Intravenous rescue analgesia in the postoperative anesthesia care unit (PACU) is the most effective method for reducing postoperative pain (POP) when perioperative multimodal analgesia fails to control it. Appropriate analgesia during these first postoperative hours may prevent morbidity associated with pain.
ObjectiveTo compare the effectiveness of intravenous morphine versus fentanyl in the PACU for reducing severe POP.
MethodsRandomized, prospective, double blind trial that included patients with severe POP using VAS in the PACU. Rescue was performed on one group with 01mg/kg morphine and with another with 1mcg/kg of fentanyl every 5min intravenously until pain was reduced from severe to mild (VAS<4). 30 patients were included in both groups.
ResultsThere were no significant differences in the percentage of patients with reduction of severe POP to mild 5min after the injection of morphine or fentanyl, or in the subsequent rescue analgesia intervals (p>0.05). Similarly, there were no significant differences in mean VAS (95% CI) in morphine or fentanyl groups beginning 5min after the first analgesic dose (p>0.05) between the groups.
There were no significant differences in side effects such as respiratory depression, nausea, vomiting or pruritus (p=1.0). There was a high satisfaction in both groups (p>0.05).
ConclusionsMorphine and fentanyl were equally effective in treating severe POP after 5min and following intervals after rescue analgesia was initiated, during 25min at PACU, with no differences in efficacy or adverse effects between groups Register # NCT02145975 clinicaltrials.gov, prospective.
La analgesia intravenosa de rescate en la unidad de cuidados postanestesicos (UCPA), es la forma más efectiva de reducir el dolor POP, cuando la analgesia mutimodal perioperatoria falla en controlarlo. Una adecuada analgesia en las primeras horas previene la morbilidad asociada al dolor.
ObjetivoComparar la efectividad para reducir el dolor POP severo de fentanilo versus morfina en recuperación postanestésica.
MetodologíaEstudio aleatorizado, prospectivo, doble ciego, en pacientes con dolor severo POP medido con la escala EVA. El rescate se hizo con un grupo morfina a 0,1mg/kg versus fentanilo a 1 mcg/kg, cada 5 minutos, vía intravenosa, hasta reducir el dolor de severo a leve (EVA <4). Se incluyeron 30 pacientes en el grupo morfina y 30 en el grupo fentanilo.
ResultadosNo se observaron diferencias en porcentaje de pacientes con reducción del dolor severo a leve desde los 5 minutos luego del rescate entre morfina o fentanilo, ó en los intervalos restantes (p>0,05). Similarmente, no se encontraron diferencias significativas en la media de EVA (IC 95%) desde los 5 minutos luego del rescate (p>0.05) entre los grupos.
No hubo diferencias en efectos adversos como depresión respiratoria, náuseas, vómitos o prurito entre grupos (p=1,0). La satisfacción fue comparable en ambos grupos (p >0,05).
ConclusionesLa morfina y el fentanilo fueron igualmente efectivos para el rescate en dolor severo desde los primeros 5 minutos, sin diferencias en los efectos adversos en ambos grupos. Registro # NCT02145975 (clinicaltrials.gov,prospectivo).
Sever postoperative pain (POP) is a problem with high incidence worldwide. Per a systematic literature review performed by Dollin and collaborators, an incidence of moderate to severe pain was found in 41% of patients in the postoperative period. Of these, only 23% experience relief.1 In Latin America, severe POP has been reported in 48% of surgical patients, and 51–60% reported some level of pain these units.2,3 In our Colombian context, the situation is similar, with a prevalence of 22.3% of severe static pain and 48.2% of dynamic pain.4 These numbers reflect insufficient management in postanesthetic care units (PACU), also called postoperative care units.
