The combination of biochemical markers, together with the design and implementation of diagnostic algorithms in laboratory computer systems could become very powerful tools in the stratification of cardiovascular risk.
ObjectivesTo implement new biochemical markers and diagnostic algorithms not yet available, in order to provide an estimation of cardiovascular risk and the diagnostic orientation of lipid alterations.
Material and methodsStudy of the implementation of Apolipoprotein B and Lipoprotein (a), as well as the inclusion of different diagnostic algorithms. This was carried out jointly by the different Lipid Units of the Spanish Society of Atherosclerosis, Hospital Virgen Macarena in Seville, Hospital Juan Ramon Jiménez, Hospital Infanta Elena, and Hospital de Rio Tinto during 2018 and 2019.
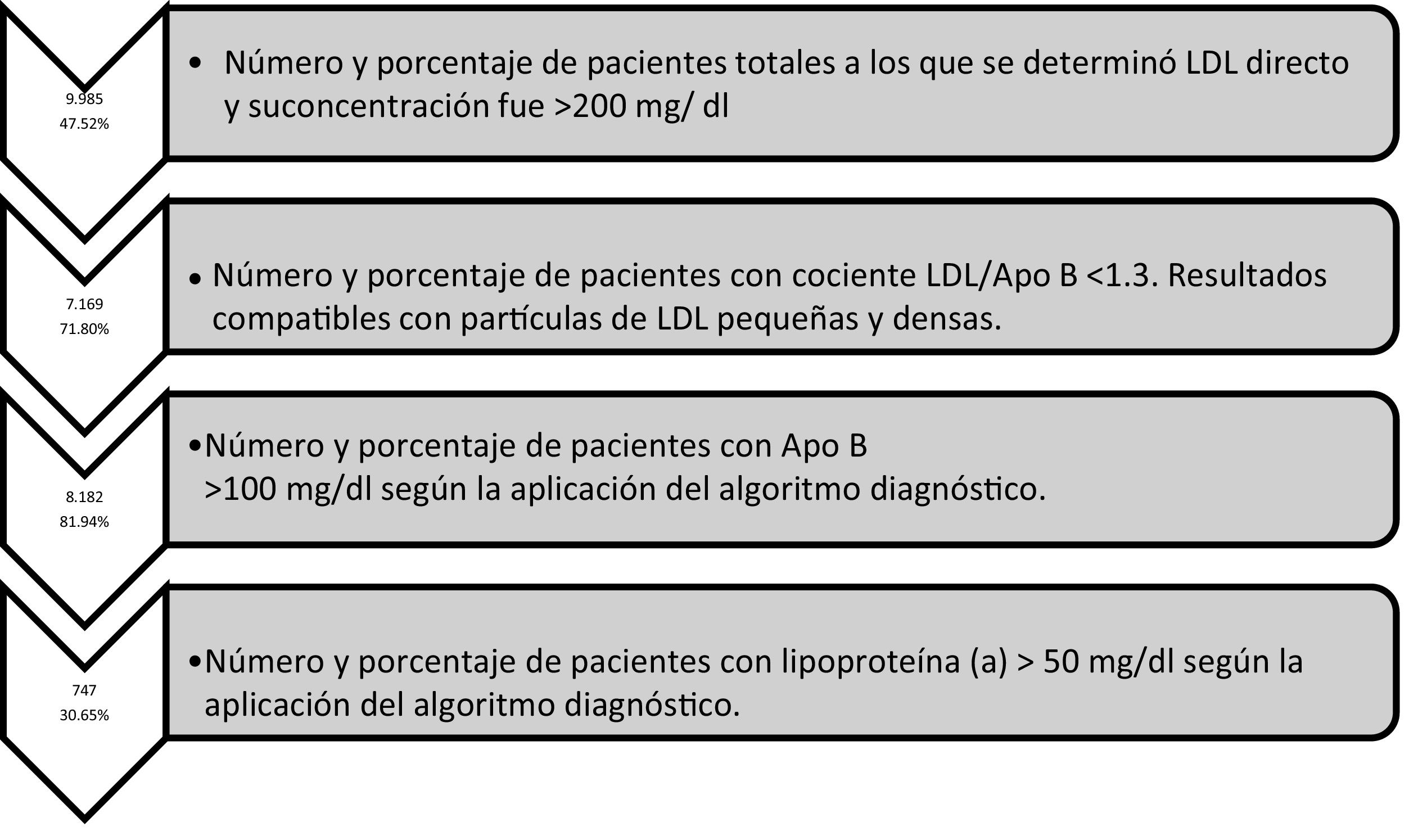
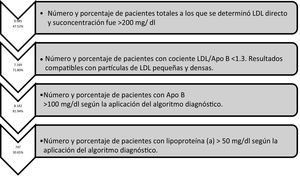
ResultsThe four diagnostic algorithms entered into the Laboratory Information System (LIS), showed a total of 9985 patients with c-LDL >200 mg/dL. The diagnostic algorithm was extended to include Apo B, with 8182 determinations showing an Apolipoprotein B > 100 mg/dL). A total of 747 lipoprotein (a) were determined, of which 30.65% were >50 mg/dL. More than two-thirds (71.80%) showed results compatible with small and dense LDL particles.
ConclusionsThe implementation of new analytical parameters and algorithms in Primary Care laboratory results can identify a considerable number of patients with different alterations in lipid metabolism. This, together with the classic risk factors, could contribute to a correct risk stratification in preventing the progression of CVD.
