During pregnancy there is a physiological increase in total cholesterol (TC) and triglycerides (TG) plasma concentrations, due to increased insulin resistance, oestrogens, progesterone, and placental lactogen, although their reference values are not exactly known, TG levels can increase up to 300 mg/dL, and TC can go as high as 350 mg/dL. When the cholesterol concentration exceeds the 95th percentile (familial hypercholesterolaemia (FH) and transient maternal hypercholesterolaemia), there is a predisposition to oxidative stress in foetal vessels, exposing the newborn to a greater fatty streaks formation and a higher risk of atherosclerosis. However, the current treatment of pregnant women with hyperlipidaemia consists of a diet and suspension of lipid-lowering drugs.
The most prevalent maternal hypertriglyceridaemia (HTG) is due to secondary causes, like diabetes, obesity, drugs, etc. The case of severe HTG due to genetic causes is less prevalent, and can be a higher risk of maternal-foetal complications, such as, acute pancreatitis (AP), preeclampsia, pre-eclampsia, preterm labour, and gestational diabetes. Severe HTG-AP is a rare but potentially lethal pregnancy complication, for the mother and the foetus, usually occurs during the third trimester or in the immediate postpartum period, and there are no specific protocols for its diagnosis and treatment.
In conclusion, it is crucial that dyslipidaemia during pregnancy must be carefully evaluated, not just because of the acute complications, but also because of the future cardiovascular morbidity and mortality of the newborn child. That is why the establishment of consensus protocols or guidelines is essential for its management.
El embarazo se caracteriza por un aumento fisiológico y esencial de los niveles plasmáticos de colesterol total (CT) y triglicéridos (TG), impulsado por el aumento de resistencia a la insulina, estrógenos, progesterona y lactógeno placentario. Durante la gestación, los TG pueden incrementarse hasta 300 mg/dl y el CT hasta 350 mg/dl, aunque se desconocen con exactitud sus valores de referencia. Cuando la concentración de colesterol excede el percentil 95 (hipercolesterolemia familiar (HF) e hipercolesterolemia materna transitoria), existe una predisposición al estrés oxidativo en los vasos fetales, exponiendo al recién nacido a mayor formación de estrías grasas y mayor riesgo de arteriosclerosis. Sin embargo, el tratamiento actual de la gestante con hiperlipidemia consiste en dieta y suspensión de fármacos hipolipemiantes
La hipertrigliceridemia (HTG) materna más prevalente es debida a causas secundarias (diabetes, obesidad, fármacos, etc.), siendo la HTG grave debida a causas genéticas, menos prevalente y representa un mayor riesgo de complicaciones maternofetales, tales como pancreatitis aguda (PA), preeclampsia, parto prematuro y diabetes gestacional. La PA por HTG grave es una complicación del embarazo poco prevalente, pero potencialmente letal para la madre y el feto, más frecuente en el tercer trimestre o en el posparto inmediato y no existen protocolos específicos para su diagnóstico y tratamiento.
Como conclusión, cabe destacar que se debe evaluar cuidadosamente la dislipemia en el embarazo, no solo por las complicaciones agudas que conlleva, sino también por la morbimortalidad cardiovascular futura en el recién nacido. Es por ello necesario el establecimiento de algoritmos o guías de consenso para su manejo.
Pregnancy is a unique physiological state in which the necessary metabolic changes must take place in the mother to ensure sufficient energy reserves (glucose, amino acids, and lipids) for adequate foetal development and growth.
Glucose is quantitatively the most important nutrient crossing the placenta, followed by amino acids,1 and foetal development depends on its availability. However, although placental transfer of lipids is limited,2 adaptations of maternal lipid metabolism that occur during gestation have particularly important consequences for foetal development. The two main consequences are the accumulation of lipids in maternal tissues3 and the development of maternal hyperlipidaemia.4
During the first two thirds of gestation there is an increase in the mother's fatty deposits,3 resulting from both hyperphagia and increased lipid synthesis.5 In this early period, the lipoprotein lipase (LPL) activity of adipose tissue does not change and may even increase,6 controlling fat uptake by the tissue and thus generating an anabolic state.
However, during the third trimester of pregnancy and coinciding with maximum foetal growth, this LPL activity decreases,2 which together with the increase in lipolytic and catabolic activity caused by insulin resistance during this period7 leads to an accelerated decrease in maternal fat deposits and an increase in maternal serum triglycerides (TG) and increases in phospholipids and cholesterol lessen.