The incidence, intensity and duration of postoperative pain varies considerably among patients, types of surgical intervention, hospitals, and even countries.5 Pain related to the postoperative period is not only important because it causes suffering or unpleasant experiences to the patient but also because of the damaging effects that it implies for multiple organs.6 In this way, stress because of pain together with surgical trauma and previous morbidity of the patient causes cardiovascular,7,8 gastrointestinal6,9–11 and respiratory12,13 dysfunction, among others. This increases the incidence of myocardial ischemia, atelectasis (25–75% after abdominal surgery), respiratory infection (pneumonia in 1–3% of patients after heart surgery), ileus, deep vein thrombosis, and cognitive dysfunction. In the same way, it has been observed that pain increases PACU stay times and the number of re-admittances through emergency for pain management. In this way, patient recuperation is delayed and morbimortality increases along with the costs of patient care.7
It is important to note that in the PACUs, when we face a patient with severe or unbearable pain,14,15 the titration of opioid analgesics is the most effective strategy for controlling postoperative pain.16,17
The most studied and used opioid currently in PACUs for analgesic rescue is morphine, which offers a good balance between the speed of the onset of action and the maintenance of the analgesia due to its pharmacokinetic properties.18 However, there are alternatives for analgesic titration, such as fentanyl, which has favorable pharmacokinetics since theoretically it has a faster analgesic response than morphine since it is lipo-soluable, facilitating its passage across the blood–brain barrier.19 Because of this, the relief of sever postoperative pain in theory may be more rapid with options like fentanyl when compared to morphine.15,16 The current study compares the effectiveness of these two opioid analgesics against severe postoperative pain at equipotent doses in 5min intervals, the time considered clinically relevant for the reduction of severe pain in postoperative analgesia.
Materials and methodsWith previous approval by the institutional Ethics Committee, an experimental, randomized, prospective, double blind study was performed in which two potent analgesic opioids were compared for severe postoperative pain, in the Hospital Universitario San Vicente Fundación.
Patients with severe postoperative pain (VAS≥7) in the PACU were selected. Two groups were formed in the following way: Group 1 was given intravenous morphine at a dose of 0.1mg/kg; Group 2 was given intravenous fentanyl at a dose of 1mcg/kg. As many doses as necessary were administered every 5min until pain was reduced from severe to mild (VAS<4).
The titration intervals were determined based on the work of Grass and collaborators yielding an onset of action of fentanyl of 4min and 8min for morphine with a variation of onset of 3min for the first drug and 5min for the second.20 After the intravenous administration of morphine to anesthetized dogs, it was detected in the cerebrospinal fluid after 2–5min and maximum concentrations were observed within 15–30min.21 The primary outcome was to determine if the analgesic rescue with fentanyl is more effective in reducing severe POP (according to the VAS) in comparison with the use of morphine in the PACU. Assuming a difference of 1.3 points in the averages of the VAS between the two groups as clinically significant, with a standard deviation of 3 on the VAS, a level of significance of 0.05 and a potency of 90%, the required sample size is 22 participants per group. This was increased by 20% for the possibility of losses, which would be equivalent to a sample of 53 participants. We rounded this up to 60 (30 per group). The calculation was performed using the program STATA 10, and the patients were randomly assigned into one of the two groups. Each group had 30 participants. All patients that complied with the inclusion criteria were selected, these criteria being: age between 18 and 65 years, admitted for urgent or elective non-ambulatory surgery under anesthesia and presenting severe postoperative pain in the PACU, having undergone a preanesthetic assessment, not being pregnant, not having a background of hypersensitivity or allergic reactions to opioids, having signed the informed consent form for voluntary participation in the study, and not having a risk of ASA >III, hypoxemia (oxyhemoglobin saturation <90%), hemodynamic instability, unexplained alteration of alertness due to anesthetic effects, or basic neurological alterations due to psychiatric disorders. These disorders could include anxiety disorders, mental retardation, congenital, metabolic, hypoxic-ischemic neurodegenerative states, or disorders related to aging that would not allow for a proper evaluation on the visual analog scale for pain assessment or a background of tolerance to opioids due to chronic consumption (defined as the use of opioids for a period longer than two weeks).
ProcedureBefore recruitment of patients began, a meeting was held with the nursing personnel and other auxiliary staff for surgical services for their training in the purposes, reach, and forms of implementation of the study so that it could be executed properly. In this meeting, all of the ethical considerations and the documentation corresponding to the clinical trial were presented.
An implementation pilot test was performed before initiating recruitment with 5% of the calculated sample to detect faults in the different phases of the study and to correct them.
An analgesic dose was applied to each patient randomly. 60 numbers were randomized with a randomized number generator, with a minimum value of 1 and maximum value of 2. 30 envelopes were marked with the number 1 and 30 with the number 2. A research assistant, who was not part of the research group, put one of the analgesic guidelines into the envelopes marked with the number 1 and put the other guidelines into the envelopes marked with the number 2. In this way, the information about the medication to use, along with the instructions for its preparation, were hidden. Next, the envelopes were marked with the number from 1 to 60 in accordance with the table of randomized numbers. As a patient was selected, the guidelines that corresponded to him or her by sequential order were applied by a nursing assistant of the PACU who was not part of the study. In this way, the researcher and the patient were unaware of the content of the medication when it was applied. At the end of the study, the substances used were revealed.