La combinación de marcadores bioquímicos así como el diseño e implementación de algoritmos diagnósticos en el sistema informático de los laboratorios podrían convertirse en herramientas muy potentes en la estratificación del riesgo cardiovascular.
ObjetivosImplementación de nuevos marcadores bioquímicos y algoritmos diagnósticos hasta ahora no disponibles para facilitar la estimación del riesgo cardiovascular y la orientación diagnostica de las alteraciones lipídicas.
Material y métodosEstudio para la implementación de Apolipoproteína B y la Lipoproteína (a) así como la inclusión de diferentes algoritmos diagnósticos. Se ha realizado conjuntamente entre las diferentes unidades de lípidos de la Sociedad Española de arteriosclerosis, Hospital Virgen Macarena de Sevilla, Hospital Juan Ramon Jiménez, Hospital Infanta Elena y Hospital de Rio tinto durante año 2018 y 2019.
ResultadosSe han aplicado 4 algoritmos diagnósticos en el SIL que mostraron 9.985 pacientes totales con c-LDL >200 mg/dl. Según algoritmo diagnóstico se amplió Apo B con 8.182 determinaciones presentaban una Apolipoproteína B > 100 mg/dl). Se determinaron 747 lipoproteína (a), de las cuales un 30.65% fueron superiores a 50 mg/dl. El 71.80% presentaban resultados compatibles con partículas de LDL pequeñas y densas.
ConclusionesLa implementación de nuevos parámetros analíticos y algoritmos en los laboratorios en atención primaria permiten identificar un número considerable de pacientes con diferentes alteraciones en el metabolismo lipídico que junto con los factores de riesgo clásicos podrían contribuir a una correcta estatificación de riesgo evitando la progresión de la ECV.
Cardiovascular diseases (CVD) are currently one of the main causes of death worldwide. In Europe alone they are responsible for over 4 million deaths.1 In Spain specifically, according to the most recent data provided by the National Institute of Statistics corresponding to December 2019, 427,721 deaths occurred.2
Changes to lipid metabolism and cardiovascular risk factors (CVR), are a key and predisposing factor in the development of cardiovascular atherosclerotic disease.3 Tables for the calculation of CVR are available for the prevention of these diseases and the progression of future cardiovascular events. The most commonly used in Europe and recommended by the European Cardiology Society and the European Atherosclerosis Society are those of the SCORE project.4 The implementation of strategies for improving the calculation of risk has a high clinical value, but presents some difficulty if they are only based on standard risk factors. There is therefore a need for new tools or for the introduction of new biomarkers to improve the prediction of CVD risk and the ability to identify people at risk, provide stability to results when they are repeated and offer therapeutic impact with early intervention.5 Recent studies have shown that some markers, including increased lipoprotein (a) (Lp[a]), homocysteine, and particularly C-reactive protein, are associated with increased risk of coronary heart disease.6,7 Also, it is well known that continuous exposure to lipoproteins which contain apolipoprotein B (ApoB) contribute to the growth and progression of atherosclerotic plaques, and it is therefore probable that the size of the plaque is determined by both the concentration of circulating LDL-C and by other lipoproteins that contain ApoB, and by the total duration of exposure to these lipoproteins.8 As occurs with other biomarkers, such as that of Lp(a), Mendelian randomization studies have consistently shown that lifetime exposure to higher levels of Lp(a) is strongly and causally associated with a higher risk of CVD.9,10 It is therefore logical to work on the development of prediction and prevention models for cardiovascular atherosclerotic diseases, aimed at determining which risk factors require intervention to reduce the probability that the patient will develop a cardiovascular event.11 At present, different guidelines are incorporating new recommendations on the usefulness of the biochemical markers in CVR evaluation. The consensus document recently published by the European Atherosclerosis Society and the European Federation of Clinical Chemistry and Laboratory Medicine12 cites the incorporation of non-HDL cholesterol into the basic lipid profile in therapeutic decision-making and assesses whether markers such as ApoB, Lp(a) or LSK particle type are useful for guiding therapeutic decisions. Along these lines, the recent Australian Society guidelines for improving care in familial hypercholesterolaemia includes, in addition to those mentioned, the calculation of remnant particles and the atherogenic lipid triad.13 The combination of biochemical markers and the design and implementation of automated diagnostic algorithms in the clinical laboratory information system (LIS) could become very powerful tools in public health to assist in the stratification of patients’ CVR from primary prevention and, thus, early detection of alterations in limpid metabolism.
The aim of this study was the implementation of new biochemical markers and diagnostic algorithms in primary prevention that are not yet available in the clinical laboratory, to help the requesting physician in the estimation of CVR and the diagnostic orientation of the resulting lipid alterations in the analytical tests.
This study for the implementation in the portfolio of primary care services of new analytical techniques was conducted in 4 laboratories belonging to the lipid units of the Spanish Society of Atherosclerosis: that of the Hospital Virgen Macarena in Seville, Hospital Juan Ramón Jiménez, Hospital Infanta Elena and Hospital de Río Tinto, all 3 in Huelva. The project began in the second half of 2018 and terminated on 31st December 2019.
