Time-lapse imaging in embryology is a recent and developing technology, which not only allows constant embryo monitoring but is also a promising non-invasive tool for embryo selection, as it permits the annotation of the embryo's kinetics throughout early development. Several external factors together with patient characteristics are reported as affecting embryo kinetics. Controversy still exists regarding whether sperm origin affects the timing of the embryo's developmental events evaluated by time-lapse monitoring. The aim of this study is to examine the effect of sperm origin on embryonic kinetics in IVF cycles.
Material and methodsA retrospective analysis of 161 IVF cycles between 2014 and 2020 were included. The morphokinetic parameters of 220 embryos obtained from couples with severe male factor infertility who underwent testicular sperm extraction (TESE), and 613 embryos from couples with fresh ejaculated spermatozoa were evaluated.
ResultsStatistically significant morphokinetic differences were observed between embryos from the TESE group compared to the normozoospermic embryos. In fact, 7 kinetic variables were eventually found to be relevant (p<.05).
ConclusionsThis study showed that embryos derived from testicular-retrieved spermatozoa presented delayed cell divisions, compared to ejaculated spermatozoa embryos.
La tecnología time-lapse en embriología es una metodología de aplicación reciente en los laboratorios de fecundación in vitro (FIV), que además de la monitorización continua de los embriones, permite realizar anotaciones sobre la morfología y la cinética del desarrollo embrionario que pueden emplearse en la selección de embriones. Se ha descrito que la cinética de los embriones en cultivo varía en función de múltiples factores, como la estimulación ovárica, los medios de cultivo empleados, las condiciones de cultivo, etc. Se desconoce cuál es el efecto paterno en la cinética embrionaria y si este efecto depende del origen de los espermatozoides. El objetivo de este estudio es evaluar el efecto del origen de los espermatozoides sobre la cinética embrionaria en los ciclos FIV.
Material y métodosPara ello, se analizaron retrospectivamente 161 ciclos de FIV entre los años 2014 y 2020. Se incluyeron 220 embriones de parejas con infertilidad por factor masculino severo que se sometieron a una biopsia para la recuperación de esperma testicular (TESE) y 613 embriones derivados del eyaculado fresco de varones normozoospérmicos.
ResultadosSe observaron diferencias estadísticamente significativas entre los embriones que se originaron en el grupo TESE, en comparación con los normozoospérmicos. De hecho, se encontraron siete variables cinéticas relevantes (p<0,05).
ConclusionesEste estudio muestra que los embriones derivados de espermatozoides recuperados por biopsia testicular presentan un patrón de división tardío, en comparación con los embriones provenientes de espermatozoides del eyaculado.
The disruption of infertility among the reproductive-aged population keeps rising year after year. It is reported that 20% of couples of reproductive age are affected by this matter.1 From those, it is estimated that around 40% are exclusively because of female factor, 40% because of male factor and the 20% remaining due to both members presenting infertility issues. At the end, the male factor may contribute in 50% of the described infertility causes.2 Over time, the study of the infertility causes has focused mainly on the study of women, who are monitored by exhaustive tests to determine the etiology of subfertility, while the study of men is usually more superficial and mostly limited to the study of semen.3 Indeed, the development of Intracytoplasmic sperm injection (ICSI) in 1992 has drastically decreased the impact of male factor, resulting in millions of pregnancies worldwide for couples who, without ICSI, would have had little chance of having their own biological child.4 However, this fertilization technique has left the study of the involved molecular mechanisms behind male factor infertility in a second place.
