To assess thrombotic risk with PAI-1 levels in patients with COVID-19, to evaluate PAI-1 differences between hyperglycemic and/or Type 2 Diabetes Mellitus (T2DM) versus non-hyperglycemic patients, and to analyze the association of plasminogen activator inhibitor-1 (PAI-1) with hyperglycemia and T2DM.
MethodsA cross-sectional study carried out in 181 patients hospitalized for COVID-19. Two groups were formed: the patients with hyperglycemia at admission and/or previously diagnosed T2DM group and the non-hyperglycemic group. Fibrinolysis was assessed by measuring PAI-1 levels by ELISA.
ResultsThe mean age was 59.4±16.1 years; 55.8% were male 54.1% of patients presented obesity, 38.1% had pre-existing T2DM and 50.8% had admission hyperglycemia and/or pre-existing T2DM. The patients with admission hyperglycemia and/or preexisting T2DM had higher PAI-1 compared with non-hyperglycemic patients [197.5 (128.8–315.9) vs 158.1 (113.4–201.4) ng/mL; p=0.031]. The glucose levels showed a positive correlation with PAI-1 levels (r=0.284, p=0.041). A multivariate logistic regression analysis showed association of PAI-1 level and hyperglycemia and pre-existing T2DM with severity of COVID-19.
ConclusionPatients hospitalized for COVID-19 infection with preexisting T2DM or hyperglycemia detected during their hospitalization presented a greater increase in PAI-1 levels, which suggests that hyperglycemia contributes directly to the hypercoagulable state and probably a worse outcome from the patients.
Este estudio busca evaluar el riesgo de trombosis en pacientes con COVID-19 mediante la medición de los niveles de inhibidor del activador del plasminógeno tipo 1 (PAI-1). También buscamos comparar los niveles de PAI-1 entre pacientes con hiperglucemia y/o diabetes mellitus tipo 2 (T2DM) y aquellos sin hiperglucemia. Además de investigar la relación entre los niveles de PAI-1 y la presencia de hiperglucemia y T2DM.
MétodosEs un estudio transversal con 181 pacientes hospitalizados por COVID-19. Los pacientes se dividieron en dos grupos: aquellos con hiperglucemia al ingreso o con diagnóstico previo de T2DM, y aquellos sin hiperglucemia. Los niveles de PAI-1 fueron cuantificados mediante ELISA para evaluar la actividad fibrinolítica.
ResultadosLa edad promedio fue de 59,4±16,1 años, con 55,8% de participantes masculinos. El 54,1% tenía obesidad, el 38,1% tenía antecedentes de diabetes mellitus y el 50,8% presentaba hiperglucemia al ingreso o diabetes mellitus preexistente. Los pacientes con hiperglucemia o T2DM preexistente mostraron niveles significativamente más altos de PAI-1 en comparación con los pacientes sin hiperglucemia (197,5 [128,8-315,9] vs. 158,1 [113,4-201,4] ng/mL; p=0,031). Se observó una correlación positiva entre los niveles de glucosa y PAI-1 (r=0,284, p=0,041). El análisis de regresión logística multivariante mostró una asociación entre los niveles de PAI-1 y la presencia de hiperglucemia y T2DM preexistente con la gravedad de la COVID-19.
ConclusiónLos pacientes hospitalizados por COVID-19 con T2DM preexistente o que desarrollan hiperglucemia durante la hospitalización experimentan un aumento significativo en los niveles de PAI-1. Esto sugiere que la hiperglucemia contribuye directamente al estado de hipercoagulabilidad, con posibles implicaciones en el pronóstico desfavorable de los pacientes.