With financing obtained from the grant by the Spanish Society of Atherosclerosis different kits were obtained for the determinations of ApoB and Lp(a), which were automated in the Roche 6000 and 8000 analysers, in keeping with the availability in each clinical laboratory, and for ApoE in the BN II Prospect nephelometer. The implementation of these analytical techniques was achieved through application of diagnostic algorithms designed in the Smarliss and Omega LIS. A diagnostic algorithm was produced for hypertriglyceridaemias with the incorporation of ApoE, hypercholesterolaemias with the incorporation of ApoB and Lp(a), and diabetic dyslipidaemias with the incorporation of ApoB.
The basic lipid profile also incorporated other calculated parameters such as calculated VLDL (VLDL-C = triglycerides/5), small, dense LDL-C particles (sd-LDL) estimated by the ratio sd-LDL = LDL/ApoB < 1.3 and non-HDL cholesterol. The following algorithms implemented in the LIS are presented below:
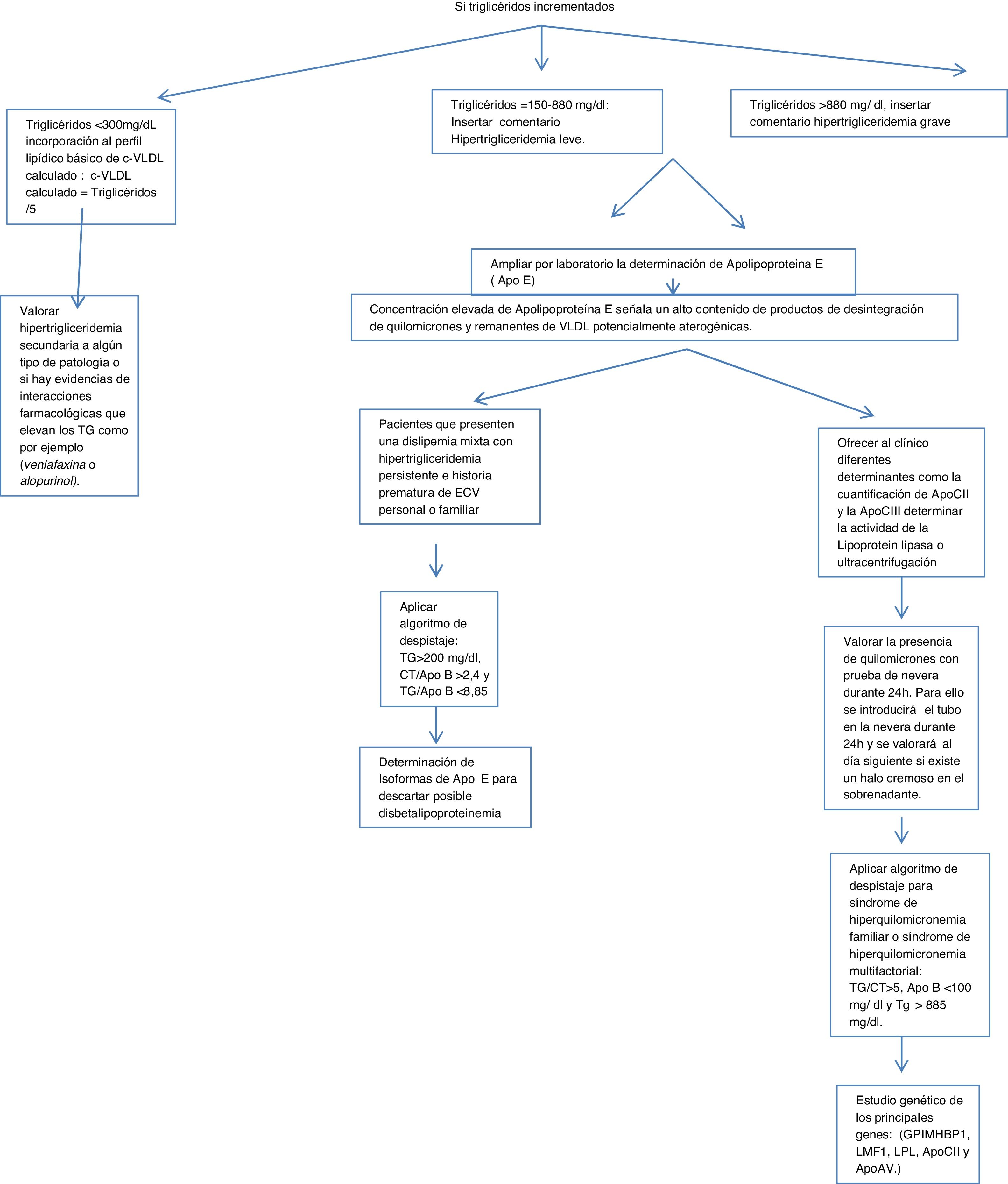
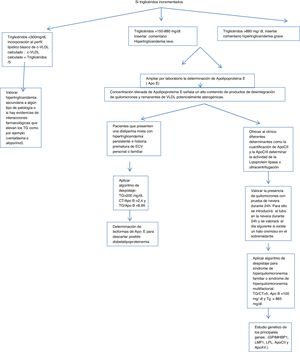
Algorithm number 1: Screening for hypertriglyceridaemias with incorporation of analytical parameter ApoE in patients with triglyceride concentrations >150 mg/dl.
In the case of elevated triglycerides based on the concentration found in routine primary care testing, calculations, alerts and ApoE expansion were inserted.
- 1)
If:
- a)
Triglycerides < 300 mg/dl, incorporation of basic lipid profile of calculated VLDL-C: calculated VLDL-C = Triglycerides/5.