Sperm role in embryo developmentContrary to the widespread idea that spermatozoa play a role just as a delivery machine of the male genome to the oocyte, high-throughput techniques have demonstrated that sperm cells carry and transfer a wide range of other molecules such as proteins and RNAs to the female gamete, which not only influence embryogenesis but also offspring development and health.5,6 In fact, paternal inheritance is observed regarding the mitotic potential and nuclear syngamy that the embryo presents.7 Concretely, the sperm cell contributes with the centriole–centrosome complex, which has been reduced during spermatogenesis, but it has retained the functional proximal centriole that is crucial for sperm aster formation and for the subsequent bipolar mitotic apparatus that forms after centriole duplication during the pronuclear stage, which will be critical for subsequent embryo cleavage.8 Actually, all microtubule formations throughout early development depend on sperm-derived centriolar integrity. It is reported that a defective centriole–centrosome complex inherited by a human oocyte may cause abnormal chromosome separation, inducing genomic instability and consequently, compromising embryo development, with embryonic aneuploidy, polyploidy, or mosaicism.9,10
Moreover, it is described how sperm also supplies the oocyte-activating factor, the sperm-specific phospholipase C (PLCζ), responsible for calcium oscillations prompting the initiation of the mitotic cycle.11 Other paternal factors such as sperm proteome influence epigenetic regulation of fertilization and early human embryo development.12
From a clinical perspective it has been described that couples affected by male factor infertility present lower rates of blastocyst formation and fertilization.13 Furthermore, a high frequency of chromosomal abnormalities has been described in testicular-retrieved sperm from men with severe male factor infertility compared to normal population.14
Time-lapse technology in the IVF laboratoryA major challenge that still remains to be solved in in vitro fertilization (IVF) cycles is the ability to non-invasively select the most competent embryo. With the introduction of time-lapse technology in IVF incubators, embryos have been settled to a constant monitoring throughout the acquisition of images, taken in pre-set intervals in multiple focal planes, providing not only a constant embryo culture but also more dynamic information about embryo development. Recently, early embryonic cell divisions have been regarded as a reflection of embryo competence.15 In this way, several authors have evaluated the kinetic parameters from the time-lapse systems as a new non-invasive approach of embryo selection prior to transfer.16 For example, some groups have correlated kinetic information to embryo ploidy status or implantation potential.17–22 However, as it has been emerging during these years of intense research, embryo kinetics may be influenced not only by inherent embryonic traits and patient characteristics but also by external factors such as the culture environment.23
In the case of the male factor, it has been suggested that type and quality of sperm injected during ICSI can affect embryo kinetics. Neyer and colleagues (2015) evaluated embryo morphokinetics and blastocyst development for distinct classes of sperm cells from a patient cohort that combined patients with male factor infertility together with recurrent implantation failures. Two-thirds of the embryos evaluated did not fulfill optimal morphokinetic criteria, and good-quality spermatozoa tended to prompt more high-quality blastocysts despite of how many morphokinetic criteria were accomplished.24 Moreover, one study reported that sperm DNA fragmentation was associated with slower embryo cell divisions.25 In the same line, Casanovas and colleagues (2018) compared kinetics from embryos obtained from semen samples with low and high single- and double-stranded sperm DNA fragmentation, ssSDF and dsSDF, respectively.26 Different patterns of delay in embryo kinetics were observed for these different types of DNA damage: dsSDF caused a delay along all stages of embryo development; however, its major effect was observed at the second polar body extrusion and morula stages. Additionally, Mangoli and colleagues (2020) described an association between some time-lapse parameters with sperm transcript levels of genes involved in apoptosis.27 Furthermore, another group assessed the impact of severe male factor infertility on embryo chromosomal status and potential association between chromosomal status and embryo morphokinetics. Higher incidences of direct uneven cleavage and slow-growing or arrested embryos were found in patients using testicular-extracted spermatozoa compared with the normozoospermia group. Nevertheless, in the testicular-retrieved sperm group, those embryos that reached the blastocyst stage did so significantly faster than the ones from the control group that presented normal sperm parameters.28 Conversely, other studies reported no impact of male factor infertility29 or the use of testicular spermatozoa23 on embryo morphokinetics, and no correlation between male age and embryo morphokinetics up to day 3 of development.30
As the consequences of male-factor infertility on IVF outcomes are still contradictory, especially in the morphokinetics field given the ease of kinetics to be affected by the extensively reported confounding factors; this study aims to shed light in this matter comparing embryo dynamics between embryos deriving from ejaculated spermatozoa and TESE spermatozoa from men with severe male factor infertility, in an homogeneous study population regarding the female partner and culture conditions.
