As it is well-known, a thrombus evolving into a disrupted/eroded atherosclerotic plaque causes most acute coronary syndromes. Plaque stabilisation via reduction of the lipid core and/or thickening of the fibrous cap is one of the possible mechanisms accounted for the clinical benefits displayed by different anti-atherosclerotic strategies. The concept of plaque stabilisation was developed to explain how lipid-lowering agents could decrease adverse coronary events without substantial modifications of the atherosclerotic lesion (‘angiographic paradox’). A number of imaging modalities (vascular ultrasound and virtual histology, MRI, optical coherence tomography, positron tomography, etc.) are used for non-invasive assessment of atherosclerosis; most of them can identify plaque volume and composition beyond lumen stenosis. An ‘aggressive’ lipid-lowering strategy is able to reduce the plaque burden and the incidence of cardiovascular events; this may be attributable, at least in part, to plaque-stabilising effects.
La patogenia de los síndromes coronarios agudos está relacionada con la rotura o erosión de una placa aterosclerótica vulnerable. La estabilización de dicha placa, por reducción del núcleo lipídico y/o aumento de la capa fibrosa, sería uno de los mecanismos potencialmente beneficiosos observados con agentes antiateroscleróticos. El concepto de estabilización de la placa de ateroma se desarrolló para explicar el efecto beneficioso del tratamiento hipolipemiante, sin cambios apreciables en el tamaño y la morfología de la lesión aterosclerótica («paradoja angiográfica»). En la actualidad, el desarrollo de nuevas técnicas de imagen no invasivas (ultrasonido vascular e histología virtual, resonancia magnética, tomografía de coherencia óptica, etc.) permite determinar el volumen, el tamaño y la composición de la placa, con lo que es posible caracterizar las placas más vulnerables y, por consiguiente, más susceptibles de rotura. Una estrategia hipolipemiante «agresiva» puede estabilizar e incluso reducir de forma significativa la carga aterosclerótica y la incidencia de episodios vasculares, al menos en parte, a través de un efecto estabilizador de la placa.
The pathogenesis of acute cardiovascular syndromes is related to the breakage or erosion of a vulnerable atherosclerotic plaque.1 Stabilising that plaque, by reducing the lipid core and/or increasing the fibrous cap, would be one of the potentially beneficial mechanisms observed with anti-atherosclerosis agents.2 The concept of stabilising atheroma plaque was developed to explain the beneficial effect of lipid-lowering treatment with no appreciable changes in the size and morphology of the atherosclerotic lesion using angiography (“angiographic paradox”).
At present, the development of new non-invasive imaging techniques (intravascular ultrasonography [IVUS]+virtual histology, optical coherence tomography [OCT], magnetic resonance imaging, positron emission tomography [PET], etc.) enable atheroma plaques in the vascular tree to be identified early, as well as their volume, size, and composition. Therefore it is possible to characterise the most vulnerable plaques and, as a result, those most susceptible to breaking and thrombosis.3 Stabilising vulnerable plaques, by reducing the lipid core and/or increasing the fibrous cap, would be one of the potentially important clinical benefits observed with some anti-atherosclerotic agents, primarily statins.
Identifying high risk/vulnerable plaquesPlaque composition, more than the degree of stenosis, is the critical determining factor for the risk of breaking and subsequent thrombogenicity. Specifically, the necrotic core, fibrous cap, and inflammation are the main factors playing a role in plaque vulnerability. Of these, a thin fibrous cap (<54μm), abundant necrotic core, and the degree of inflammatory infiltrate are considered to be the best discriminating factors of vulnerability.4 Advances in understanding the cellular and molecular bases of plaque progression have enabled the physiopathological role of inflammation in vulnerable plaques to be established.5 At present, it is known that inflammatory mediators associated with leucocyte activation promote progression; a clear example is interleukin 6 (IL-6), a cytokine associated with increased production of C-Reactive Protein, which is an established marker of cardiovascular risk.6,7
Better understanding the physiopathology of atherosclerosis and the development of new imaging techniques has enabled high-risk plaques to be better characterised.8
Coronary angiography has traditionally been the imaging test that best determines the degree of stenosis and it continues to be the technique used to guide revascularisation procedures, both surgical and intravascular catheterisation. Angiography provides information about the number and size of vascular stenoses; however, a low correlation has been observed between angiographic findings and risk factor modification, and moreover, it does not tell us anything about the plaque's composition.