- b)
Triglycerides = 150−880 mg/dl: commentary was inserted: mild hypertriglyceridaemia; assess possible secondary causes.
- c)
Triglycerides > 880 mg/dl, commentary was inserted: severe hypertriglyceridaemia.
- a)
If triglycerides are above 150, the determination of ApoE by the laboratory was expanded, inserting the following caption into the LIS: laboratory expanded lipid study. The elevated concentration of ApoE indicates a high content of chylomicron breakdown products and potentially atherogenic VLDL remnants.
- 1)
Assess the presence of chylomicrons with a refrigerator test for 24 h. For this purpose the tube shall be placed in the refrigerator for 24 h and assessed the next day for the presence of a creamy halo in the supernatant.
- 2)
Apply screening algorithm for familial or multifactorial hyperchylomicronaemia: triglycerides/total cholesterol >5, ApoB < 100 mg/dl and triglycerides > 885 mg/dl.14
- 3)
To determine the origin of this hypertriglyceridaemia different determinants can be offered to the clinician, such as the quantification of ApoCII and ApoCIII (cofactors of the lipoprotein lipase enzyme, which hydrolyses the triglycerides) by immunological techniques which are not normally available in clinical practice clinical laboratories, but are in external laboratories. Lipoprotein lipase activity is determined or ultracentrifugation of serum is used. When lipoprotein lipase activity is deficient a genetic study of the main genes can be requested: (GPIMHBP1, LMF1, LPL, ApoCII and ApoAV.)
- 4)
Assess secondary hyper trigliceridaemia to some type of pathology or whether there is evidence of drug interactions that elevate the triglycerides, such as, for example, venlafaxine or allopurinol.
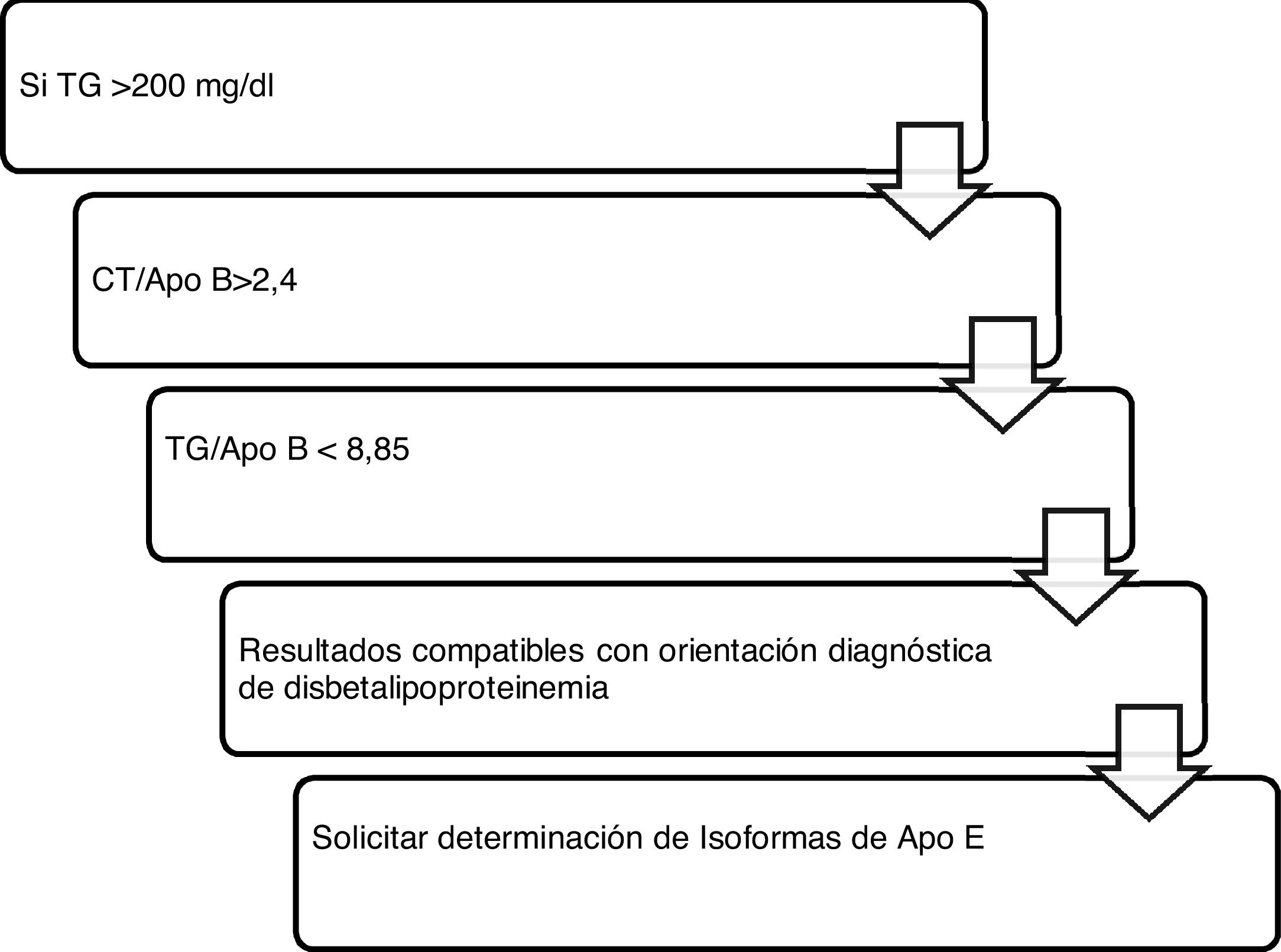
For those patients who present with mixed dyslipidaemia with persistent hypertriglyceridaemia and a premature history of personal or familial CVD, the determination of ApoE isolforms would be interesting to rule out possible dysbetalipoproteinaemia (Fig. 1).