Materials and methodsStudy designThe study population consisted of women undergoing intracytoplasmic sperm injection (ICSI) who were ≤35 years old at the moment of the IVF cycle, presenting ≤25.5kg/m2 of body mass index (BMI). They were allocated to two groups according to the sperm source and analysis: normal ejaculated spermatozoa according to WHO criteria (World health organization 2010, 5th edition) (N=116 couples) and severe male factor infertility (<1M/ml), reason for which biopsy testicular sperm extraction (TESE) was performed (N=45 couples). Morphokinetic data from zygotes cultured in the EmbryoScope time-lapse chamber were retrospectively reviewed. Data collection for this study was approved by our Institutional Review Board.
Ovarian stimulation and embryo culture conditionsOvarian stimulation protocol was stablished according to patient age, serum anti-Mullerian hormone levels and antral follicle count. Women were treated with either a gonadotropin releasing hormone (GnRH) agonist or antagonist to suppress ovulation until follicle maturity was attained. Final follicular maturation was triggered with human chorionic gonadotrophin and/or a GnRH agonist when at least two lead follicles measured 18mm in mean diameter. Oocytes were collected 36h later by transvaginal ultrasound-guided needle aspiration of follicles.
Sperm handling proceduresAfter 3–5 days of sexual abstinence, semen samples were collected by masturbation and analyzed according to WHO (2010) guidelines. For TESE, freshly retrieved tissue was washed with the use of buffered medium (HEPES, LifeGlobal®). After this, it was disrupted with the use of a tissue homogenizer to obtain a suspension of small pieces. The suspension was then centrifuged at 1500rpm for 10min, and the pellet was assessed under the inverted microscope to evaluate the presence of sperm.
Time-lapse incubator and embryo kinetic analysesInjected oocytes by ICSI were individually cultured in a 12-well culture dish (Embryoslide, Vitrolife), filled with of continuous single-culture media (Global total®, LifeGlobal) and overlaid with mineral oil (OVOIL®, Vitrolife), until day five of embryo development. The time-lapse-monitored incubator (EmbryoScope, Vitrolife) was set at 37°C, 6% O2 and 7% CO2. Images were captured at seven different focal planes in 10min intervals. Morphokinetic parameters were annotated until D+5; however, only kinetic parameters until D+3 were taken into account in this study. Kinetic data from embryo development were based on time series of the following discrete events in the hours post-ICSI as previously defined: time of second polar body extrusion (tPB2), time of pronuclei appearance (tPNa), time to pronuclear fading (tPNf), time of division to 2, 3, 4, 5, 6, 7, 8 cells (t2, t3, t4, t5, t6, t7, t8) and some relevant intervals reported in the literature (S2,S3,cc2). The process of annotating embryo kinetics was performed following the good practice recommendations of ESHRE Working group on Time-lapse technology.31
Statistical analysisA descriptive analysis of the kinetic parameters between the studied groups was performed. Continuous data are presented as absolute values, mean, standard deviation (SD). Categoric variables are presented as absolute values, percentage, and 95% confidence interval (CI). To assess the actual distinctness between the variability of the two groups, a Levene's test or Fligner–Killeen for equality of variances was first performed. According to the result obtained in the previous test, and in order to test the two population means, a classic t-test or corrected t-test for unequal variances (Welch two sample t-test) was done. Mann-Whitney U test was used to assess whether the median values in the exact timings of morphokinetic parameters were significantly different between normal and severe male factor groups when a normal data distribution was not followed. A p-value of ≤0.05 was considered to denote a statistical significant difference. All analyses were performed by R software v4.0.5.
ResultsA total of 833 embryos were analyzed, 613 embryos included in the control group, and 220 embryos in the TESE group. Demographic characteristics for patients having ICSI with TESE sperm or normozoospermic fresh ejaculated sperm are shown in Table 1. The paternal mean age of patients included in the study was comparable between TESE and normozoospermic groups (36.818±5.457 vs 36.338±4.361, respectively). In the same line, no statistically significant differences were found in the studied ovarian reserve markers, neither basal FSH, E2 nor antral follicle count (AFC). Conversely, the normozoospermic group had a higher maternal age compared to the TESE group (32.901±2.476 vs. 31.727±2.476, respectively; p<0.009).