Following the declaration of the COVID-19 pandemic in March 2020, healthcare systems were overwhelmed with dramatic health and mortality consequences.1 According to the World Health Organization (WHO), 765,903,278 confirmed cases of COVID-19 and 6,927,378 deaths have been reported worldwide (May 16, 2023); in Mexico, more than 7.5 million cumulative cases have been reported, with 333,960 deaths (WHO, May 16, 2023).2 Despite widespread COVID-19 vaccination use, which is highly efficacious at preventing severe disease and hospitalization, the pandemic has evolved into an endemic and global challenge with new variants, breakthrough infections, and long term health consequences.3
Type 2 Diabetes Mellitus (T2DM) is highly prevalent in COVID-19 patients.4 Both T2DM and hyperglycemia, when detected at the time of hospital admission, have been associated with increased severity and mortality in COVID-19 patients.5 Patients with T2DM are prone to thrombotic events due to factors such as platelet hypersensitivity, modifications of coagulation factors, and hypofibrinolysis. Hyperglycemia, insulin resistance and other comorbidities (e.g., obesity, dyslipidemia, non-alcoholic fatty liver disease) contribute to the hypercoagulable state in these patients.6
It is well described that coagulopathy and thromboembolic events are common in COVID-19 patients and are associated with a poor prognosis (severity and mortality). Despite these findings, information on the association of thrombotic risk and diabetes/hyperglycemia specifically in patients with COVID-19 remains scarce.7–13
T2DM is characterized by immunological alterations that contribute to viral disease progression. T2DM is a proinflammatory, prothrombotic and hypercoagulable state and usually involves the innate immune system.6 In addition, SARS-CoV-2 virus potentiates this proinflammatory state, leading to increased insulin resistance and endothelial dysfunction, while diminishing the anti-atherogenic role of the vascular endothelium.14–16
There are several mechanisms by which SARS-CoV-2 infection can cause macrovascular and microvascular occlusions, including cytokine-mediated activation of leukocytes, endothelium, platelets, and potentiation of thrombosis by neutrophil extracellular traps (NETs). Fibrinolysis is a tightly controlled process whereby a fibrin-rich thrombus is degraded and remodeled by the protease plasmin. Plasminogen activators and inhibitors regulate the conversion of plasminogen to plasmin, alterations in this balance result in modifications in fibrinolysis. The interaction of plasminogen activators, both tissue-type as tissue plasminogen activator (t-PA) and Urokinase-type Plasminogen Activator (u-PA), and their main inhibitor, Plasminogen Activator Inhibitor-1 (PAI-1), plays a key role in the regulation of fibrinolytic activity. It has been suggested that fibrinolysis is impaired in patients with COVID-19, which may increase thrombotic risk.17
PAI-1 is a molecule belonging to the superfamily of serine protease inhibitors (SERPIN) that acts as the main inhibitor of fibrinolysis and is secreted by vascular endothelial cells, epithelial cells, hepatocytes, and adipocytes.18 PAI-1 forms complexes with t-PA to control its catalytic ability. PAI-1 concentrations are elevated in various endocrine diseases, such as obesity, diabetes mellitus and metabolic syndrome.19,20 Hyperglycemia and insulin resistance lead to elevation PAI-1, resulting in reduced fibrinolytic factors.6 The excess of adipose tissue leads to an overexpression of proinflammatory cytokines such as TNF-α, IL-6 and IL-1 beta, which inhibit insulin signaling and increase PAI-1 levels, a phenomenon observed in a significant proportion of patients with T2DM.21
The objectives of this study were: (a) to measure serum PAI-1 levels to assess thrombotic risk in patients with COVID-19, (b) to evaluate PAI-1 differences between hyperglycemic and/or diabetic versus non-hyperglycemic patients, and (c) to analyze the association of PAI-1 with hyperglycemia and T2DM.
MethodsStudy populationAn observational, cross-sectional study was conducted in adult patients hospitalized with a diagnosis of COVID-19 at the Centro Medico Nacional “Siglo XXI” of the Instituto Mexicano del Seguro Social (IMSS) from July to December 2021. All participants were informed of the purpose of this study and provided written informed consent.
Inclusion criteria included patients older than 18 years with clinical symptoms associated with COVID-19 and confirmed by a positive SARS-COV-2 test (real-time PCR) who were hospitalized. SARS-CoV-2 testing was performed according to WHO recommendations.22
Data collectionMedical history, vital signs and laboratory reports were obtained from electronic medical records and then evaluated and analyzed. The information collected was age, gender, comorbidities, body mass index (BMI), triglycerides, high-density cholesterol, D-dimer (at admission and discharge), glucose (at admission and discharge), triglyceride/HDL ratio, triglyceride/glucose ratio, and hospital outcomes (survival vs mortality). The glycated hemoglobin (HbA1c) was calculated using the formula: (Glucose (mg/dL)+46.7)/28.7).23
Severity was assessed using criteria proposed by the Mayo Clinic Proceedings as follows: (a) Mild: temperature<38°C, slightly symptomatic, with no imaging compatible with pneumonia. (b) Moderate: fever, respiratory symptoms and imaging compatible with pneumonia. (c) Severe: respiratory rate of 30 breaths per minute or more, oxygen saturation less than 93% without oxygen supplementation, blood pressure index of oxygen on inspired fraction of oxygen (Kirby's Index) less than 300. (d) Critical: respiratory failure in need of mechanical assistance, shock and/or organ failure that requires management in the intensive care unit.24 Improvement or death was recorded as discharge reasons.
Biochemical determinations and proceduresBiochemical analysis was performed using blood samples collected upon admission from the antecubital vein. The samples were obtained in tubes with sodium citrate and then were centrifuged at 3000rpm for 15min at 4°C. The aliquots of plasma were stored at −70°C until the test was performed.