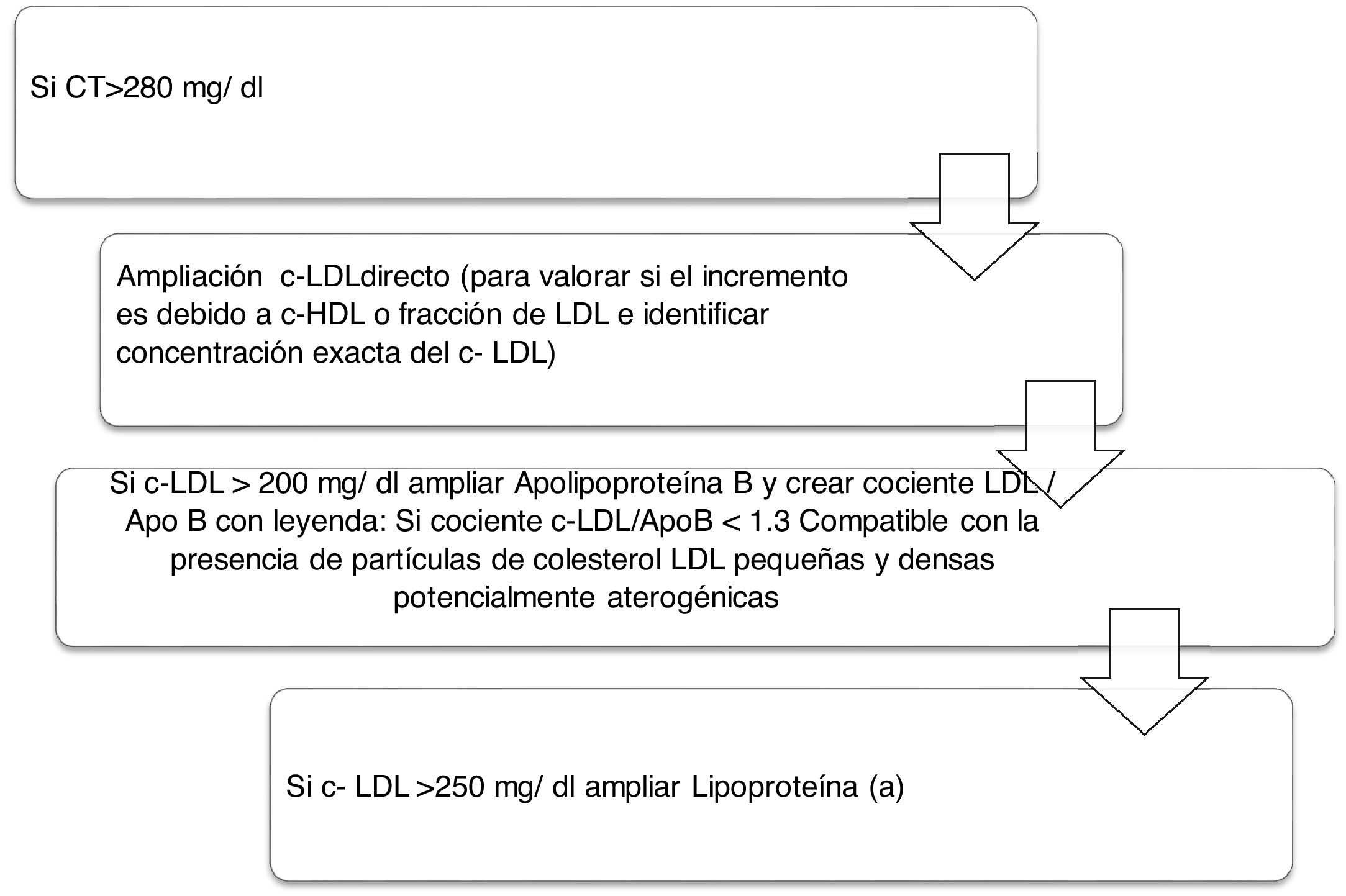
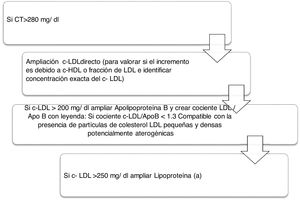
Algorithm number 2: Incorporation of the parameter Lp(a) for detection of patients with high CVR: identify total cholesterol in patients increasing the type of LDL-C and whether there is also an increase of Lp(a) which enhances the patient’s CVR (Fig. 2).
