Heavy menstrual bleeding is the major complaint in women with symptomatic fibroids. Ulipristal acetate (UPA) is a “selective progesterone receptor modulator” in which mixed agonist and antagonist activity appears to offer a novel approach to medical management of symptomatic uterine fibroids. There are currently no studies that have assessed its effect on the mammary gland in humans.
MethodsProspective observational study conducted in a tertiary hospital, in which 14 consecutive patients diagnosed with uterine fibroids by transvaginal ultrasound were included. The patients received two cycles of UPA 5mg/day for 3 months each, with a rest period of two months between both cycles. To assess the safety on the mammary gland, 3 mammary ultrasounds were performed at three different points: prior to the first cycle of UPA, between the two cycles and at the end of the second cycle.
ResultsOf the 14 recruited patients, 9 completed the study period. Breast ultrasound reports were normal in all patients. In 4 patients (44.4%), the 3 breast ultrasounds were classified as BI-RADS 2. In 2 patients (22.2%) the 3 breast ultrasounds were classified as BI_RADS1; and in 3 patients (33.3%), the first breast ultrasound was classified as BI-RADS 3 and the last two as BI-RADS 2.
ConclusionIn conclusion, our study showed that 2 cycles of treatment with UPA in patients with symptomatic uterine fibroids is safe for normal breast tissue with an acceptable adverse effects profile. Nonetheless, further research is required to confirm our findings.
El sangrado menstrual abundante es el principal síntoma en mujeres con miomas uterinos. Acetato de ulipristal (AUP) es un «modulador selectivo del receptor de progesterona» que parece ofrecer una nueva opción al manejo médico de los miomas debido a su acción mixta agonista y antagonista. Actualmente no hay estudios que evalúen su efecto en la glándula mamaria en humanos.
MétodosEs un estudio prospectivo observacional realizado en un hospital terciario, en el cual se incluyeron 14 mujeres con diagnóstico de miomas uterinos mediante ecografía transvaginal. Las pacientes recibieron 2 ciclos de AUP 5mg/día durante 3 meses cada ciclo, con un período de descanso de 2 meses entre ambos ciclos. Para evaluar el impacto en la glándula mamaria se realizaron 3 ecografías mamarias en 3 momentos diferentes: previo al inicio del primer ciclo, entre los 2 ciclos y al terminar el segundo ciclo.
ResultadosDe las 14 pacientes reclutadas, 9 completaron el período de estudio. Las ecografías mamarias en todas las pacientes fueron normales. En 4 pacientes (44,4%), las 3 ecografías mamarias fueron catalogadas como BI-RADS® 2. En 2 pacientes (22,2%) las 3 ecografías fueron catalogadas como BI-RADS® 1, y en 3 pacientes (33,3%), la primera ecografía mamaria fue catalogada como BI-RADS® 3 y las 2 últimas BI-RADS® 2.
ConclusiónEn conclusión, nuestro estudio muestra que 2 ciclos de tratamiento con AUP en pacientes con miomas uterinos sintomáticos es seguro para el tejido mamario sano con efectos adversos aceptables. Sin embargo, hacen falta más estudios para confirmar nuestros resultados.
Uterine fibroids are benign tumors of the smooth muscle of the uterus.1 These are the most frequent benign uterine tumors, with an incidence of up to 70% in women of reproductive age.2 Uterine fibroids are usually asymptomatic, but in certain cases (40%) they can cause abnormal uterine bleeding, pelvic pain, infertility, constipation or even bladder compression, depending on its location and size.3
Fibroids treatment can be medical or surgical and will depend on several factors such as the patient's symptoms, age and wish to conceive.
Ulipristal acetate (UPA) is a medical treatment option for symptomatic fibroids. UPA is a steroidal compound belonging to the class of selective progesterone receptor modulators (SPRMs), which main pharmacodynamic property is the reversible blockade of the progesterone receptor (PR), exhibiting direct tissue-specific partial progesterone antagonist effects. UPA acts specifically on the pituitary gland (inhibiting LH surge and ovulation), on the endometrium (stopping uterine bleeding) and directly on uterine fibroids (inhibiting cell proliferation and inducing apoptosis). In addition, it has extensively shown its clinical efficacy and safety4–7 and is indicated both for repeated intermittent treatment and for the preoperative treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age.
However, little is known regarding the effect of UPA in other target tissues. In the endometrium, its administration has been associated with a non-proliferative, non-physiological, benign histological pattern of the endometrium which is called progesterone receptor modulator associated endometrial changes (PAEC), which have been shown to be reversible.5,6 To our knowledge, no previous studies have assessed the safety of UPA in breast tissue.