Plasmatic concentrations of PAI-1 were measured by enzyme-linked immune-sorbent assay (SERPINE1 Human ELISA Kit OKBB00347, Aviva Systems Biology, Corp. San Diego, CA, USA) where the plasma was incubated (37°C) with a biotinylated detection antibody, specific for human PAI-1. Then, an Avidin-Biotin-Peroxidase Complex was added, incubated and the unbounded complexes were washed away. Finally, the enzymatic reaction was visualized through the addition of 3,3′,5,5′-Tetramethylbenzidine (TMB), which was catalyzed by horseradish peroxidase (HRP). The combination of TMB and hydrogen peroxide causes the oxidation of TMB, resulting in the formation of a blue color. After, the blue-colored product that changes to yellow when adding acidic stop solution. The absorbance density of yellow coloration was obtained by absorbance at 450nm which was quantitatively proportional to the amount of human PAI-1 captured in the well microtiter plate.
Statistical analysisParametric variables were expressed as mean±standard deviation and non-parametric variables such as median and interquartile range (IQR). Differences between groups were evaluated by the Student's t test, Wilcoxon signed-rank test or Mann–Whitney U test, as appropriate. The correlation among variables was identified by Spearman's tests. Multiple logistic regression analysis was performed to estimate factors associated with mortality, sex, obesity, hypertension (HTN), T2DM and PAI-1. All analyses were performed with the statistical package SPSS v.21. A statistically significant p value was considered as p<0.05.
ResultsCharacteristics of COVID-19 patientsOf 301 adult patients hospitalized with a diagnosis of COVID-19, there were patients with incomplete data in medical records than were excluded, also, pregnant women were not eligible for the study. So, patients with chronic diseases were excluded: kidney failure, cancer, neurological, heart disease. Thus, in this study were selected 181 patients.
The mean age was 59.4±16.1 years; 55.8% were men and 44.2% were women of 181 patients were included. According to BMI, 54.1% of the patients were with obesity and 45.9% were normal weight or overweight, 50.82% (92/181) had HTN, 38.1% had pre-existing T2DM and 50.8% had admission hyperglycemia and/or pre-existing diabetes (Table 1).
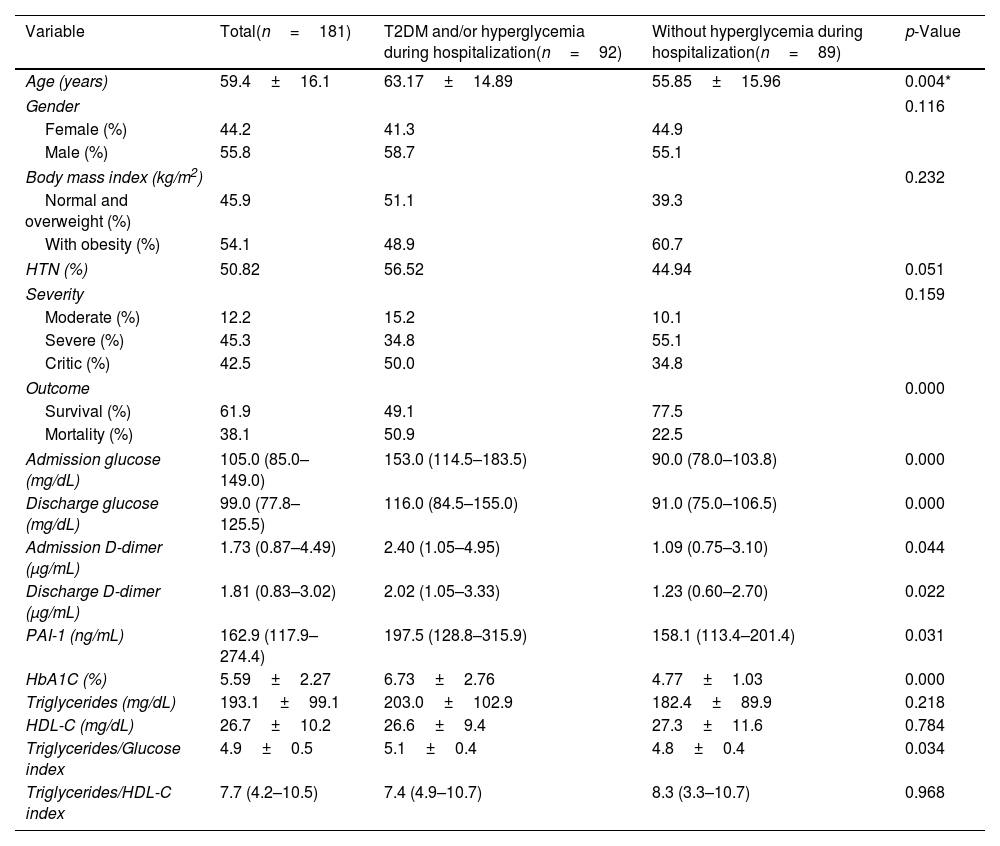
Characteristics of hospitalized COVID-19 patients with T2DM and/or hyperglycemia during hospitalization vs patients without hyperglycemia during hospitalization.