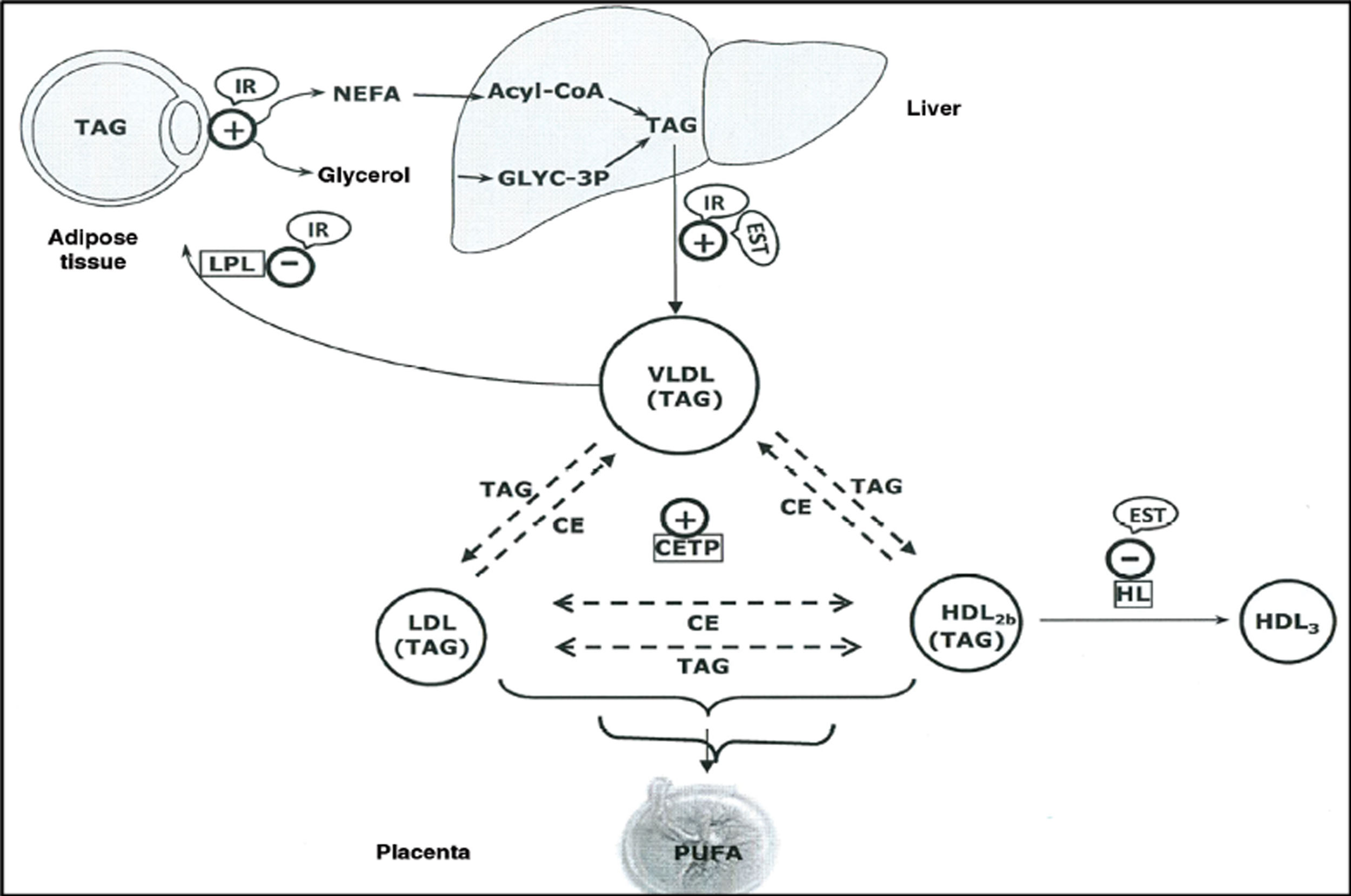
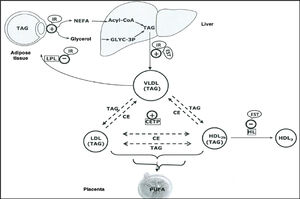
The elevated production of oestrogens during pregnancy leads to an increase in the liver’s production of very low density lipoproteins (VLDL),8 and TG-enriched VLDL,9 which together with the increased activity of cholesterol ester transfer protein around halfway through gestation,10,11 and decreased liver lipase activity in late pregnancy, appear to contribute to the accumulation of TG in low-density lipoproteins (LDL) and high-density lipoproteins (HDL),9 and to maternal hypertriglyceridaemia (HTG) (Fig. 1).
Schematic representation of the lipoprotein interactions occurring during the third trimester of pregnancy.
CE: Cholesteryl esters; CETP: Cholesteryl ester transfer protein; HDL: High density lipoproteins; HL: Hepatic lipoprotein; IDL: Intermediate density lipoproteins; IR: Insulin resistance; LDL: Low density lipoproteins; OEST: Oestradiol; TAG: Triacylglycerides; VLDL: Very low-density lipoproteins.
Source: Modified from Leiva et al.
The maternal TG that carry plasma lipoprotein are hydrolysed and taken up by the placenta, whose trophoblast cells express proteins related to VLDL-Apo E and LDL receptors, re-esterifying to provide a fatty acid reservoir.11 This is how the foetus obtains the essential fatty acids necessary for its development. After intracellular hydrolysis of TG, the released fatty acids diffuse into foetal plasma and are transported by alpha-fetoprotein12 to the foetal liver where they are re-esterified and secreted back into the circulation as TG.