The main objective of the current study was to evaluate the radiological changes by ultrasound produced by treatment with UPA in breast tissue. This knowledge could provide valuable information to complete the safety profile of UPA when prescribing this treatment.
Material & methodsStudy designA pilot, prospective, observational and descriptive study of women treated with UPA was designed. The study was carried out at the Department of Gynecology and Obstetrics of Hospital Universitari Sagrat Cor (HUSC), in Barcelona, Spain, between November 2017 and March 2019. The study was approved by the local Ethical Committee, according to the prevailing regulations. Informed consent was requested from all patients prior to inclusion in the study.
Participants and study proceduresA total of 14 consecutive patients who were candidates for treatment with UPA were invited to participate. All patients were ≥30 and <50 years old with ultrasound diagnosis of one or more uterine fibroid between 2 and 8cm and moderate to severe symptoms referred to our unit during the study recruitment period.
The exclusion criteria included patients with asymptomatic fibroids, patients with symptomatic fibroids outside the pre-defined study range (<2 to >8cm), hypersensitivity to the active substance and/or the excipients, pregnancy and breastfeeding, genital hemorrhages of unknown etiology, and patients with endometrial, cervical, ovarian or breast cancer.
The diagnosis of uterine fibroid was confirmed using a 2D-3D TVS, with an endovaginal probe (type RIC5-9, Voluson V730 Expert, GE Healthcare, Milwaukee, WI, USA). The procedures were performed by two expert sonographers following the Morphological Uterus Sonographic Assessment (MUSA) consensus statement.8 For the purpose of this study, uterine fibroids were classified as described in FIGO classification.9
Patients received two courses of 3 months of AUP 5mg/day, with a drug-free 2-month period between them.
To assess the safety on the mammary gland, 3 breast ultrasounds were performed in three different time points: a baseline point before starting of the first treatment course, an intermediate point between the first and the second treatment course, and a final point after completing the second treatment course (up to 4 weeks after completing it).
Breast ultrasounds were performed using a Esaote mylab 70 ultrasound and were performed by one expert sonographer and reported following the Breast Imaging Reporting and Data System (BI-RADs).10
Either on the baseline ultrasound or during the follow-up, a report of Birads 4 or 5 involved the exclusion of the patient from the study and their management according to the corresponding HUSC breast pathology protocol, with mammography (Mx) and lesion biopsy.
MeasuresSociodemographic and gynecological data were collected at baseline (age, parity, gynecological symptoms such as abnormal uterine bleeding, pelvic pain and/or compression symptoms). In order to evaluate patient‘s symptoms, women were asked to rate the current intensity of dysmenorrhea, dyspareunia, dyschezia, non-menstrual pelvic pain and dysuria by means of a 0–10-point numerical rating scale (NRS), with “0” indicating no pain and “10” indicating the worst possible pain. Additionally, we used a dichotomus variable (i.e. “yes”, encoded 1 and “no”, encoded 0) to assess whether (i) the patient had or not moderate to severe symptoms (defined as the patient reporting some type of pain≥4 in the NRS, as previously described11) and/or (ii) the patient had heavy menstrual bleeding (HBM) (defined as the patient having lost≥80ml in a menstruation). We assessed quantitatively menstrual bleeding using the Pictorial Blood Loss Assessment Chart (PBAC).
A radiological change in a breast ultrasound was considered if at least 1 of these 3 conditions were met: (1) increase in a Birads category, (2) increase in the size of a known nodule and/or (3) appearance of a new nodule.
Data on menstrual bleeding, symptoms, size and location of the fibroid were collected at three time points: before starting the first UPA course, between the first and the second treatment courses and after completing the second treatment course. In case of more than one uterine fibroid was identified, only the largest one was considered for size measurement (the largest diameter being reported).
According to the SPC, liver tests were performed before every UPA course was started and on a monthly basis during the two courses. Patients were asked by adverse events during the treatment period at every visit.
Data analysis strategyThe statistical analysis was performed using the Statistical Package for the Social Sciences software, release 25.0 for Windows (SPSS, Chicago, Illinois). Continuous variables are presented as mean±standard deviation. Categorical variables are presented as total count and relative percentages (%). Since this was a pilot study, a descriptive statistical analysis was carried out. Statistical significance was defined as a P-value<0.05, only for descriptive purposes.