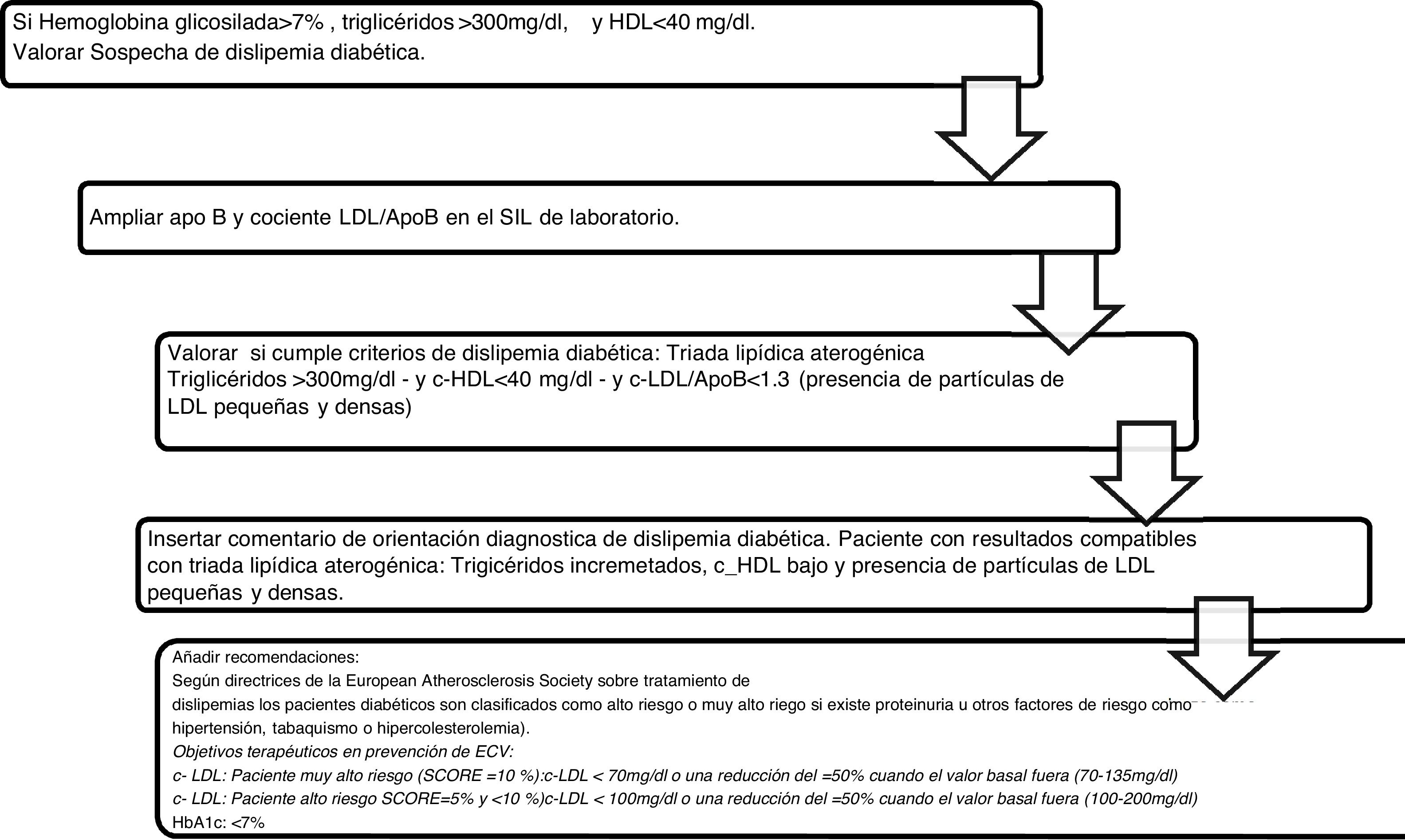
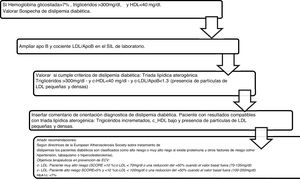
Algorithm number 3: Incorporation of the parameter ApoB for the diagnostic algorithm of diabetic dyslipidaemia (Fig. 3).
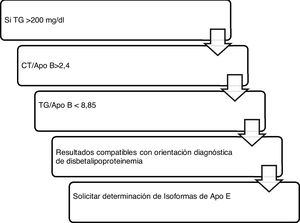
Algorithm number 4: Incorporation of the parameter ApoB for the diagnostic algorithm of dysbetalipoproteinaemia15 (Fig. 4).
Algorithm number 4: Incorporation of the parameter ApoB for the diagnostic algorithm of dysbetalipoproteinaemia.15
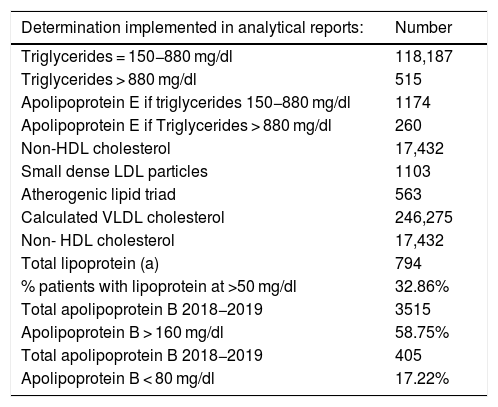
Table 1 shows the results of the number of biochemical markers used de novo in the LIS, the percentage of patients with lipoprotein above normal levels, the number of patients and the percentage of ApoB according to different cut-off points, and the number of patients according to the concentration of triglycerides obtained. Fig. 5 shows the number of biochemical markers according to the application of algorithm number 2 with implementation of LDL/ApoB, Lp(a) and ApoB.