Cholesterol also plays an important role in embryonic and foetal development. It is an essential component of cell membranes and a precursor of bile acids and steroids. The foetus can perform important endogenous total cholesterol (TC) synthesis,13 although it also uses maternal TC. The physiological barrier that the maternal CT must overcome to reach the foetus is the yolk sac up to the fourth week of gestation and later the syncytiotrophoblast and the foetal micro-vessels of the placenta.14
The presence of various lipoprotein receptors in the placenta15 and to a lesser extent in the yolk sac16 allows these tissues to take up TC from the maternal lipoproteins, although the transport is completed with other active and passive pathways and the use of other receptors, binding proteins and placental LPL.14 However, the exact mechanism of this process is not yet known.
Reference values for lipids during pregnancyChanges in lipid concentration during pregnancy begin to occur as early as the twelfth week, progressing as the pregnancy advances,17 and although it has been shown repeatedly that lipid concentration increases considerably during pregnancy, it is still difficult to define what the range of normality is within this period.
Ordovas et al.4 already described in 1984 that TC increased by about 49% during pregnancy, reaching a maximum between 33 and 36 weeks, and that TG reached levels around 3 times those usual in non-pregnant women between 37 and 40 weeks of gestation. However, the reference ranges reported by the different authors vary widely.18–20
Piechota and Staszewski20 conducted a study in Poland in 1992 involving 719 healthy pregnant women, in which they were able to confirm that the concentration of all the lipids, including apolipoprotein A (Apo A) and apolipoprotein B (Apo B), increased significantly during the second and third trimesters of pregnancy, the most pronounced increase being in TG, which reached levels 2.7 times the upper reference limit during the third trimester. HDL cholesterol (HDL-C) concentration already started to increase in the first quarter, reaching its maximum elevation (25%) during the second quarter and decreasing in the third.
Ying et al. in 201521 later conducted another study in China in which they measured the lipid concentration in 3200 pregnant women compared with 3200 healthy non-pregnant women of childbearing age, finding significantly higher levels in all lipid parameters in the pregnant women group compared to the control group (p < .05). TG were the parameter that rose the most, increasing gradually throughout pregnancy (p < .01) and the LDL-C/HDL-C ratio was lower than that of the non-pregnant women in all stages of pregnancy.
The Life Child study, published in 2019,22 was performed in a cohort of healthy pregnant women representative of the Caucasian population, according to trimester of pregnancy. A total of 748 pregnant women were studied between July 2011 and August 2018 and all the serum lipid parameter levels except for Apo A showed a significant increase between the second and third trimester, observing the most pronounced increase in TG, as in the other studies.
The concentration of LDL cholesterol (LDL-C), TG, Apo A and Apo B was approximately .1–.5 mmol/l higher than that of the Piechota and Staszewski study, a divergence that could be due to the difference in the upper reference limit proposed by both authors (97.5 vs. 95 percentile); however, the concentration of HDL-C remains .3–.4 mmol/l higher in the Life Child study after adjusting the ranges. In this study, unlike that by Piechota and Staszewski, no increase in Apo A levels was observed between trimesters.
The LDL-C/HDL-C ratio measured in the second and third quarter was lower than the recommended cut-off of 1.523,24; however, higher values were found in the Chinese study by Ying et al.21
Based on the low ratio found in this cohort, and the fact that lipid concentration returns to baseline levels after pregnancy, it could be considered that there is no evidence of increased risk of cardiovascular events in Caucasian pregnant women,25 although no conclusions can be drawn about the atherogenic risk of pregnant women, based on data from non-pregnant women.
The mean values found in the Life Child study are also similar to those found by Lippi et al.26 in Italy in a significantly lower number of pregnant women (n = 57). However, other studies carried out in different populations show differences mainly in the concentration of HDL-C, which is considerably lower in the study by Landázuri et al.27 performed in Colombia and in the study by Pusukuru et al.28 performed in pregnant women of Indian origin.
There is also no unanimity that HDL-C concentration increases during pregnancy, and while some authors argue that it does, others have not detected such an increase. In a study published in 2019 and undertaken in China by Wang et al.29 on 1283 healthy pregnant women, as well as in the study by Shen et al.30 and Piechota and Staszewski,20 HDL-C was found to increase during the first and second trimester of pregnancy and slightly decrease during the third trimester.
It can generally be stated that lipid levels during pregnancy are influenced multifactorially by ethnic and geographical factors,31,32 and therefore national reference levels must be established for serum lipids in pregnant women, but at present there is no consensus on normal lipid levels during pregnancy.
However, the changes observed in lipids and lipoproteins during pregnancy in the Life Child study are consistent with most of the data in the literature,20,21,28 thus confirming previous research studies.