| Variable | Total(n=181) | T2DM and/or hyperglycemia during hospitalization(n=92) | Without hyperglycemia during hospitalization(n=89) | p-Value |
|---|---|---|---|---|
| Age (years) | 59.4±16.1 | 63.17±14.89 | 55.85±15.96 | 0.004* |
| Gender | 0.116 | |||
| Female (%) | 44.2 | 41.3 | 44.9 | |
| Male (%) | 55.8 | 58.7 | 55.1 | |
| Body mass index (kg/m2) | 0.232 | |||
| Normal and overweight (%) | 45.9 | 51.1 | 39.3 | |
| With obesity (%) | 54.1 | 48.9 | 60.7 | |
| HTN (%) | 50.82 | 56.52 | 44.94 | 0.051 |
| Severity | 0.159 | |||
| Moderate (%) | 12.2 | 15.2 | 10.1 | |
| Severe (%) | 45.3 | 34.8 | 55.1 | |
| Critic (%) | 42.5 | 50.0 | 34.8 | |
| Outcome | 0.000 | |||
| Survival (%) | 61.9 | 49.1 | 77.5 | |
| Mortality (%) | 38.1 | 50.9 | 22.5 | |
| Admission glucose (mg/dL) | 105.0 (85.0–149.0) | 153.0 (114.5–183.5) | 90.0 (78.0–103.8) | 0.000 |
| Discharge glucose (mg/dL) | 99.0 (77.8–125.5) | 116.0 (84.5–155.0) | 91.0 (75.0–106.5) | 0.000 |
| Admission D-dimer (μg/mL) | 1.73 (0.87–4.49) | 2.40 (1.05–4.95) | 1.09 (0.75–3.10) | 0.044 |
| Discharge D-dimer (μg/mL) | 1.81 (0.83–3.02) | 2.02 (1.05–3.33) | 1.23 (0.60–2.70) | 0.022 |
| PAI-1 (ng/mL) | 162.9 (117.9–274.4) | 197.5 (128.8–315.9) | 158.1 (113.4–201.4) | 0.031 |
| HbA1C (%) | 5.59±2.27 | 6.73±2.76 | 4.77±1.03 | 0.000 |
| Triglycerides (mg/dL) | 193.1±99.1 | 203.0±102.9 | 182.4±89.9 | 0.218 |
| HDL-C (mg/dL) | 26.7±10.2 | 26.6±9.4 | 27.3±11.6 | 0.784 |
| Triglycerides/Glucose index | 4.9±0.5 | 5.1±0.4 | 4.8±0.4 | 0.034 |
| Triglycerides/HDL-C index | 7.7 (4.2–10.5) | 7.4 (4.9–10.7) | 8.3 (3.3–10.7) | 0.968 |
Parametric variables are represented as mean±SD.
Non-parametric variables are represented as median and interquartile range (IQR).
PAI-1=plasminogen activator-inhibitor type-1.
HDL-C=high-density lipoprotein cholesterol.
HbA1C=glycated hemoglobin A1C.
HTN=hypertension.
The clinical and biochemical characteristics of COVID-19 patients with hyperglycemia on admission and/or pre-existing T2DM vs patients without hyperglycemia are compared in Table 1. Age (63.1±14.8 vs 55.8±15.9; p=0.004) was higher in patients with admission hyperglycemia and/or pre-existing T2DM compared to non-hyperglycemic patients. There were no significant differences in gender, BMI, HTN, or severity of COVID-19.
On biochemical evaluation, patients with admission hyperglycemia and/or pre-existing T2DM had higher levels of glucose at admission [153.0 (114.5–183.5) vs 90.0 (78.0–103.8) mg/dL; p=0.0001], glucose at discharge [116.0 (84.5–155.0) vs 91.0 (75.0–106.5) mg/dL; p<0.001], HbA1C [6.73±2.76 vs 4.77±1.03%, p<0.001]; D-dimer at admission [2.40 (1.05–4.95) vs 1.09 (0.75–3.10) μg/mL; p=0.044], D-dimer at discharge [2.02 (1.05–3.33) vs 1.23 (0.60–2.70) μg/mL; p=0.022], PAI-1 [197.5 (128.8–315.9) vs 158.1 (113.4–211.4) ng/mL, p=0.031].
Characteristics of COVID-19 patients according to the outcomeThe clinical and biochemical characteristics of COVID-19 patients according to the outcome (survival vs mortality) are shown in Table 2. There were no differences in gender, HTN, and obesity. When comparing the survival to mortality group, the mortality group had significantly higher age (64.6±14.6 vs 56.7±15.9 years; p=0.004), glucose at admission [125.5 (90.0–168.0) vs 97.0 (80.8–130.2) mg/dL; p=0.04], glucose at discharge [112.0 (79.5–142.0) vs 90.5 (76.3–111.5) mg/dL; p=0.013], D-dimer at admission [2.70 (1.44–9.39) vs 1.00 (0.68–2.14) μg/mL; p=0.001], D-dimer at discharge [2.64 (1.82–3.97) vs 1.02 (0.61–2.20) μg/mL; p<0.001] and HbA1c (6.2±2.68 vs 5.2±2.05%; p=0.015).