ResultsSample descriptionThree patients left the first treatment cycle when the statement on “liver damage” was reported (in all liver profile was requested resulting normal); 1 patient was lost during the follow-up and in 1 patient, a Birads 4a resulted in the first breast ultrasound so was excluded from the study. Therefore, 9 patients completed the study and were definitely included.
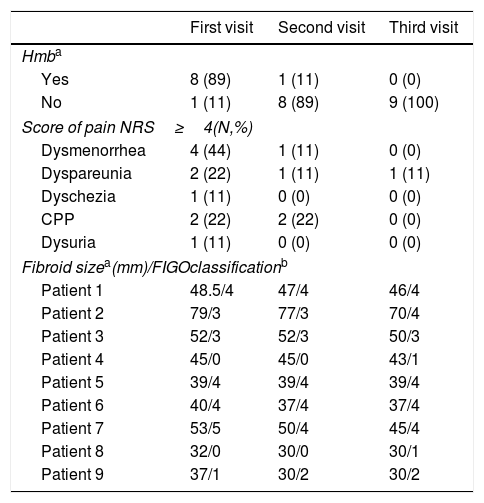
The mean age of the 9 patients included in the study was 45±2.70 years. Body mass index was 22.09±3.32. Six of the nine patients were nulliparous and 3 patients had one or more live births. The main reason for consultation was HMB in 8 patients and metrorrhagia in one. Data on menstrual bleeding, symptoms and, size and location of the uterine fibroid at the three time points are shown in Table 1.
Data of clinical variables in the three visits.
| First visit | Second visit | Third visit | |
|---|---|---|---|
| Hmba | |||
| Yes | 8 (89) | 1 (11) | 0 (0) |
| No | 1 (11) | 8 (89) | 9 (100) |
| Score of pain NRS≥4(N,%) | |||
| Dysmenorrhea | 4 (44) | 1 (11) | 0 (0) |
| Dyspareunia | 2 (22) | 1 (11) | 1 (11) |
| Dyschezia | 1 (11) | 0 (0) | 0 (0) |
| CPP | 2 (22) | 2 (22) | 0 (0) |
| Dysuria | 1 (11) | 0 (0) | 0 (0) |
| Fibroid sizea(mm)/FIGOclassificationb | |||
| Patient 1 | 48.5/4 | 47/4 | 46/4 |
| Patient 2 | 79/3 | 77/3 | 70/4 |
| Patient 3 | 52/3 | 52/3 | 50/3 |
| Patient 4 | 45/0 | 45/0 | 43/1 |
| Patient 5 | 39/4 | 39/4 | 39/4 |
| Patient 6 | 40/4 | 37/4 | 37/4 |
| Patient 7 | 53/5 | 50/4 | 45/4 |
| Patient 8 | 32/0 | 30/0 | 30/1 |
| Patient 9 | 37/1 | 30/2 | 30/2 |
0: intracavitary pedunculate, 1: <50% intramural, 2: =50% intramural, 3: contact the endometrium 100% intramural, 4: intramural 5: subserous =50% intramural, 6: subserous <50% intramural, 7: pedunculated subserous 8:other (e.g cervical, parasitic); HMB, heavy menstrual bleeding; NRS, numerical rate scale; CPP, chronic pelvic pain.
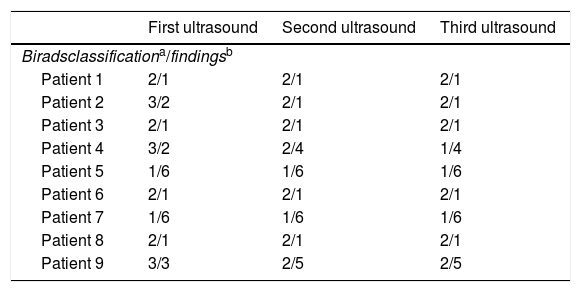
Breast ultrasound reports were normal for all the patients at the three time points (Table 2). The 3 breast ultrasounds were Birads 2 for 4 patients (44.4%); two patients (22.2%) had Birads 1 in the 3 ultrasounds and three patients (33.3%) had Birads 3 on the first ultrasound and Birads 2 on the last two.