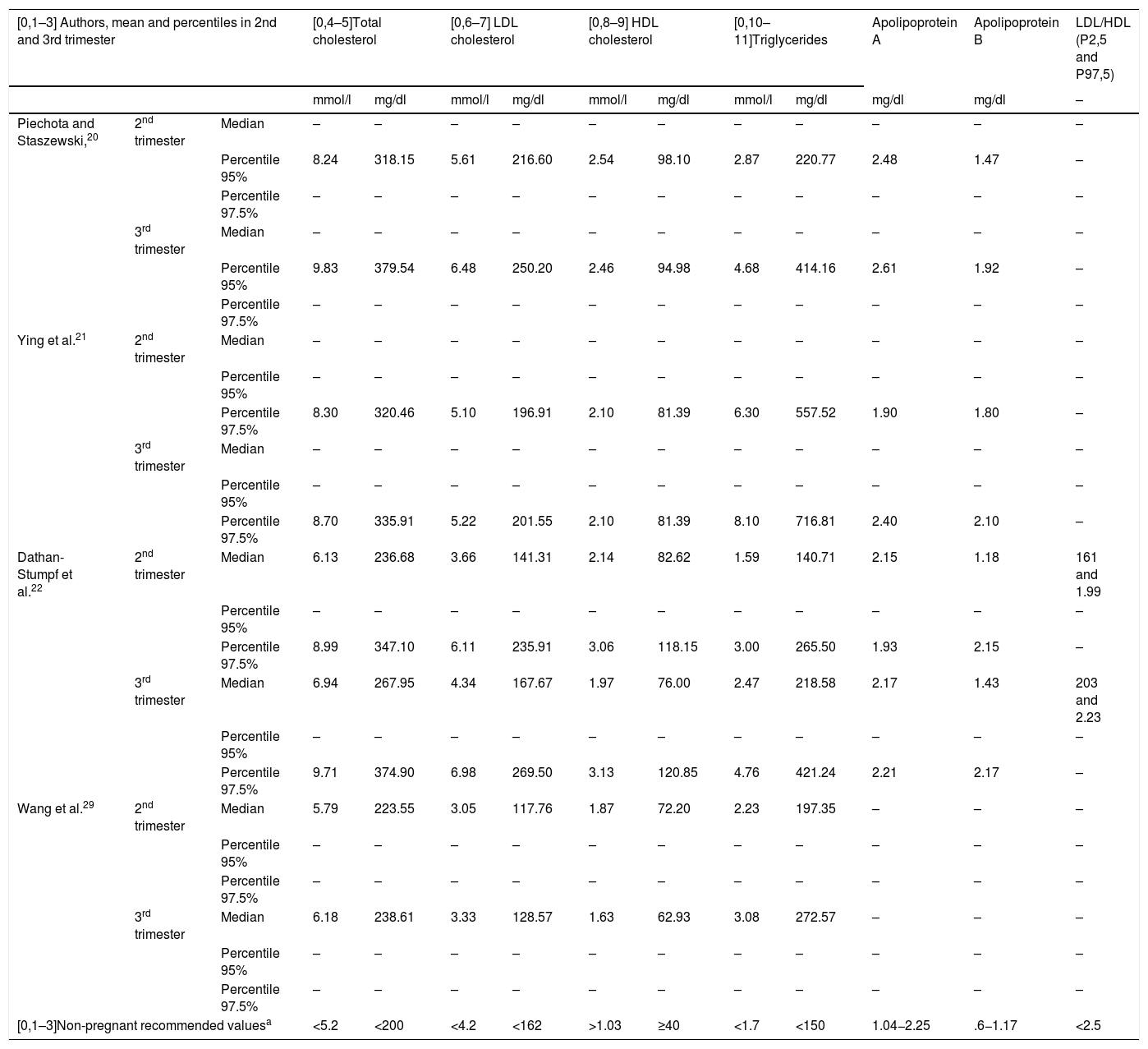
Table 1 shows the upper reference limit and mean values of the different lipid parameters during the second and third trimesters of pregnancy, according to the most significant studies.
Mean and percentiles of the lipid profile in the 2nd and 3rd trimesters of pregnancy, according to different authors.
| [0,1–3] Authors, mean and percentiles in 2nd and 3rd trimester | [0,4–5]Total cholesterol | [0,6–7] LDL cholesterol | [0,8–9] HDL cholesterol | [0,10–11]Triglycerides | Apolipoprotein A | Apolipoprotein B | LDL/HDL (P2,5 and P97,5) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mmol/l | mg/dl | mmol/l | mg/dl | mmol/l | mg/dl | mmol/l | mg/dl | mg/dl | mg/dl | – | |||
| Piechota and Staszewski,20 | 2nd trimester | Median | – | – | – | – | – | – | – | – | – | – | – |
| Percentile 95% | 8.24 | 318.15 | 5.61 | 216.60 | 2.54 | 98.10 | 2.87 | 220.77 | 2.48 | 1.47 | – | ||
| Percentile 97.5% | – | – | – | – | – | – | – | – | – | – | – | ||
| 3rd trimester | Median | – | – | – | – | – | – | – | – | – | – | – | |
| Percentile 95% | 9.83 | 379.54 | 6.48 | 250.20 | 2.46 | 94.98 | 4.68 | 414.16 | 2.61 | 1.92 | – | ||
| Percentile 97.5% | – | – | – | – | – | – | – | – | – | – | – | ||
| Ying et al.21 | 2nd trimester | Median | – | – | – | – | – | – | – | – | – | – | – |
| Percentile 95% | – | – | – | – | – | – | – | – | – | – | – | ||
| Percentile 97.5% | 8.30 | 320.46 | 5.10 | 196.91 | 2.10 | 81.39 | 6.30 | 557.52 | 1.90 | 1.80 | – | ||
| 3rd trimester | Median | – | – | – | – | – | – | – | – | – | – | – | |
| Percentile 95% | – | – | – | – | – | – | – | – | – | – | – | ||
| Percentile 97.5% | 8.70 | 335.91 | 5.22 | 201.55 | 2.10 | 81.39 | 8.10 | 716.81 | 2.40 | 2.10 | – | ||
| Dathan-Stumpf et al.22 | 2nd trimester | Median | 6.13 | 236.68 | 3.66 | 141.31 | 2.14 | 82.62 | 1.59 | 140.71 | 2.15 | 1.18 | 161 and 1.99 |
| Percentile 95% | – | – | – | – | – | – | – | – | – | – | – | ||
| Percentile 97.5% | 8.99 | 347.10 | 6.11 | 235.91 | 3.06 | 118.15 | 3.00 | 265.50 | 1.93 | 2.15 | – | ||
| 3rd trimester | Median | 6.94 | 267.95 | 4.34 | 167.67 | 1.97 | 76.00 | 2.47 | 218.58 | 2.17 | 1.43 | 203 and 2.23 | |
| Percentile 95% | – | – | – | – | – | – | – | – | – | – | – | ||
| Percentile 97.5% | 9.71 | 374.90 | 6.98 | 269.50 | 3.13 | 120.85 | 4.76 | 421.24 | 2.21 | 2.17 | – | ||
| Wang et al.29 | 2nd trimester | Median | 5.79 | 223.55 | 3.05 | 117.76 | 1.87 | 72.20 | 2.23 | 197.35 | – | – | – |
| Percentile 95% | – | – | – | – | – | – | – | – | – | – | – | ||
| Percentile 97.5% | – | – | – | – | – | – | – | – | – | – | – | ||
| 3rd trimester | Median | 6.18 | 238.61 | 3.33 | 128.57 | 1.63 | 62.93 | 3.08 | 272.57 | – | – | – | |
| Percentile 95% | – | – | – | – | – | – | – | – | – | – | – | ||
| Percentile 97.5% | – | – | – | – | – | – | – | – | – | – | – | ||
| [0,1–3]Non-pregnant recommended valuesa | <5.2 | <200 | <4.2 | <162 | >1.03 | ≥40 | <1.7 | <150 | 1.04−2.25 | .6−1.17 | <2.5 | ||
In a normal pregnancy, the woman shows a 30%–50% physiological increase in plasma TC in a process referred to as maternal physiological hypercholesterolaemia.33 In familial hypercholesterolaemia (FH) and in some cases in women with normal TC prior to pregnancy, TC during pregnancy rises above the physiological range, with values exceeding 280−300 mg/dl.34 The latter is termed maternal supraphysiological hypercholesterolaemia (MSPH)35,36 by some authors and its prevalence in previously normolipaemic women is unknown, as the lipid profile of pregnant women is not usually tested. There is a positive correlation between maternal and foetal blood TC during the second and third trimesters of pregnancy, but new-born TC from mothers with MSPH is low,37 unless there is a genetic lipid metabolism defect, as in the case of FH.