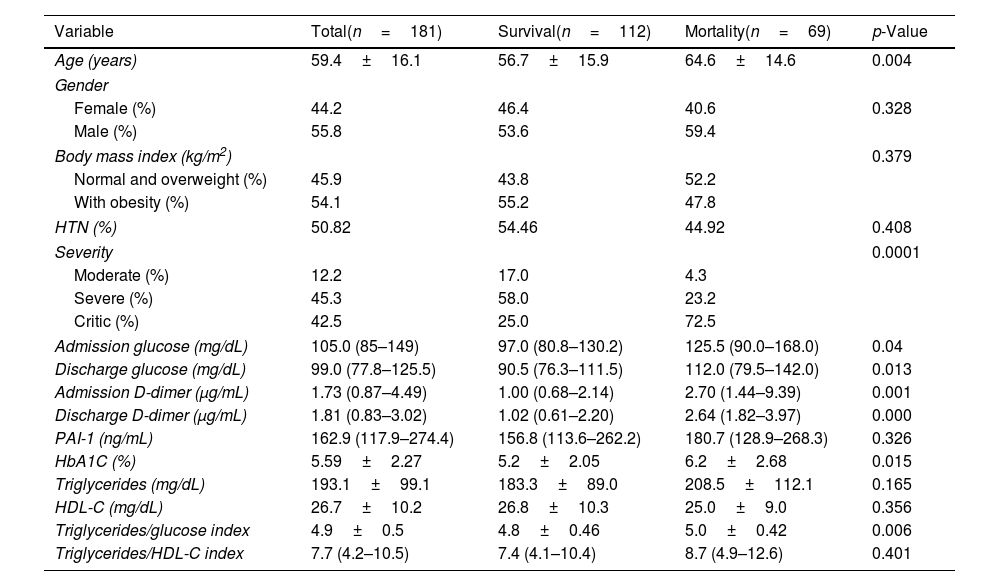
Characteristics of COVID-19 patients according to the outcome.
| Variable | Total(n=181) | Survival(n=112) | Mortality(n=69) | p-Value |
|---|---|---|---|---|
| Age (years) | 59.4±16.1 | 56.7±15.9 | 64.6±14.6 | 0.004 |
| Gender | ||||
| Female (%) | 44.2 | 46.4 | 40.6 | 0.328 |
| Male (%) | 55.8 | 53.6 | 59.4 | |
| Body mass index (kg/m2) | 0.379 | |||
| Normal and overweight (%) | 45.9 | 43.8 | 52.2 | |
| With obesity (%) | 54.1 | 55.2 | 47.8 | |
| HTN (%) | 50.82 | 54.46 | 44.92 | 0.408 |
| Severity | 0.0001 | |||
| Moderate (%) | 12.2 | 17.0 | 4.3 | |
| Severe (%) | 45.3 | 58.0 | 23.2 | |
| Critic (%) | 42.5 | 25.0 | 72.5 | |
| Admission glucose (mg/dL) | 105.0 (85–149) | 97.0 (80.8–130.2) | 125.5 (90.0–168.0) | 0.04 |
| Discharge glucose (mg/dL) | 99.0 (77.8–125.5) | 90.5 (76.3–111.5) | 112.0 (79.5–142.0) | 0.013 |
| Admission D-dimer (μg/mL) | 1.73 (0.87–4.49) | 1.00 (0.68–2.14) | 2.70 (1.44–9.39) | 0.001 |
| Discharge D-dimer (μg/mL) | 1.81 (0.83–3.02) | 1.02 (0.61–2.20) | 2.64 (1.82–3.97) | 0.000 |
| PAI-1 (ng/mL) | 162.9 (117.9–274.4) | 156.8 (113.6–262.2) | 180.7 (128.9–268.3) | 0.326 |
| HbA1C (%) | 5.59±2.27 | 5.2±2.05 | 6.2±2.68 | 0.015 |
| Triglycerides (mg/dL) | 193.1±99.1 | 183.3±89.0 | 208.5±112.1 | 0.165 |
| HDL-C (mg/dL) | 26.7±10.2 | 26.8±10.3 | 25.0±9.0 | 0.356 |
| Triglycerides/glucose index | 4.9±0.5 | 4.8±0.46 | 5.0±0.42 | 0.006 |
| Triglycerides/HDL-C index | 7.7 (4.2–10.5) | 7.4 (4.1–10.4) | 8.7 (4.9–12.6) | 0.401 |
Parametric variables are represented as mean±SD.
Non-parametric variables are represented as median and interquartile range (IQR).
PAI-1=plasminogen Activator-Inhibitor type-1.
HDL-C=high-density lipoprotein cholesterol.
HbA1C=glycated hemoglobin A1C.
HTN=hypertension.