Demographics of patients in the TESE and normozoospermic treatment groups. p-value <0.05 considered to be statistically significant. Note: data are mean±SD unless stated otherwise.
| TESE | NORMO | p-value | |
|---|---|---|---|
| Maternal age (years) | 31.727±2.912 | 32.901±2.476 | 0.009 |
| Maternal BMI (kg/m2) | 22.237±1.947 | 22.801±2.388 | 0.132 |
| Paternal age (years) | 36.818±5.457 | 36.338±4.361 | 0.549 |
| Retrieved oocytes | 10.520±4.870 | 9.710±3.350 | 0.685 |
| MII rate (%) | 84.570±12.846 | 85.989±14.177 | 0.390 |
| Fertilization rate (%) | 70.280±22.460 | 72.740±20.610 | 0.497 |
| Basal fsh (UI/L) | 7.810±2.697 | 7.209±3.733 | 0.356 |
| AFC (n follicles) | 15.450±10.380 | 17.550±10.000 | 0.137 |
| E2 (pg/mL) | 1970.450±859.020 | 1873.070±902.850 | 0.705 |
Statistically significant outcomes (bold) for p<0.05.
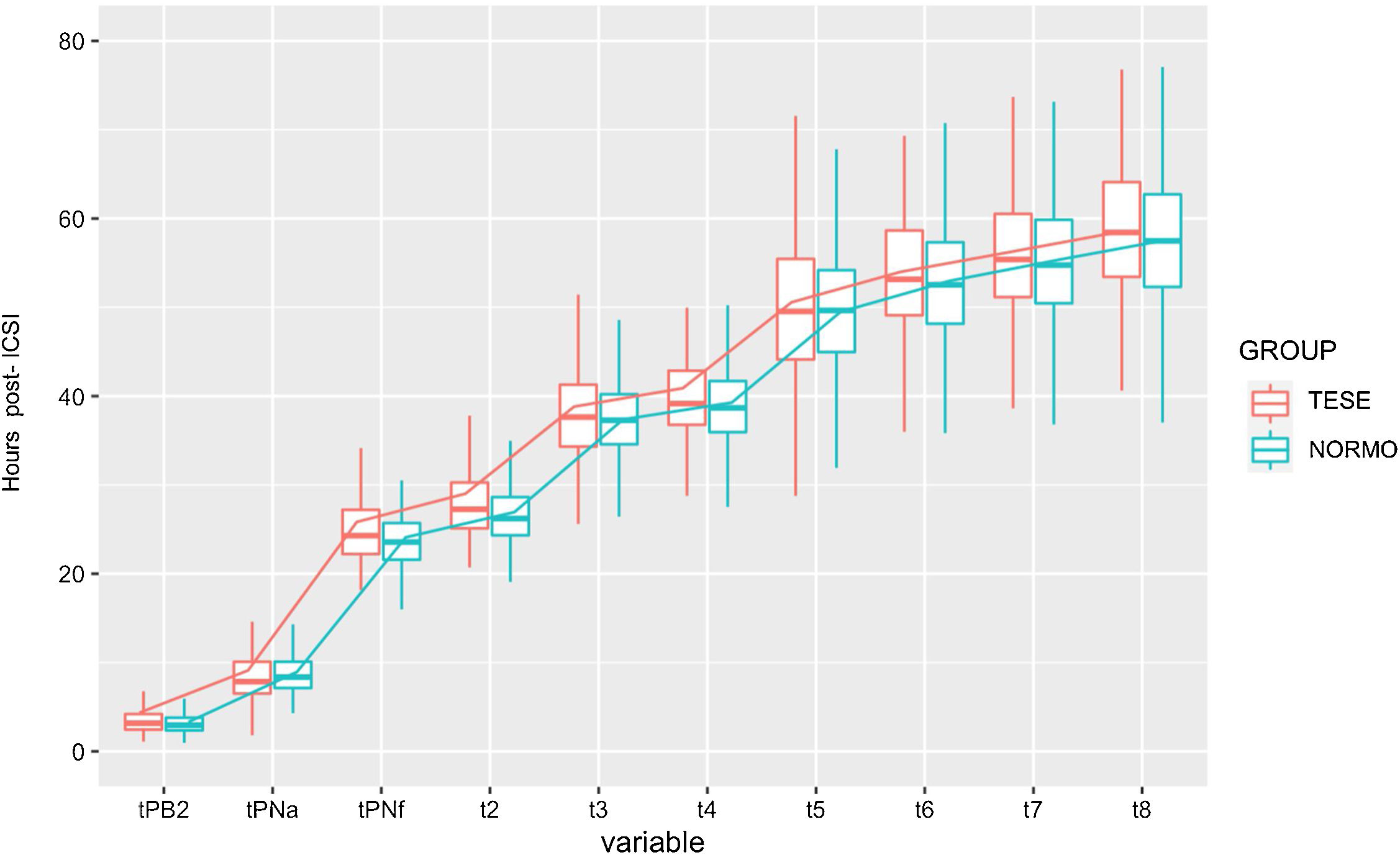
Concretely, a descriptive analysis of the kinetic parameters was made between the groups studied. Regarding embryo kinetics, Table 2 shows a brief descriptive of outcomes obtained in single variable analysis between the groups. Some kinetic variables were found to differ from both mean and standard deviation (SD), while others just present dissimilarities in mean or SD suggesting the complexity when dealing with this type of data. Indeed, most assessed distributions of the distinct morphokinetic parameters (and the pairwise differences variables) from severe male factor embryos were characterized by significantly larger variances than the distributions of variables from the normozoospermic group variables (Table 2). Embryos derived from testicular sperm appeared to be impaired in the first steps