Data of breast ultrasounds at the three time points.
| First ultrasound | Second ultrasound | Third ultrasound | |
|---|---|---|---|
| Biradsclassificationa/findingsb | |||
| Patient 1 | 2/1 | 2/1 | 2/1 |
| Patient 2 | 3/2 | 2/1 | 2/1 |
| Patient 3 | 2/1 | 2/1 | 2/1 |
| Patient 4 | 3/2 | 2/4 | 1/4 |
| Patient 5 | 1/6 | 1/6 | 1/6 |
| Patient 6 | 2/1 | 2/1 | 2/1 |
| Patient 7 | 1/6 | 1/6 | 1/6 |
| Patient 8 | 2/1 | 2/1 | 2/1 |
| Patient 9 | 3/3 | 2/5 | 2/5 |
0: incomplete, 1: normal study, 2: benign findings, 3: probably benign findings, 4: no classic malignancy, but suspicious enough to recommend biopsy, 5: highly suspicious findings, biopsy is recommend, 6: proven malignancy.
1: simple bilateral infracentrimetric cysts, 2: new benign nodule in the right breast, 3: new benign nodule in the left breast, 4: benign nodule already known (e.g. fibroadenoma, simple cyst) in the right breast, 5: benign nodule already known (e.g. fibroadenoma, simple cyst) in the left breast, 6: no nodules.
Liver profiles (assessed by monthly liver lab tests) were normal for all patients, and no one presented signs or symptoms compatible with liver damage. One patient had hot flushes in her first course with UPA, with spontaneous remission. There were not reporting of other adverse events.
DiscussionIn this pilot study we found that the use of UPA for the treatment of symptomatic fibroids did not showed any suspicious radiological change on breast ultrasound. In addition, we confirmed UPA efficacy in these patients, observing a reduction in the volume of menstrual bleeding as well as in the volume of the fibroid.
There are few studies that have assessed the safety of UPA in the breast tissue.12,13 Communal L. et al. evaluated UPA actions mediated by PR and glucocorticoid receptor (GR) in normal and cancer breast tissue samples xenografted in athymic mice. The analysis of PR and GR reporter gene transactivation and their respective endogenous target genes indicated that UPA exerted anti-progestational and anti-glucocorticoid activity in both types of cells with a more pronounced effect in cancer cells. This study concludes that UPA administration would not be deleterious to normal breast tissue at least for a course (28 days) of continuous administration.12
In Pohl et al. study, it was evaluated carcinogenetic properties of UPA in two different experimental models (mice and rats). Rats receiving UPA had decreased incidences of fibroadenomas and adenocarcinomas in the mammary gland; in mice, no tumor of any type increased at UPA exposures up to 313 times of therapeutic exposure.13
Interestingly, there are some studies that showed UPA inhibition of human breast cancer cell proliferation.14,15 Esber et al. in 2015 showed that UPA decreased cell proliferation and repressed BCL2-L1 expression, and anti-apoptotic factor that contributes cancer cell proliferation.14 The same group in 2016 evaluated the anti-tumor activity of UPA in a novel preclinical approach based on patient-derived breast tumor xenografted in nude mice and demonstrated that UPA treatment for 42 days led to a significant 30% reduction in tumor weight, accompanied by a significant 40% retardation in tumor growth.15
To our knowledge, this is the first study assessing the safety of UPA in the normal mammary gland in humans. These results suggest that UPA does not exert growth stimulation on normal breast tissue. In this regard, similar results were found in a previous study with mifepristone, another member of the SPRM family with a similar mechanism of action, which assessed its effects on the mammary gland and concluded that it is a potent anti-proliferative compound on normal breast cells.16
As expected, there was an improvement with regard to menstrual bleeding and symptoms in all patients, as well as a reduction in uterine fibroids size. Similar findings have been previously described in studies evaluating UPA treatment in patients with symptomatic fibroids.4–7,17
We believe that good point of our study was the assessment after several months and using not only one, but two different timepoints. Another strength of the present study was the strict inclusive and exclusive criteria, which ensured that only patients with uterine fibroids were analyzed. Additionally, the sonographic diagnosis was performed by the same two expert sonographers.
However, this study has several limitations. First, the safety in the mammary gland was assessed using breast ultrasound, but no anatomopathological samples were analyzed. This may underestimate the diagnosis. Second, we did not perform mammographies during the study as an established procedure, an essential technique when evaluating the mammary gland, especially in patients>40 years old: nevertheless, a recent mammography (one year prior to inclusion at the most) was required before the patient inclusion, and in case it could not be obtained, then a new one was performed. Finally, the sample size is too small, even for a pilot study, and the restricted use of UPA after the last European Medicines Agency (EMA) statements makes the possibility to perform a larger study in the future very low.18
In conclusion, our study showed that two cycles of treatment with UPA in patients with symptomatic uterine fibroids is safe for normal breast tissue. Further research is needed to confirm our findings.
Funding statementThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors declare that they have no conflicts of interest and nothing to disclose.