In the case of mortality (50.9 vs 22.5%, p=0.0001) compared to non-hyperglycemic patients. Besides, PAI-1 levels were higher in the mortality group, although no statistical significance was found [180.7 (128.9–268.3) vs 156.8 (113.6–262.2) ng/mL, p=0.326].
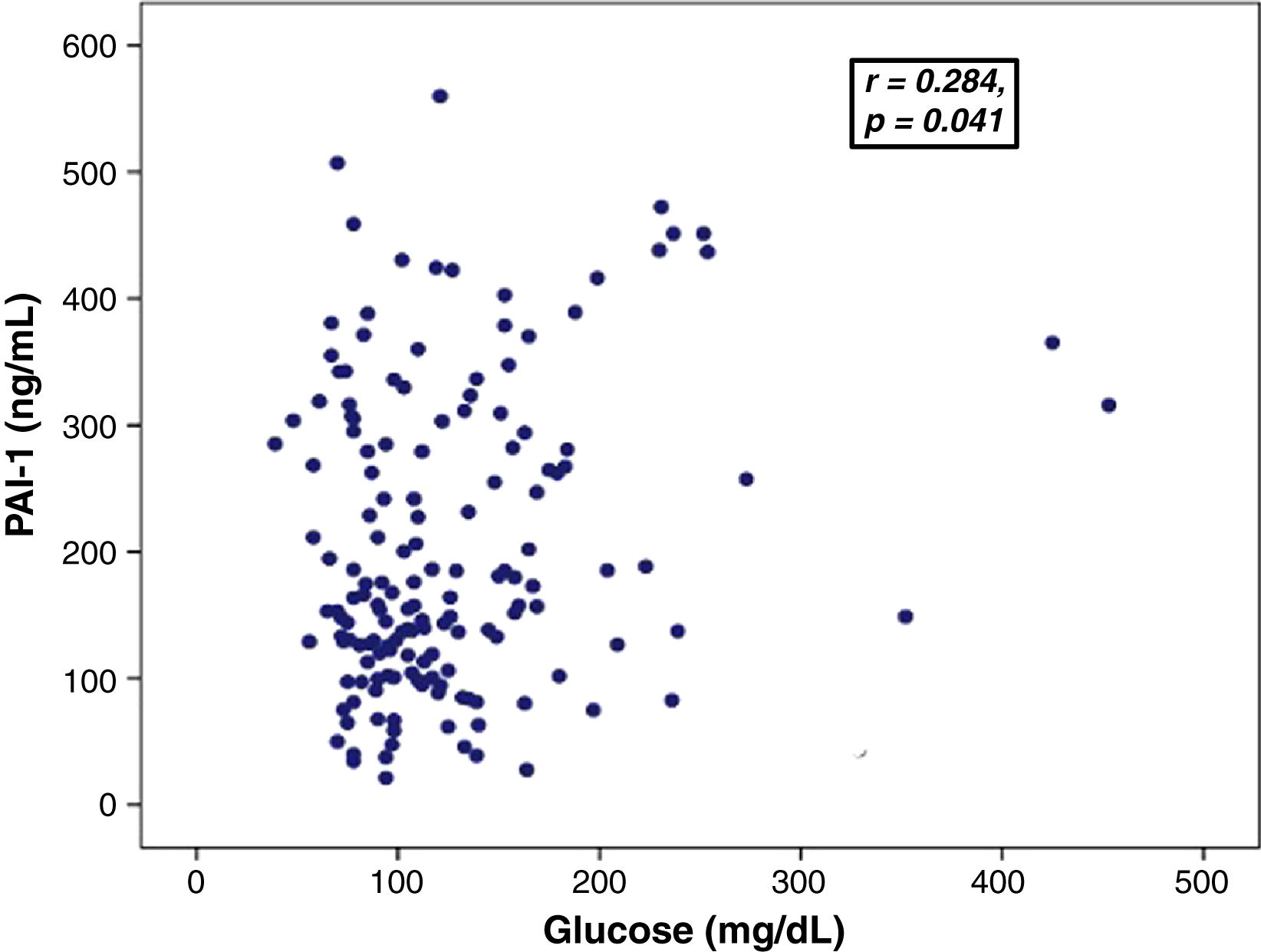
Association between PAI-levels and hyperglycemiaGlucose levels showed a positive correlation with PAI-1 levels (0.284, p=0.041) (Fig. 1) and D-dimer (r=0.186, p=0.037). There was no significant correlation between PAI-1 and D-dimer levels.
As previously discussed, patients with hyperglycemia on admission and/or pre-existing T2DM had higher PAI-1 levels compared to non-hyperglycemic patients. In a subset analysis according to severity within the group of non-hyperglycemic patients, patients with critical COVID-19 had higher PAI-1 levels compared to those with moderate/severe disease [177.2 (147.2–227.6) vs 134.9 (89.0–176.0) ng/mL; p=0.037]. PAI-1 levels were markedly elevated in patients with hyperglycemia on admission and/or pre-existing T2DM. However, there was no difference within the groups in PAI-1 levels according to COVID-19 severity [critical disease 231.0 (127.3–316.1) vs moderate/severe disease 179.7 (118.2–292.1) ng/mL; NS].