Endothelial cells synthesize nitric oxide (NO), a potent vasodilator generated by NO synthases (NOS), and proceed with the oxidation of l-arginine in a process dependent on the bioactivity of several cofactors, including tetrahydrobiopterin (BH4).38,39 A low concentration of BH4 and/or its low activity favours the generation of the superoxide anion (O22―) instead of NO, causing endothelial dysfunction.40,41 Patients with hypercholesterolaemia present low levels of NO, BH4 and an increase in arginase activity,34,36 which results in reduced dilation of the umbilical vein and is one of the mechanisms of onset of arteriosclerosis in the foetus.
Thus, endothelial dysfunction, the initial phase of the development of arteriosclerosis and the first arteriosclerotic lesions in the form of fatty striae, begin during intrauterine life in foetal vessels, as a consequence of increased TC levels in mothers with hypercholesterolaemia34,42 and may be a consequence of the described imbalance between vasodilators and circulating vasoconstrictors in fetoplacental circulation,43–45 since there is no autonomous innervation in the placenta or in the vessels of the umbilical cord. Lipid changes during pregnancy are accompanied by an increased oxidant/antioxidant profile46 and a reduction in the activity of paraoxonase-147 suggesting that maternal hyperlipidaemia may lead to increased susceptibility to oxidative stress during pregnancy.14
There is a theory that maternal hypercholesterolaemia programmes TC metabolism in the new-born. This fact has been supported by epidemiological studies,34 even if maternal hypercholesterolaemia is only temporary during pregnancy. Some lipidomics studies on the composition of phospholipids for the synthesis of eicosanoids in gestational hypercholesterolaemia also help support this theory.48 The FELIC study (Fate of Early Lesions in Children),49 which analysed the findings of autopsies carried out in 156 children aged between 1 and 14 years with normal TC, concluded that maternal hypercholesterolaemia was associated with greater progression of arteriosclerosis in the child. In another study, mothers with extremely high LDL-C levels from the second trimester of pregnancy had children with elevated LDL-C at the age of 6–13 years.50 Maternal hypercholesterolaemia and associated oxidative stress can lead to an accumulation of oxidized LDL in fatty striae and a deregulation of the genes involved in new-born lipid metabolism, by epigenetic mechanisms, and increase postnatal susceptibility to arteriosclerosis.37,50,51
Furthermore, numerous studies conclude that HTG during pregnancy is involved in the development of pre-eclampsia, although hypercholesterolaemia may participate in its physiopathology through endothelial dysfunction and arterial inflammation.14,52
Heterozygous familial hypercholesterolaemiaThe normal physiological 30% increase in TC levels during pregnancy is also observed in women with FH53,54 and severely worsens baseline hypercholesterolaemia. In addition, pregnant women should discontinue statin therapy.55
However, in women with no known lipid profile before pregnancy, hypercholesterolaemia during pregnancy should never make us consider a diagnosis of FH or study for mutation during this period. From a theoretical point of view, maternal hypercholesterolaemia results in a more pro-coagulant profile than in women without FH, because there is less decrease in utero-placental vascular resistance than that observed in healthy women56 and consequently it could lead to premature births in mothers with FH. However, women with FH do not appear to be at increased risk of preterm delivery, low birth weight infants or congenital malformations according to the results of a Norwegian retrospective cohort study with 2319 births from 1093 women with FH.57
There are no studies on the influence of pregnancy on the onset of cardiovascular disease among women with FH, as extensive cohort studies would be needed to reach any conclusions in this regard.