Intravascular ultrasonography (IVUS) plus virtual histology enable atheroma plaques in the vascular tree to be identified, and can quantify the size, volume, composition, and distribution of the plaque. Similarly, IVUS can detect lesions in vessels without stenosis in the angiographic test and precisely identify areas with positive and negative remodelling, as well as the atherosclerotic “burden”.9
Optical coherence tomography (OCT) enables several variables related to plaque morphology and composition to be detected, as well as the presence of calcium deposits, which are correlated with the extent of the atherosclerosis, although there is some controversy regarding the correlation between calcification and vulnerability.9
Magnetic resonance imaging possesses advantages over other imaging techniques, by not requiring the use of intravascular ionic contrasts. It also enables images to be obtained on several planes and provides information about vascular tissue composition. Recent research has demonstrated that it is possible to obtain images of not only the vascular lumen, but also the composition of the artery wall.10
Carotid ultrasonography detects the presence of focal atherosclerotic plaques and quantifies the carotid intima-media thickness (IMT). This is a non-invasive procedure that is standardised and has been validated in several studies. IMT, preferably measured in the common carotid artery, has been correlated with cardio- and cerebrovascular risk in different risk groups.11
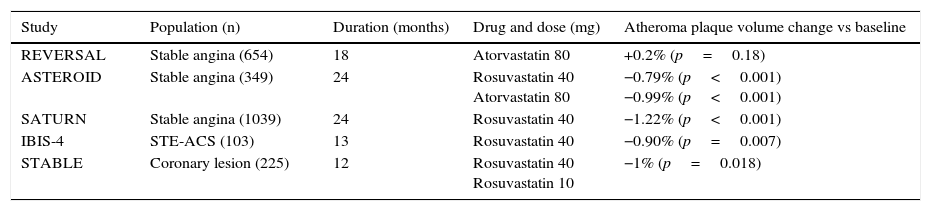
Measuring plaque response to systemic therapyStatins have changed the natural history of atherosclerotic disease in general and coronary and cerebrovascular syndromes in particular, as demonstrated by the reduction in cardiovascular morbimortality in primary and secondary prevention studies.12,13 What seems clear is that an “aggressive” lipid-lowering strategy to maintain LDLc levels <70mg/dL is the one that achieves the best results (Table 1). Nevertheless, it has been postulated that statins act independently of the reduction in cholesterol (pleiotropic effects),14,15 without these potential effects having demonstrated any significant clinical repercussions.
Summary of studies with IVUS that determine the impact of an “aggressive” lipid-lowering strategy on atherosclerotic plaque regression.
| Study | Population (n) | Duration (months) | Drug and dose (mg) | Atheroma plaque volume change vs baseline |
|---|---|---|---|---|
| REVERSAL | Stable angina (654) | 18 | Atorvastatin 80 | +0.2% (p=0.18) |
| ASTEROID | Stable angina (349) | 24 | Rosuvastatin 40 Atorvastatin 80 | −0.79% (p<0.001) −0.99% (p<0.001) |
| SATURN | Stable angina (1039) | 24 | Rosuvastatin 40 | −1.22% (p<0.001) |
| IBIS-4 | STE-ACS (103) | 13 | Rosuvastatin 40 | −0.90% (p=0.007) |
| STABLE | Coronary lesion (225) | 12 | Rosuvastatin 40 Rosuvastatin 10 | −1% (p=0.018) |
IVUS: intravascular ultrasonography; STE-ACS: ST-segment elevation acute coronary syndrome.