When the patient arrived to the recovery room, they were evaluated with the visual analog scale for pain. If pain was above 7, the envelope from the sequence was taken and opened to assign the intervention. The opaque, sealed and sequentially numbered envelopes were opened by a nursing assistant who was not part of the study. The envelopes contained information about the medication that would be applied to the corresponding patient with preparation instructions (morphine 10mg in up to 10ml of 0.9% saline, held in a 10ml syringe, or 100mcg fentanyl in up to 10ml of 0.9% saline, held in a 10ml syringe). This paper with instructions was discarded. The envelopes also contained a number (1 or 2) that corresponded to one of the two interventions and that the researcher recorded in the survey. In this way, the researcher did not know which intervention had been applied. The nursing assistant applied the medication at a dose of 0.1ml/kg of weight, following the researcher's instructions. The medication was prepared in identical syringes and at equi-analgesic concentrations in accordance with protocol. After the administration of the medication, pain measurement was begun in 5min intervals in accordance with the VAS. This was recorded in a table and another dose of medication was administered if pain was >3 until the patient manifested having a pain level below 4 (mild pain, per the VAS). The total quantity of medication used in the intervention in ml was then recorded.
The sociodemographic data, type of surgery, and the duration of surgery were recorded. Also recorded was the time spent in the recovery room in minutes.
Also, the presence of secondary effects from the opioids was evaluated (hypotension, bradycardia, respiratory depression, nausea, vomiting, pruritus).
Furthermore, a Likert-Style Satisfaction Scale was used to subjectively evaluate how satisfied each patient was with the control of their postoperative pain as soon as the intervention was started by giving a score of 1 to refer to complete satisfaction, of 2 if they were simply satisfied, a score of 3 if they were neither satisfied nor dissatisfied, and 4 if they were completely dissatisfied.