The mechanisms of epigenetics, which include DNA methylation, histone modification, chromatin remodelling and microRNA alterations, are hereditary disorders in gene activity that do not involve changes in the genetic code but allow cells to respond differently to environmental changes.58,59 With respect to the new-born, epigenetics can explain the foetal programming of the metabolism of its cholesterol due to the impact of maternal hypercholesterolaemia, which acts as an intrauterine risk factor for the subsequent development of cardiovascular disease during adulthood.58,59
Mendelian inheritance of FH causes it to be transmitted equally to both sexes. Some studies have investigated the importance of inheritance on the FH phenotype, as new-borns with FH inherited from the mother have been exposed to higher levels of TC in the uterus than those inherited from the father.60–65 Half of these studies have observed no differences in plasma TC levels in the new-born between those who inherited FH from the mother or father,60,61 whereas the remaining studies did observe differences. Of these studies, that by Van der Graaf et al.65 with 2339 adults with FH found a slight increase in TC and LDL-C in the order of 0.16 and 0.19 mmol/l, respectively, among those who inherited maternally compared to those who inherited paternally. A Dutch pedigree study63 that followed the descendants of 161 individuals with a specific mutation of the LDL receptor for 7 generations showed higher mortality among those who inherited FH from the mother.
The type of LDL receptor mutation conditions the lipid profile of adult individuals with FH and should be considered the dominant factor in the FH phenotype.66 However, the above-mentioned studies on maternal or paternal inheritance indicate that exposure to hypercholesterolaemia in the womb may, in the long term, adversely affect the phenotype in terms of atherosclerotic manifestations.59
Thus, the genes, diet and lifestyles shared by mothers and children with FH, and the direct effects of exposure to maternal hypercholesterolaemia along with other possible associated factors, will largely determine cardiovascular health during growth.50
Statins and pregnancyThe main clinical guidelines and expert consensus documents agree that statins should not be taken during pregnancy as they are contraindicated due to the risk of teratogenesis, especially during the first trimester.67–70 This is because all statins are considered by the Food and Drug Administration (FDA) to be in category X, and therefore their discontinuation should be advised at least one month before conception and during pregnancy and lactation, as several studies in animal models with rats and rabbits have shown central nervous and limb abnormalities with the use of high doses of statins.71 The information available in humans related to congenital abnormalities initially came from case reports, patient records and small cohort studies, although these were uncontrolled studies and limited by selection bias.72,73
In recent years there has been greater prescription of statins to women of childbearing age due to an increase in obesity, physical inactivity, high-fat diets, type 2 diabetes, and wider diagnosis of FH. It is also estimated that half of all pregnancies are unplanned.74,75 Therefore, some women can be considered to have started their pregnancies exposed to statin treatment. Case series studies,76 cohort studies,77,78 registry-based studies,57 a small randomized controlled study79 and several systematic reviews71,74,75,80 found that the prevalence of congenital abnormalities in mothers exposed to statins was similar to that of the rest of the pregnant population in the control groups. In contrast to the first case studies, most of these studies are controlled for confounding factors such as diabetes and obesity, which are themselves associated with increased teratogenesis. However, with the information currently available, discontinuation of statins during pregnancy should continue to be recommended.76
Regarding other complications such as premature birth, foetal death, or miscarriage in the first trimester of pregnancy, the small number of patients included in the studies makes it difficult to reach any conclusions.71,78 But a large UK study based on a primary care data registry81 showed a higher proportion of pregnancies that ended in miscarriage when statins were used, compared to pregnant women who did not take statins.
Of note is the emerging evidence of the potential role of statins in preventing pre-eclampsia due to their vasodilatory effect on the umbilical veins.82–84 In particular, hydrophilic statins such as pravastatin of which concentrations are found below the limit of detection in the umbilical cord and may have fewer adverse effects on the foetus than other statins. The studies underway to demonstrate the prevention and reversal of placental failure with this drug84 will increase our knowledge of this clinical condition.
