10 mg/day showed a 27% fall in the hospitalization rate due to HF. Zelniker et al. published a meta-analysis and systematic review of the CV outcome studies of SGLT2 inhibitors in primary and secondary prevention.139 In this meta-analysis SGLT2 inhibitors were associated with a fall of more than 20% in the combined outcomes of hospitalization due to HF or death with a CV cause in diabetic patients with or without a history of HF. SGLT2 inhibitors are the first class of antidiabetic drug which has been proven to reduce the risk of hospitalization due to HF in patients with DM2. Secondary analyses of CV safety studies suggest that SGLT2 inhibitors reduce the risk of hospitalization due to HF for patients already diagnosed with HF and those who have not been so diagnosed. SGLT2 inhibitors should there be the first-lime medication in diabetics with known HF and in those at high risk of developing it.2,139–141 The same author undertook a meta-analysis of studies to compare GLPT-RA and SGLT2 inhibitors, finding that that treatment with glycogen peptide 1 receptor agonists (GLP1-RA) and SGLT2 inhibitors reduce atherosclerotic MACE to a similar degree in patients with established atherosclerotic CVD, while renal glucose cotransporter 2 (SGLT2) inhibitor has a more marked effect on the prevention of hospitalization due to heart failure and the progression of renal disease. Thus the different clinical benefit profiles should be taken into account during the decision-making process when treating patients with DM2.140
When the available CV results from the Cardiovascular Outcome Trials (CVOT) with SGLT2 inhibitor or GLP-1 RA for patients with DM2 and high vs. low levels of HbA1c are compared, this leads to the conclusion that there is no reason not to add an agent to lower blood glucose with proven heart protection properties in high risk patients with DM2, even though they are in the HbA1c target level with metformin.142
The Food and Drug Administration (FDA) approved empagliflozine and dapagliflozine for the treatment of heart failure with a reduced ejection fraction. Recent publications show evidence that empagliflozine and dapaglifloxine reduce the incidence of new or recurring episodes of auricular fibrillation and auricular flutter (AF/AFL), both of which are associated with diabetes.
Meta-analyses of the trials reported to date suggest that GLP-1 receptor agonists and SGLT2 inhibitors reduce the risk of major adverse atherosclerotic events in patients with DM 2 and established atherosclerotic cardiovascular disease (ASCVD).143 SGLT2 inhibitors also seem to reduce the risk of hospitalization due to heart failure and the progression of renal disease in patients with established ASCVD, multiple risk factors for ASCVD or diabetic renal disease.144 We recommend that patients with DM2 and ASCVD, multiple risk factors for ASCVD or diabetic renal disease should be given a SGLT2 inhibitor with proven CV benefits to reduce the risk of major adverse CV events and/or hospitalization due to heart failure. Patients with DM2 and established ASCVD or multiple risk factors for ASCVD are recommended to take a peptide 1 receptor agonist similar to glycogen with proven CV benefits, to reduce the risk of major adverse CV events. It is appropriate for many patients to use a SGLT2 inhibitor or a GLP-1 receptor agonist to reduce CV risk.2
RecommendationsSGLT2 inhibitors are good drugs to reduce CV mortality and HF in diabetic individuals with and without established HF (1/++).
We recommend that patients with DM2 and established HF with a reduced ejection fraction should take a SGLT2 inhibitor with a proven benefit in this population of patients to reduce the risk of worsening heart failure and CV death (1/+++).
We recommend that patients with DM2 and established atherosclerotic CVD or multiple risk factors for the same should take a GLP-1 RA with proven CV benefits to reduce the risk of major adverse CV events (1/+++).
ConclusionsControlling CVRF prevents and/or delays the development of CVD. However, epidemiological studies clearly that these factors are not controlled in patients with DM2. It is fundamental to view and treat patients holistically, as well as to plan therapeutic strategy on an individual basis. A Mediterranean diet (in our country) and aerobic exercise benefit diabetic patients, helping to control CVRF and to attain therapeutic targets. Based on the available evidence there is no need for antioxidant or vitamin supplements if there is no deficiency in them. Plasmatic levels of vitamin B12 and folic acid should be determined in diabetic patients, especially if they are under treatment with metformin.140,145 Bariatric surgery seems to increasingly effective, reducing the comorbidities and complications of DM over the short and long term. It also reverses the progress of the disease in a significant number of patients with DM2 and obesity. We should attempt to increase patient adherence to therapies, as well as greater involvement and more realistic targets in the guides, personalising treatment and its targets, adapting them to patients’ circumstances and deciding on treatment and its objectives with them. The target level of HbA1c should stand at around 6.5%, determined on an individual basis according to age and the risk of hypoglycaemia, and HbA1c levels of up to 8,5% would be acceptable. Metformin is still the first line drug, and the indications for SGLT2 inhibitor or GLP-1 RA should also be evaluated as the first or second option in patients with CVR, or in combination with metformin. ABP should be lower than 140/90 mmHg or even 130/80 mmHg if there is no associated risk, with LDL cholesterol levels set according to CVR, using statins as the first option and adding ezetimibe to achieve LDL cholesterol targets. If these targets are not achieve using the maximum tolerated dose or in case of statin intolerance, PCKS9 inhibitors should be used. We recommend the use of ASA as secondary prevention (75-162 mg/day). For diabetic patients at risk of HF we emphasise the use of treatment with a SGLT2 inhibitor as the first line of treatment. Age is not a contraindication for treating CVRF, and therapies should be determined on an individual basis in each case. In patients over the age of 65 years with DM we should screen them for the early detection of mild cognitive deterioration or dementia and aid their adherence to treatment. Given the complexity of the task, it is fundamental to consider including a specialist in Endocrinology and Nutrition in Cardiac Rehabilitation Units for a correct diagnostic and therapeutic assessment of the metabolic alterations which form the pathogenic basis for CVD. These mean that the role of a professional with in-depth knowledge of how to manage such alterations is increasingly necessary to improve the cardiometabolic control of patients with DM2. This would ensure that the highest possible number of CVRF will achieve their treatment targets, favouring a holistic view in the therapeutic approach.146 Lp (a) should be determined for all diabetic individuals, to assess their overall CV risk, as is shown by the CV risk equation in safeheart in the population with congenital family hypercholesterolaemia.147
Please cite this article as: Arrieta F, Pedro-Botet J, Iglesias P, Obaya JC, Montanez L, Maldonado GF, et al. Diabetes mellitus y riesgo cardiovascular: actualización de las recomendaciones del Grupo de Trabajo de Diabetes y Enfermedad Cardiovascular de la Sociedad Española de Diabetes (SED, 2021). Clin Investig Arterioscler. 2022;34:36–55.