Several clinical studies have found a clear correlation between reducing cholesterol and clinical benefit in several patient categories, from those with established heart disease to asymptomatic subjects with cardiovascular risk and subjects with carotid artery disease, which cannot be explained in quantitative terms (plaque anatomy), but only qualitative terms (plaque biology).16–23
A prospective study incorporating IVUS and OCT has demonstrated a significant decrease in plaque volume and an increase in the fibrous cap in the group treated with 4mg of pitavastatin versus the control group.18 The YELLOW study compared IVUS and infrared spectroscopy in 87 patients undergoing coronary intervention who were randomised to rosuvastatin (40mg/day) or standard therapy. After 7 weeks of treatment, it was observed that the high statin doses were associated with a significant reduction in the necrotic core and decreased atheroma plaque progression.19 Subanalysis of the Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin Versus Atorvastatin (SATURN) evaluated the effect of aggressive lipid-lowering therapy on the plaque characteristics identified via virtual histology in 1039 patients. Rosuvastatin 40mg was compared against atorvastatin 80mg over a 24-month period in patients with stable atherosclerotic disease. Although no changes in the percentage of the plaque occupied by fibrotic tissue were observed, an increase in the percent of intraplaque calcium was reported.20,21 In subjects receiving an intracoronary stent due to myocardial infarction in the IBIS-4 study, a high-dose statin regimen (rosuvastatin 20–40mg/day) was associated with a considerable reduction in the lesion volume measured with virtual histology.22 In the STABLE study, a double-blind, randomised study in patients with an indication for angiography or percutaneous intervention, one year of treatment with rosuvastatin 40mg (and also 10mg) significantly reduced the percent of necrotic core (from 21.3% to 18%), increased fibrofatty tissue volume (from 11.7% to 14.8%), and decreased the plaque volume percent (from 51.4% to 50.4%)23 (Table 1). Lastly, a recent meta-analysis of prospective studies investigating the effect of statins on plaque volume and composition using IVUS and virtual histology (9 studies with 16 treatment arms with statins and 830 patients) concluded that the treatment induced favourable changes in the plaque, especially in the elastic membrane and fibrous tissue, with no significant effect on the lesion volume or necrotic core content.24
There is also evidence that intensive treatment with statins can have beneficial effects on plaque morphology in the carotid artery, which can be detected using ultrasound, magnetic resonance imaging, or PET. Different studies have also demonstrated that statins reduce carotid IMT progression and have a favourable impact in symptomatic patients undergoing carotid endarterectomy, although what the best technique is for quantifying changes in the atherosclerotic plaque has not been established. Magnetic resonance angiography and PET can provide additional information on the degree of plaque inflammation and identify the most vulnerable.25,26
Mechanisms by which statins may stabilise atheroma plaquesSome of the results cannot be attributed exclusively to a reduction in LDLc, but rather to pleiotropic effects related to higher fibrous tissue content, as well as decreased thrombogenicity and inflammation in the plaque.27 In this sense, in the IBIS-4 study, necrotic core stabilisation in myocardial infarction patients who received high statin doses was limited to those who achieved a greater reduction in CRP levels during treatment.28
A new frontier: PCSK9 inhibitionProprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition represents one of the major advances that have occurred in the last two years with a spectacular reduction in LDL-c.29 An interesting fact is that in addition to decreasing LDL-c, PCSK9 is expressed in human atherosclerotic plaques,30 likely playing a role in the vascular inflammation process and apoptosis. Moreover, the ATHEROREMO-IVUS study demonstrated that serum levels of PCSK9 were correlated with necrotic core volume as measured by IVUS and virtual histology.31 On-going studies with PCSK9 inhibitors (GLAGOV) will enable the scale of the LDL-c reduction and the other actions of these drugs on the composition and behaviour of high-risk plaques to be established.32,33
ConclusionResearch in recent years has enabled the molecular and cellular mechanisms underlying the biology of atheroma plaques to be established. The development of new vascular imaging techniques has contributed to this. Statins have changed the natural history of atherosclerosis, since there is clinical evidence that an “aggressive” lipid-lowering treatment can promote stabilisation and even regression of the atherosclerotic plaque, which is an important milestone in cardiovascular medicine. The atheroma plaque regression process includes favourable changes in the plaque's morphology and composition, more than its size or the degree of stenosis. On-going studies will enable the plaque response to systemic therapy to be more precisely defined.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments were conducted on human beings or animals for this research.
Confidentiality of dataThe authors declare that they have followed the protocols of their work site regarding the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data is contained in this paper.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Páramo JA, Civeira F. ¿Es posible la regresión de la placa aterosclerótica? Clin Invest Arterioscler. 2017;29:46–50.