Other treatments in pregnancyThere is little data on the teratogenic effect of other lipid-lowering drugs. Ezetimibe, fibrates, and nicotinic acid have been associated with malformations in animal studies and classified as category C of the FDA and the Pregnancy and Lactation Labelling Rule (PLLR).14 Their use is not recommended during pregnancy or while breastfeeding.
Drugs in category B include bile acid sequestering resins, cholesevelam and mipomersen, on which there have been some controlled studies in pregnant women, without adverse effects on the foetus. Despite the contradiction that cholestyramine is classified as group C, the only drugs accepted for use during pregnancy are cholesevelam and cholestyramine because they do not pass into systemic circulation and should not increase the risk of congenital malformations.54,55,69,85
There are no studies of PCSK9 inhibitors in pregnancy or of foetal damage when given to pregnant women with FH. Studies in primates with evolocumab showed that it can cross the placental barrier, without embryonic or foetal abnormalities being observed. At present and in view of the lack of information, PCSK9 must be discontinued before conception.
In pregnant women with heterozygous FH with ischaemic heart disease, homozygous FH, and acute pancreatitis (AP) due to HTG, there is a broad consensus to use lipoprotein apheresis as treatment, if indicated considering individual clinical circumstances.86
Homozygous familial hypercholesterolaemiaPregnancy in women with homozygous familial hypercholesterolemia (HoFH) is poorly documented because published cases are exceptional and fewer than a hundred. The increase in cardiac volume and output and the other physiological changes that occur in pregnancy can worsen pre-existing arterial lesions and trigger acute cardiovascular episodes in 30% of cases.87 This is why a detailed evaluation of the cardiovascular situation of women with HoFH who wish to become pregnant and which could contraindicate pregnancy is recommended.88
There are no clinical guidelines to manage pregnancy in women with HoFH, but lipoprotein apheresis is recommended to lower HDL-C and prevent complications.89 The American Society of Apheresis indicates it in HoFH, with a category I and grade of evidence IA90 in all its modalities. The results of published cases with this treatment report good tolerance and no greater side effects than in non-pregnant women.87,91
How to approach pregnant women with HoFH is a medical challenge that requires multidisciplinary planning by specialist personnel, cardiologists, obstetricians, lipidologists, intensive care doctors and psychologists, among others, as well as the support of the administrations to implement all the necessary techniques for their management.92,93
Hypertriglyceridaemia and pregnancyLipid metabolism during pregnancy and pathogenesis of hypertriglyceridaemia-induced pancreatitisAs mentioned above, normal pregnancy is characterised by adaptive changes in lipid metabolism to ensure placental needs and the glucose and lipid requirements of the growing foetus.94,95 In women whose lipoprotein metabolism is abnormal, changes in this lipid metabolism can lead to severe HTG and may precipitate AP.96
During pregnancy there is an increase in plasma TG, especially during the second and third trimesters of pregnancy. Plasma TG concentration usually increases 2–4 times in the last trimester,97 and does not usually exceed 300 mg/dl, and therefore its clinical relevance tends to be low.52,98
Severe HTG associated with pregnancy is a rare condition, which usually occurs during the third trimester of pregnancy.97 Severe HTG is defined as plasma TG greater than 1000 mg/dl; such pregnancies show an increased risk of acute complications and also have a risk of hyperlipidaemia in the future.99 It should be noted that this clinical situation threatens maternal-foetal prognosis by increasing the incidence of complications such as foetal macrosomia52,97 and exposes the mother to its major complication, AP.100 However, the prevalence of AP is one in 1000–12000 pregnancies.101–103 The most common causes of AP during pregnancy are cholelithiasis, severe HTG and idiopathic. Other complications may also occur, such as hyperviscosity syndrome, preeclampsia52,104,105 or placental abruption.106 This clinical situation requires urgent care based mainly on dietary measures and extraction of the foetus if the gestational age permits.107,108
The most prevalent causes of HTG during pregnancy are secondary, such as diabetes with poor metabolic control, metabolic syndrome and obesity, alcoholism and drugs (corticosteroids, beta-blockers, diuretics, tamoxifen and antipsychotics, among others).109,110 However, most of the cases of severe gestational HTG that have been previously described, even though they are less prevalent, were caused by familial chylomicronaemia syndrome (FCS) or familial HTG.111,112 To date, rare mutations in the LPL, APO E, APO C2, and APO A5 genes have been described that condition chylomicronaemia in pregnancy.113–115 Cases of HTG secondary to pregnancy itself have also been described in the literature.108,116
Keys to a diagnosis of acute pancreatitis during pregnancyDiagnosing BP during pregnancy is difficult because the symptoms can mimic abdominal pain from any other cause, including onset of labour. Sometimes the AP itself can trigger the onset of labour due to peritoneal irritation.96 Blood tests are recommended initially, as they will help establish the diagnosis of AP and assess its severity. For specific analytical parameters, both amylase and lipase are considered reliable markers of AP during pregnancy. Plasma amylase concentration is normal or slightly increased during pregnancy and lipase is unchanged.117 A simultaneous increase in alkaline aminotransferase >3 times the upper limit of normal will point to a biliary aetiology.118
Abdominal ultrasound is the initial technique of choice to rule out biliary aetiology119 and it does not irradiate the foetus. In pregnant women, nuclear magnetic resonance (NMR) or cholangiopancreatography MRI (non-ionising) is preferred, avoiding the ionising radiation that may be emitted by X-ray or computerised axial tomography (CT).120
It is important to make a differential diagnosis of pathologies such as myocardial infarction, peptic ulcer, appendicitis, cholecystitis, acute mesenteric ischaemia, pyelonephritis, etc. Of course, obstetric complications such as preeclampsia, HELLP syndrome, placental abruption and uterine rupture must also be ruled out.121 In cases of term pregnancy with peripancreatic collection, it is usually exceedingly difficult to reach a diagnosis.122 Occasionally in some cases HTG-induced AP is difficult to diagnose, because levels decrease after a few days of fasting, which is necessary as a treatment for AP.