of early development. TESE embryos were slower to extrude the second polar body (95%CI 0.0382–0.383), to appear (95%CI 0.805–0.085) and disappear (95%CI 0.333–1.416) the pronuclei. In fact, the first division, t2, was delayed occurring approximately 2h later in the embryos originating from testicular sperm (95%CI 0.527–1.643; p<0.05). Furthermore, the 4 cell-stage variable was similarly delayed in the testicular sperm group (95%CI 0.068–1.505; p<0.05).
Summary of mean and standard deviation (SD) values of kinetic variables between normozoospermic (NORMO) and severe male factor (TESE) groups. Most variables differ in variability but slightly in mean values. Results are expressed mean±SD in hpi (hours post insemination).
| TESE (n=220) | NORMO (n=613) | 95% CI | p-value | ||
|---|---|---|---|---|---|
| Low | Upper | ||||
| tPB2 | 4.350±4.996 | 3.298±1.551 | 0.038 | 0.383 | 0.017 |
| tPNa | 9.115±5.023 | 8.952±2.719 | −0.805 | −0.085 | 0.015 |
| tPNf | 25.833±7.238 | 24.103±3.872 | 0.333 | 1.416 | 0.002 |
| t2 | 29.016±6.756 | 26.941±4.171 | 0.527 | 1.643 | <0.0001 |
| t3 | 38.818±7.709 | 37.445±5.359 | −0.3171 | 1.2596 | 0.251 |
| t4 | 40.890±7.013 | 39.340±5.594 | 0.068 | 1.505 | 0.029 |
| t5 | 50.810±9.808 | 49.542±7.417 | −0.725 | 1.857 | 0.402 |
| t6 | 54.699±9.727 | 53.015±7.133 | −0.120 | 2.296 | 0.079 |
| t7 | 57.159±10.254 | 55.617±7.800 | −0.351 | 2.223 | 0.158 |
| t8 | 59.709±9.420 | 58.043±8.262 | −0.195 | 2.859 | 0.091 |
| t8-t5 (S3) | 10.894±7.419 | 9.343±7.605 | 0.426 | 2.777 | 0.006 |
| t4-t3 (S2) | 2.504±3.916 | 1.906±3.404 | −0.006 | 1.202 | 0.233 |
| t3-t2 (cc2) | 9.764±4.840 | 10.609±4.146 | −1.521 | −0.172 | 0.002 |
Hours post-ICSI.