A multivariate logistic regression analysis was performed to estimate factors associated with the outcome (severity and mortality). A first model evidenced an association between PAI-1 level (β=0.07; Exp B=0.993; p=0.011) age, and hyperglycemia and pre-existing T2DM (β=2.1; Exp B=8.7, p=0.051) with severity. A second model, adjusted by age, gender, and BMI evidenced an association between hyperglycemia and pre-existing T2DM (β=0.99, Exp B=2.69; p=0.05), and severity of COVID-19 (β=1.52, Exp B=4.81; p<0.001) with mortality.
DiscussionThis study showed that hospitalized COVID-19 patients with T2DM and/or admission hyperglycemia had higher PAI-1 levels compared to non-hyperglycemic patients, with a corroborated association between PAI-1 and glucose, suggesting that hyperglycemia contributes to the hypercoagulable state in patients with SARS-CoV-2 infection, which may worsen prognosis. In addition, hyperglycemia present on admission, pre-existing T2DM, and PAI-1 were associated with the severity of COVID-19, while pre-existing T2DM and severity were associated with mortality.
T2DM and COVID-19 are prone to coagulopathy and thromboembolic events, both as separate and coexisting entities. PAI-1 is one of the possible factors related to this association according to our findings.6,7,24
Before the COVID-19 pandemic, alterations in balance between coagulation and fibrinolysis pathways were shown to play an important role in the pathogenesis of SARS-CoV infection.25
Currently, alterations in the coagulation and fibrinolysis pathways after SARS-CoV infection have been corroborated in patients with COVID-19.7,8 The histopathological findings observed in the lungs of patients with COVID-19 pneumonia include the presence of venous and arterial thrombi in small and large vessels, and a pattern of acute fibrinous and organizing pneumonia, with extensive intra-alveolar fibrin deposition.26,27
This pulmonary pathology is a marked microvascular thrombosis and hemorrhage linked to extensive alveolar and interstitial inflammation that shares features with macrophage activation syndrome and could be considered a diffuse pulmonary intravascular coagulopathy. It has been suggested that increased tissue factor, together with reduced fibrinolysis induced by increased PAI-1, promotes the thrombin generation and intra-alveolar fibrin deposition seen in these patients.27,28
These findings are related to high levels of PAI-1, a marker of endothelial dysfunction and fibrinolysis. In patients with severe COVID-19, there is an elevated release of PAI-1 by human pulmonary microvascular endothelial cells (HPMEC) in response to uptake of recombinant SARS-CoV-2 protein (S1).29 Persistent fibrin deposition in the lung parenchyma and alveolar spaces of patients with COVID-19 could suggest that elevated PAI-1 levels may overcome the local release of tPA.28 Moreover, hypofibrinolysis due to elevated PAI-1 levels should be considered a risk factor for thrombosis.29,30
Fibrinolytic activity in patients with COVID-19 has been evaluated in different studies, however, information about the association of thrombotic biomarkers with hyperglycemia or diabetes is missing.31,32 Also, in COVID-19 patients, the increase in PAI-1 levels due to STAT3 activation may efficiently inhibit both t-PA and u-PA, leading to coagulopathy and thrombosis.27
Zuo Y. et al. in a cohort of 118 hospitalized patients with COVID-19 found elevated t-PA and PAI-1 levels, which were associated with worse respiratory status.18 In that study, the frequency of diabetes was 43%; however, the association of diabetes or hyperglycemia with PAI-1 was not evaluated.
In the American population, patients with COVID-19 had higher levels of D-dimer and PAI-1 compared with healthy controls, lower levels of D-dimer compared with patients with sepsis, and higher levels of PAI-1 compared with patients with sepsis. That study proposes the role of PAI-1 in promoting thrombosis in patients with COVID-19 due to simultaneous activation of coagulation and inhibition of fibrinolysis.31
In a Mexican population, a study with a cohort of 119 hospitalized patients with COVID-19 showed higher levels of D-dimer and PAI-1 in patients with severe COVID-19, as well as higher prothrombotic biomarkers in patients with a fatal outcome. This study also did not evaluate the association of diabetes or hyperglycemia and prothrombotic biomarkers.32
In contrast to these findings, our study demonstrated the association between hyperglycemic state and increased PAI-1. Importantly, the increase in PAI-1 was not only observed among patients with T2DM, but also among those without hyperglycemia and increased disease severity. This is consistent with our finding in the logistic regression model, which demonstrated an association between increased PAI-1 and worsened severity in all included patients. This denotes that it is not only important to consider prothrombotic biomarkers in the patient with COVID-19, but also factors that may aggravate the thrombotic risk, such as hyperglycemia.