Data analysisThe count of the categorical variables was reported in absolute numbers and in percentages. The differences in the base characteristics and post intervention variables were calculated with hypothesis testing. For qualitative variables, the chi-squared test and Fisher's exact test were used as appropriate. The assumption of normality for the analysis of outcomes was not fulfilled, so the Mann–Whitney test was used. The base characteristics of both groups will be presented in a table through measures of frequency and proportions for qualitative variables and measures of central tendency and dispersion for quantitative variables. In quantitative variables, normality was evaluated with the Shapiro–Wilk test and group comparison with ANOVA. This analysis was supported with SPSS software version 2.0.
Ethical aspectsThe study complies with the Helsinki Declaration and the norms in force in Colombia (Resolution No. 008430 of 1993 of the Ministry of Health and Resolution No. 2378 of 2008 about good clinical practice). No actions were taken that would not comply with the ethical proposals recorded therein. An evaluation by the Ethics Committee of the University Hospital where the study took place was required. The committee gave their approval to proceed with the development of the study and patients were asked individually to provide informed consent.
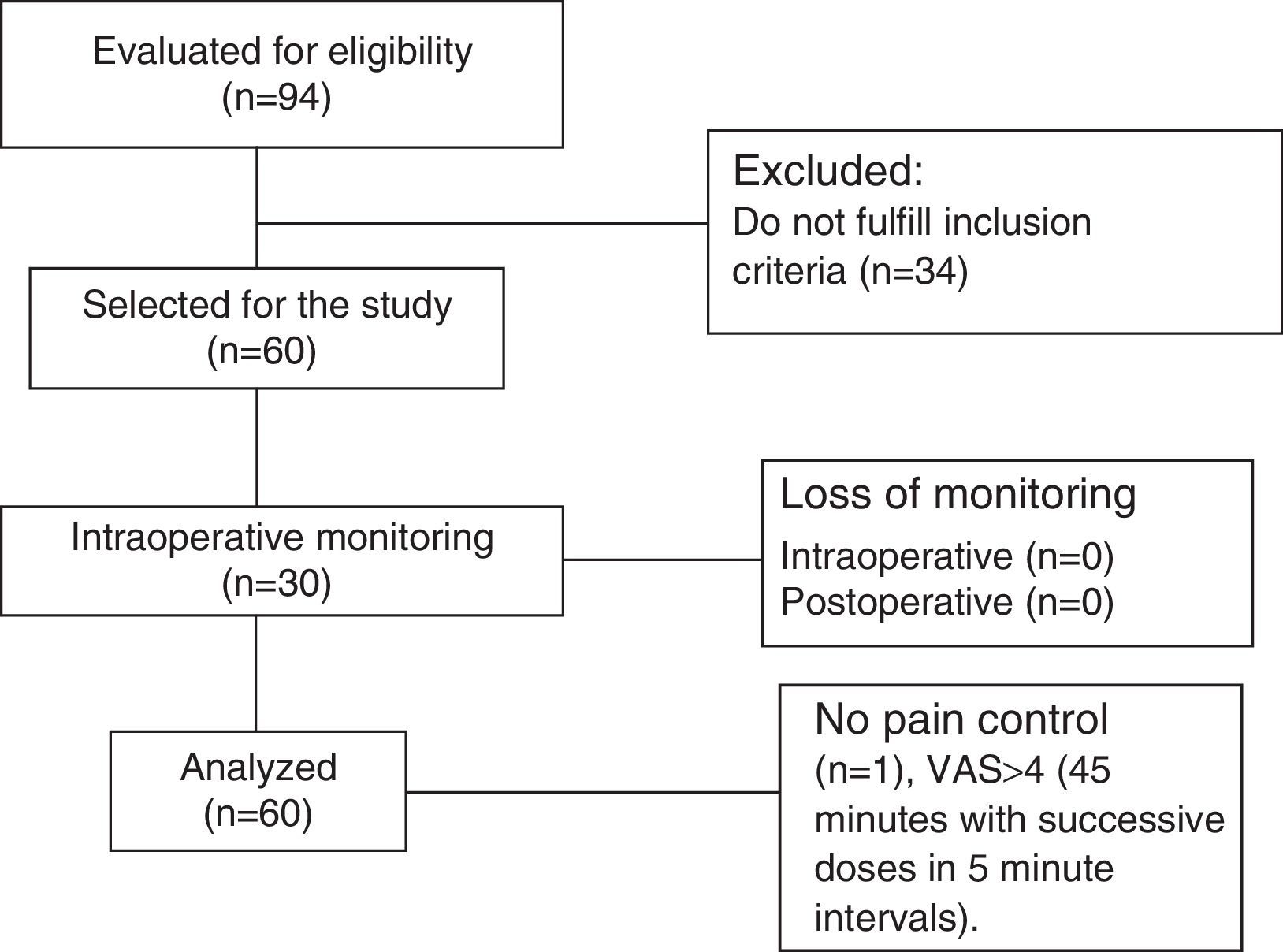
ResultsA total of 94 patients complied with the selection criterion of presenting severe pain in the PACU. Of these, 60 patients complied with the inclusion criteria and did not comply with any exclusion criteria, yielding 30 patients per intervention group (Fig. 1).
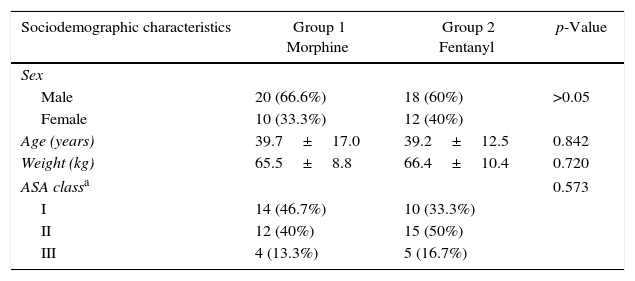
The two groups were similar in sex, age, weight, ASA class (p>0.05). As an exclusion criterion, patients had to have an ASA class>III (Table 1). The most frequent procedures were orthopedic surgery (28 patients) and general surgery (15 patients). There were also procedures of thoracic surgery (6 patients), plastic surgery (4 patients), otorhinolaryngology (3 patients), vascular surgery (2 patients), and maxillofacial surgery (2 patients). The majority were elective procedures, with no statistically significant difference between the two groups (p=0.165).