95% CI: 95% confidence interval.
Statistically significant outcomes (bold) for p<0.05.
From the interval morphokinetic variables, most parameters were found to be unequal in regard to variance between the two groups (Table 2). Besides, differences in mean were also confirmed. Interestingly, the delayed pattern observed in the interval morphokinetic variables of the embryos deriving from testicular sperm was mainly observed at S2 (t8–t5) (p<0.05) and t3-t2 (cc2) (p<0.05). In general, TESE embryos presented a delayed pattern of cell divisions as represented in Fig. 1.
DiscussionThe application of time-lapse technology for embryo monitoring, especially for cases with underlying male factor infertility, may be a useful and non-invasive tool in understanding where and how specific sperm origin and possible defects influence human embryo development. The idea of correlation between embryo kinetics and viability relies on the existence of cell cycle checkpoints that monitor the progress of the whole process.32,33 Concretely, these are stages at which the completeness of the prior phases of the cell cycle is examined. Given an alteration, the cycle is delayed, arrested or resumed according to the severity level of the problem. Consequently, a slower developmental rate could be the result of an auto-defense mechanism against DNA damage or aneuploidy.32 Particularly, our results showed delayed dynamics of early embryo development. One possible explanation for this outcome could be the increased aneuploidy reported in testicular sperm cells that could alter the normal dynamics of cell cycle as some authors stated.34 However, further molecular research to elucidate this fact.
In the same line, Desai and colleagues also determined a slowed rhythm of embryo development, as in cc2 (t3–t2), when male factor infertility is present in the couple. Other studies also suggested that normal ejaculated spermatozoa reached the later morphokinetic events such as synchronous division (S3, t8–t5) and time to morula (tM), faster than embryos obtained from testicular retrieved spermatozoa.32
Immaturity of testicular sperm may be expressed as deficits in sperm centrosome function, sperm aster formation or/and sperm methylation necessary for full function.35 Furthermore, it is known that the acquisition of motility occurs during sperm transit through the epididymis. In fact, some authors showed lower odds of live birth with testicular sperm, suggesting that passage through the epididymis was essential for proper sperm maturation.36 Recently, substantial changes during testicular and post-testicular maturation involving the small RNA payload of sperm have been also described. Actually, these RNAs are modulated by paternal environmental conditions and potentially delivered to the zygote at fertilization, where they can regulate early embryonic development.37 The reported molecular differences between testicular sperm and fresh ejaculated sperm could explain the impairment of embryo dynamics in early embryo development between the studied groups. However, further research is needed to elucidate the concrete mechanisms behind this delay in humans. Kinetic differences are found among viable embryos from severe male factor couples compared to normozoospermic ones, especially in syngamy involved parameters such as tPB2, tPNa, tPNf or t2. As all female patients included in the study were younger than 35 years old and all ovarian reserve factors analyzed were comparable between groups, the slight difference found in maternal age may not explain the general delayed dynamics in TESE embryo group. Thus, caution should be taken regarding sperm origin when constructing predictive models based on embryo kinetics, as variables reported as predictors of blastocyst formation38 such as t8–t5 are found to be delayed in TESE patients. Our findings provided evidence that a paternal impact exists on embryo dynamics according to the origin of sperm cells used during fertilization; although the clinical relevance clearly needs further investigation not only in a prospective way, but also with a larger population of individuals. Nonetheless, our results may not be generalizable to patients under-represented in our clinic population or laboratories with distinct environmental conditions, given the susceptibility of embryo kinetics to external conditions. Future projects should also assess the impact of male hormonal status, together with sperm morphology such as head versus flagellum defects or DNA fragmentation on embryo kinetics. Further research is needed, not only to keep deciphering the complexity of kinetic data but also the possible molecular mechanisms behind these kinetic disparities in embryo development.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis research has not received specific aid from public sector agencies, commercial sector or non-profit entities.
Conflict of interestThe authors declare that they have no conflict of interest.