The combination of metabolic diseases such as diabetes, obesity or HTN, and factors such as hyperinsulinism, triglycerides, hyperglycemia and inflammatory cytokines promotes elevated PAI-1 expression.33
Diabetes, as well as infection with COVID-19, is associated with a prothrombotic state. T2DM and hyperglycemia modify coagulation hemostasis and fibrinolysis, resulting in a prothrombotic state characterized by platelet hypersensitivity, coagulation disorders and hypofibrinolysis.34 The hypofibrinolytic state in T2DM is attributed to the formation of fibrinolysis-resistant clots and alterations of the fibrinolytic system.6
In rats with diabetes, a significant reduction in glucose levels has been following specific inhibition of PAI-1 by the synthetic molecule PAItrap3.35 Clinically, a direct association between T2DM risk and PAI-1 levels has been demonstrated, which appears independent of other metabolic risk factors.35
As can be seen in our findings, this hyperglycemia-PAI-1 relationship is also evident in patients with COVID-19. In addition, hyperglycemia increases plasminogen glycation, inhibiting plasmin generation. In turn, plasminogen acts as a proinflammatory factor, which promotes insulin resistance and exacerbates the prothrombotic state as a vicious cycle.6 These pathophysiological processes, together with those described for the prothrombotic state during SARS-CoV-2 infection, create a catastrophic environment in the patient with COVID-19 and hyperglycemia.
On the other hand, D-dimer levels have been shown to have predictive value for COVID-19 mortality. In a study conducted in Wuhan, it was established that a D-dimer level higher than 2.0μg increases in-hospital mortality from COVID-19.36 Likewise, an association between D-dimer levels and glucose concentrations has been reported.36–40 In our study, we found that patients with T2DM and/or admission hyperglycemia had higher D-dimer levels compared to patients without hyperglycemia, with an association between glucose levels and D-dimer.
It is important to consider antithrombotic strategies in patients with COVID-19, from the most generalized (therapeutic anticoagulation, e.g. low molecular weight heparin), to selective inhibition of PAI-1, which may be more promising for improving outcomes beyond thrombosis, especially given the multifaceted role of PAI-1 in COVID-19.29,30 In addition, the management of hyperglycemia and other factors that can worsen the thrombotic risk in these patients is critical, also it's a borderline situation for cardiovascular complications. It has been shown that achievement of euglycemia could reverse coagulation alterations, highlighting the importance of glucose control, together with anticoagulant therapy, to improve thrombotic risk in patients with COVID-19 and T2DM and/or hyperglycemia.6
Strengths and limitationsThis study measured PAI-1, which is the main inhibitory molecule of fibrinolysis, but still requires broadening of knowledge about other coagulation proteins.
The present study included a significant number of patients and coagulation samples taken upon patient admission to the hospital. Original data from the study include the identification of a positive association between PAI-1 and glucose levels in patients with COVID-19 infection, as well as the fact that patients with previous diagnosis of T2DM or hyperglycemia were observed during hospitalization to show a significant increase in PAI-1 compared with non-hyperglycemic patients.
It is critical that institutions continue gathering information on COVID-19 infections, along with other viral infections during pandemic events, in order to properly be able to analyze pathophysiological and epidemiological trends. By gathering this information healthcare institutions will be able to better combat pandemics, and improve long term health outcomes for patients.
In conclusion, in patients hospitalized by SARS-CoV-2 infection, high PA1-levels may be explained by the severity of the disease, and these are associated with diabetes, hyperglycemia and probably endothelial damage. Therefore, it is important to insist on glucose control in patients with COVID-19 with and without previous diagnosis of diabetes, since hyperglycemia may detonate an increase in the prothrombotic state.
Authors’ contributionsLB, LMA, CMM, JCAG and MGMO have made substantial contributions to conception and acquisition of data, analysis investigation. LIMO, JZ and LBH methodology. LB and LMA writing of original draft. AR and SO review and editing of the manuscript. LB and LMA validation and visualization. Authors read and approved the final manuscript.
Availability of data and materialsThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participateAlso, this study was approved by the ethical committee and the Local Research and Health Committee, with registered R 2020-3601-232.
EthicsThe study was approved by the institutional ethics and research committee (registration number R 2020-3601-232) of Instituto Mexicano del Seguro Social (IMSS), and the study was conducted according to the guidelines of the Declaration of Helsinki.
FundingThis study was supported by Instituto Mexicano del Seguro Social (IMSS) (Grant No. IMSS 2023-3-71/R 2020-3601-232). The funder had no role on the design of study and collection, analysis, and interpretation of data and in writing the manuscript in this study.
Conflict of interestsThe authors declare that there is no conflict of interest regarding the publication of this article.
We acknowledge the support Instituto Mexicano del Seguro Social (IMSS) (IMSS 2023-3-71) and SNI/CONAHCYT.