Number of determinations implemented de novo in the laboratory information system.
| Determination implemented in analytical reports: | Number |
|---|---|
| Triglycerides = 150−880 mg/dl | 118,187 |
| Triglycerides > 880 mg/dl | 515 |
| Apolipoprotein E if triglycerides 150−880 mg/dl | 1174 |
| Apolipoprotein E if Triglycerides > 880 mg/dl | 260 |
| Non-HDL cholesterol | 17,432 |
| Small dense LDL particles | 1103 |
| Atherogenic lipid triad | 563 |
| Calculated VLDL cholesterol | 246,275 |
| Non- HDL cholesterol | 17,432 |
| Total lipoprotein (a) | 794 |
| % patients with lipoprotein at >50 mg/dl | 32.86% |
| Total apolipoprotein B 2018−2019 | 3515 |
| Apolipoprotein B > 160 mg/dl | 58.75% |
| Total apolipoprotein B 2018−2019 | 405 |
| Apolipoprotein B < 80 mg/dl | 17.22% |
Current recommendations by the Spanish Society of Artherosclerosis16 on the prevention of cardiovascular atherosclerotic disease in clinical practice are aimed at the assessment of the global CVR of each patient. Along these lines, the joint consensus panel of the European Atherosclerosis Society and the EFLM12 recently addressed present and future challenges in the laboratory diagnosis of athrogenic lipoproteins. Both highlighted that the set of determinations, such as total cholesterol, triglycerides, high density lipoprotein cholesterol (HDLC), LDL and non-HDL cholesterol make up the primary lipid panel to estimate the risk of cardiovascular atherosclerotic disease and includes several recommendations, among which they highlight the creation of a “standard lipid profile” and an “expanded lipid profile” which includes Lp(a) or ApoB in selected cases. With the aim of adapting to the recommendations of the aforementioned clinical guidelines, the direct determination of 2 analytical parameters, ApoB and Lp(a), as well as other estimated markers, has been implemented in routine analyses with this project.
The importance of the joint determination of LDL-C and ApoB is a key factor in assessing the posible presence of potentially atherogenic sd-LDL because these particles have a lower affinity for the LDL receptor, a higher susceptibility to oxidation and a greater affinity for the proteoglycans of the arterial wall. The current project has reported a total of 1103 patients with compatibility with the presence of sd-LDL sent to the requesting physician in the routine tests for the appropriate clinical and therapeutic management of each patient. Therefore, this marker, combined with other classic lipid markers in routine tests is useful for the application of another algorithm to characterise the atherogenic dyslipdaemia. One study conducted in southern Europe, The Hortega-Liposcale Follow-up Study17 published that sd-LDL showed the strongest association with cardiovascular events when the composition of the particles was researched instead of the total concentration, so that a change in the initial composition of the LDL particles from large to medium and small was associated with a higher CVR.
The combination of reduced HDL-C, the predominance of sd-LDL and increased triglycerides is the so-called “atherogenic lipid triad”,18 which increases the risk of CVD from its 3 components. Five hundred and sixty three alerts of results compatible with the atherogenic lipid triad with their corresponding interpretation in the analytical report have been implemented. Atherogenic dyslipidaemia is under diagnosed, under treated and, consequently, under controlled both in Europe and in Spain.19,20 In the European EUROASPIRE III21 register of secondary prevention with a representative population of 22 countries, it was concluded that over a third presented with atherogenic dyslipidaemia and in the Dyslipidaemia International Study (DYSIS)14 on the Spanish population, the percentage was 13.1%. The EDICONDIS-ULISEA15 study on the control of dyslipidaemia in lipid units and vascular risk by the Spanish Society of Atherosclerosis revealed that only one out of every 6 patients with atherogenic dyslipidaemia achieved therapeutic objectives in HDL-C and triglycerides.
The Canadian Cardiovascular Society guidelines place LDL-C as the main objective but also introduce ApoB and non-HDL cholesterol as alternative objectives and highlight that the latter may even be a better objective than the ApoB due to its simple calculation.22 Seventeen thousand four hundred thirty-two determinations of non-HDL cholesterol were included in the primary care basic lipid profile, which provided added information of all particles causing cardiovascular diseases, i.e. LDL, VLDL, IDL and Lp(a) in the non-fasted state, also including cholesterol in chylomicrons and their remnant particles.12 One of the most novel indications is the recommendation to use ApoB for risk assessment, which may be preferable to the non-HDL cholesterol in people with mild to moderate hypertriglyceridaemia (from 2 to 10 mmol/L), diabetes, obesity or metabolic syndrome, or very low LDL-C < 1,8 mmol/L.23 The most recent quantification data of ApoB in Andalusia correspond to the study “Dieta y Riesgo de Enfermedades Cardiovasculares en Andalucía” (DRECA) (Diet and risk of cardiovascular diseases in Andalusia) which classifies them according to blood pressure and BMI in mean concentrations, but there are no records of ApoB in patients with severe hypercholesterolaemias.24 Based on the results of this study and the guidelines, we see its inclusion in the portfolio of clinical laboratory services as beneficial, as its determination is superior to LDL-C and non-HDL measurements and calculations for the assessment of exposure to the number of atherogenic lipoprotein particles in the circulation. Furthermore, as with non-HDL cholesterol, it may be measured without fasting and is not affected by the biological variability of triglycerides.