General aspects of the management and treatment of acute pancreatitisIt should be noted that there are no specific obstetric guidelines for the treatment of AP secondary to HTG in pregnancy. The clinical presentation of AP secondary to HTG does not differ from other aetiologies and once a correct diagnosis has been made, the algorithms to manage pregnant patients are the same as for the general population.96 If the pregnancy is considered at term, the decision is usually to bring it to an end. Plasma TG values must be normalised as soon as possible. However, there are currently restrictions on the prescription of lipid-lowering drugs in pregnancy, due to the lack of consistent studies carried out in humans. After delivery, general population protocols are again used. The following will have to be considered: specific very low-fat diet, plasma TG control, usually every 1–2 weeks according to severity and especially in the third trimester,115 and of course strict obstetric control. Admission is recommended in the case of TG concentrations >1500 mg/dl. Therefore, it is important to create multidisciplinary units for the adequate management of these cases.123
Low fat diet and nutritional supplementsIn pregnancy or otherwise, the mainstay of managing severe HTG is a very low-fat diet (<20% of total calorie intake). Sometimes this restriction results in significant weight loss, which can lead to risks such as low birthweight, prematurity, and maternal complications. In some cases, to control severe gestational HTG, hospital admission is required for administration of serotherapy and sometimes total parenteral nutrition, until plasma TG values drop by at least 50%.
In turn, low-fat diets can induce deficiencies of essential fatty acids124; therefore, medium chain TG (MCTs) or omega-3 fatty acids are an essential part of treatment. In addition, MCTs are densely packed with calories (3.8 kcal/g) and their subsequent products, such as acetyl coenzyme A, can play a role in cerebral myelination of the foetus.
Lipid-lowering drugs to manage HTG in pregnancyLipid-lowering drugs are the first therapeutic line in AP secondary to HTG in nonpregnant women. However, there are few studies in pregnant women and therefore we have little information about their teratogenic effects.
Omega-3 polyunsaturated fatty acids containing eicosapentaenoic acid and docosahexaenoic acid reduce plasma TG by 25%–30% through various mechanisms. On the one hand, they can directly stimulate LPL, improving the elimination of TG-rich lipoproteins, and regulate genes for liver lipogenesis and those which stimulate fatty acid oxidation in the liver and skeletal muscle. Despite being FDA class C, they are considered safe and their use is recommended.125
The use of fibrates during pregnancy is limited because there is scanty data available from well-designed studies and more studies are required to support their use in pregnant women.126 However, several cases have been described in which fibrates were used during pregnancy and no teratogenic effects were observed.115
Statins, as mentioned above, should not be administered during pregnancy.126
Insulin and heparinInsulin increases the activity of LPL and leads to degradation of chylomicron, which reduces HTG,127 while heparin stimulates the release of LPL, which binds to endothelial cells and lowers plasma levels of TG.128 One case of a pregnant woman was described, in whom chronic use of heparin depleted the LPL on the surface of the endothelial cells, causing a hypertriglyceridaemic effect and thus triggering AP.128 More cases of HTG secondary to heparin use have been described,129 and therefore its use is not recommended.115
Plasma exchangeThis is indicated to decrease plasma TG levels, as well as to reduce inflammatory cytokines and to replace LPL or apolipoprotein deficiency. In AP secondary to HTG, plasma exchange can be used as established by the American Society of Apheresis guidelines.90 There are several studies and some clinical cases that have evaluated the safety and efficacy of plasma exchange in pregnant patients.126,130–132 Studies comparing the effect of plasma exchange versus conservative treatment on morbidity and mortality in cases of HTG-induced AP found no statistically significant differences.
In conclusion, we highlight that, although hyperlipidaemia is physiological in pregnancy, pregnant women with monogenic hypercholesterolaemia and especially those with severe HTG pose a challenge in clinical management that ultimately involves endocrinologists, internists, nutritionists, and gynaecologists. This implies the need to create management algorithms or consensus guidelines. Furthermore, as lipid-lowering drugs are clearly contraindicated during pregnancy, diet, plasma exchange or total parenteral nutrition are therapeutic options to be considered.
FundingNo funding was received in drafting this article.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Mauri M, Calmarza P, Ibarretxe D. Dislipemias y embarazo, una puesta al día. Clin Investig Arterioscler. 2021;33:41–52.