A complementary parameter is the introduction of VLDL-C calculation in routine tests, in our case, 246,275. This provides additional information regarding CVR estimation of the patients, since their presence in human and rabbit atherosclerotic plaques has also been reported.25 Mendelian randomization studies indicate that elevated serum concentrations of lipoproteins rich in triglycerides or their remnants is causally associated with a higher risk of CVD and all-cause mortality.26 In the case of patients with hypertriglyceridaemia, due to the huge biological variability of this parameter so dependent on recent dietary intake or associated secondary diseases, the application of the algorithm as well as the corresponding ApoE expansion is performed with the purpose of alerting the requesting physician of values that would pose a risk to the patient if they did not revert to normal. The recent publication of the CAMAGUE study (adherence of laboratories to the recommended guidelines for dyslipidaemia in Europe) has shown that only 23% of laboratories add an alarm when the risk of pancreatitis due to hypertriglyceridaemia is high.27 In our case, 118,187 alarms of mild or moderate hypertriglyceridaemias were introduced with their corresponding 1174 ApoE, 90% of which were higher than the normal level and 515 of which were severe hypertriglyceridaemia alerts with 260 ApoE, all within pathological range. The characterisation of the type of hypertriglyceridaemia, and the extension of the ApoE, apart from the possible risk of pancreatitis, provide information about the number of potentially atherogenic remnant particles and improve the management of this disease which is difficult to diagnose. Non-availability of the determination of the ApoE isoforms in many laboratories is a major disadvantage for confirmation of suspected disease.28,29
Regarding the results obtained in the quantification of Lp(a), a total of 747 determinations were made. The most relevant and unpromising data is that, after the application of algorithm number 4, 30.65% of patients with LDL-C > 220 mg/dl presented with Lp(a) > 50 mg/dl concentrations. The risk means a LDL-C above double the clinical guideline, low risk is potentiated in 30.65% of patients with hyper-Lp(a). In addition, 13 patients showed Lp(a) higher than 180 mg/dl. As shown by the latest European guidelines from the European Atherosclerosis Society, patients with extremely high Lp(a) > 180 mg/dl (>430 nmol/L) levels may have a lifelong higher risk of cardiovascular atherosclerotic disease, similar to that of people with heterozygous familial hypercholesterolaemia. Up until now, the largest study to analyse Lp(a) levels and CVD in the context of familiar hypercholesterolaemia was the Dutch cohort, which reported a significant association between levels of Lp(a) > 30 mg/dl and CVD.30 The Spanish register with the highest number of quantified Lp(a) corresponds to that of SAFEHEART (cohort study of Spanish familial hypercholesterolaemia), which includes 2, 917 subjects (1960 patients with familial hypercholesterolaemia and 957 family members without it): the number of subjects with levels of Lp(a) > 50 mg/dl was significantly higher in the group of familial hypercholesterolaemia (P < .001).31 Since around 90% of the Lp(a) levels of a person is inherited, extremely high Lp(a) may represent a new hereditary lipid disorder which is associated with a very high risk of lifelong CVD and is 2 times more common than heterozygous familial hyper cholesterolaemia. This workgroup highlights that the screening of high Lp(a) may be valuable in patients without familial hypercholesterolaemia or with it, since these patients have a greater risk of cardiovascular atherosclerotic disease, especially with coexisting familial hypercholesterolaemia.32 The standards of the Spanish Atherosclerosis Society33 for global control of CVR coincide in its relevant role in the increased vascular risk presented by some patients with familial hypercholesterolaemia, and in subjects with premature or recurrent ischaemic disease, despite good control of their CVR factors. In these circumstances, its determination would be indicated.
The inclusion of this new marker in the automated laboratories was pioneering in the province of Huelva, thanks to the financing of this project. Almost a third of the patients analysed had pathological figures, or their biochemical and genetic CVR was increased, and was known at an early stage thanks to the application of biochemical algorithms.
ConclusionsThe implementation of new analytical parameters such as the ApoB and Lp(a) in laboratories and the application of diagnostic algorithms in primary care facilitate the identification of a considerable number of patients with different alterations in lipid metabolism. This, together with the classic risk factors, may contribute to correct risk stratification in preventing the progression of CVD.
FinancingStudy financed by a grant from the Spanish Society of Atherosclerosis in 2018 to research atherosclerosis in primary care: Introduction of a biochemical and genetic screening programme for familial hypercholesterolaemia and other dyslipidaemias in the Lipid Unit of the Hospital Virgen Macarena in Seville and in the Lipid Unit of the Hospital Infanta Elena in Huelva. Laboratory involvement in the achievement of therapeutic goals. ARIAN study.
Conflict of interestsThe authors have no conflict of interests to declare.
Begoña Gallardo Alguacil, Eva Nadiejda Gutiérrez Cortizo, Francisco Javier Caballero Granado, Elena Sánchez Ruiz Granados, Guadalupe Bueno Rodríguez, Marta Rico Rodríguez, Francisco Gonzalvo López, Pilar Carrasco Salas, Miguel Ángel Castaño López, Antonio León Justel.
Please cite this article as: Arrobas Velilla T, Bonet Estruch E, Roa Garrido J, Romero Jiménez M, Varo Sánchez GM, Vázquez Rico I. Incorporación de parámetros bioquímicos y algoritmos diagnósticos en el sistema informático de laboratorio para la detección precoz de alteraciones lipídicas desde las unidades de lípidos. Clin Investig Arterioscler. 2021;33:273–281.