Sociodemographic characteristics of the population.
| Sociodemographic characteristics | Group 1 Morphine | Group 2 Fentanyl | p-Value |
|---|---|---|---|
| Sex | |||
| Male | 20 (66.6%) | 18 (60%) | >0.05 |
| Female | 10 (33.3%) | 12 (40%) | |
| Age (years) | 39.7±17.0 | 39.2±12.5 | 0.842 |
| Weight (kg) | 65.5±8.8 | 66.4±10.4 | 0.720 |
| ASA classa | 0.573 | ||
| I | 14 (46.7%) | 10 (33.3%) | |
| II | 12 (40%) | 15 (50%) | |
| III | 4 (13.3%) | 5 (16.7%) | |
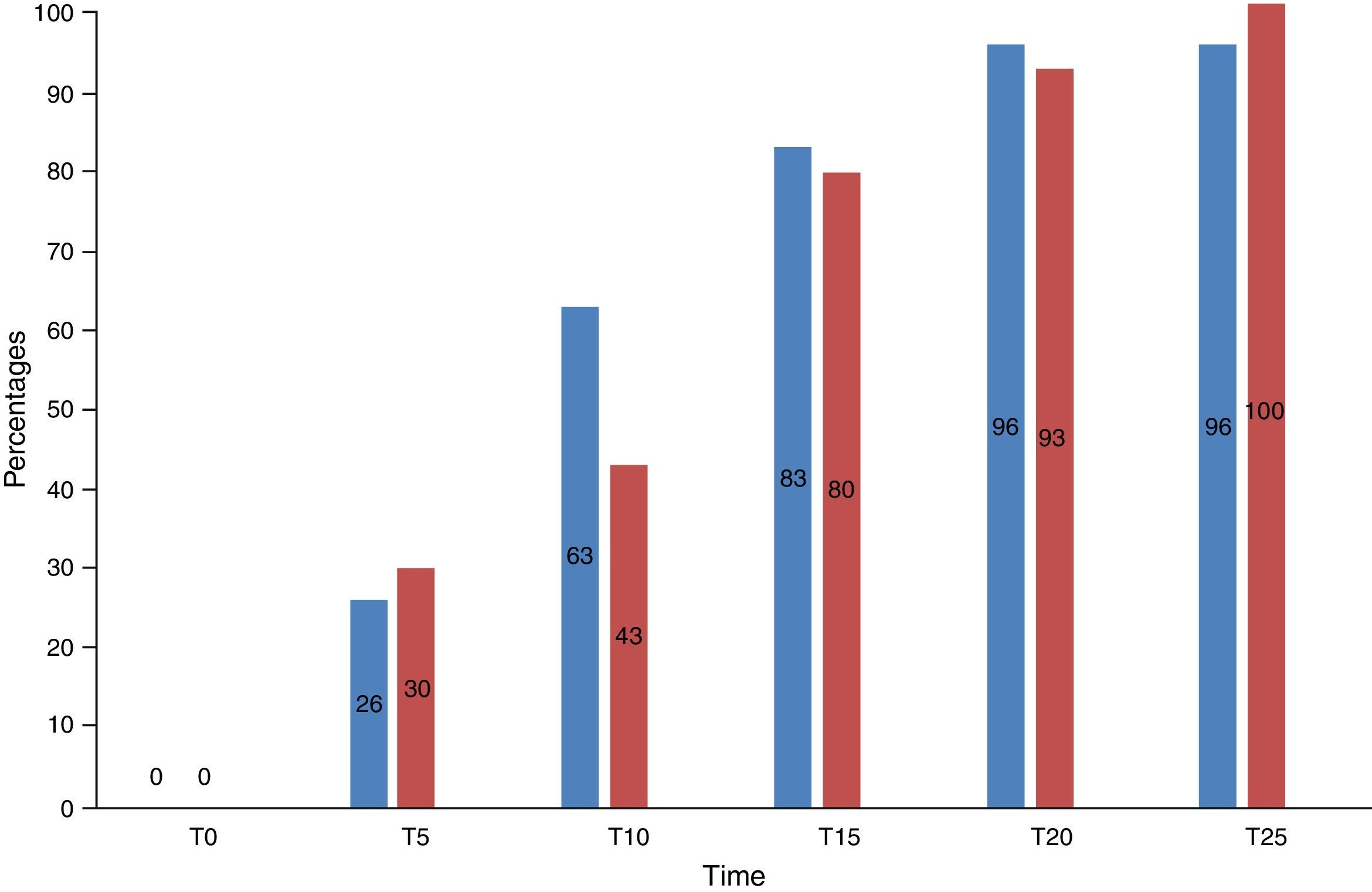
30 patients were included in the morphine group and 30 patients in the fentanyl group. 26% (n=8) versus 30% (n=9) of the patients in the morphine and fentanyl groups respectively felt mild pain within 5min of the first dose (p>0.05). Similarly, 63% (n=19) versus 43% (n=13) of the patients in the morphine and fentanyl groups respectively felt mild pain after 10min (p>0.05). In the following intervals, up to 25min after the first titration, no significant differences between the groups were observed (Fig. 2).
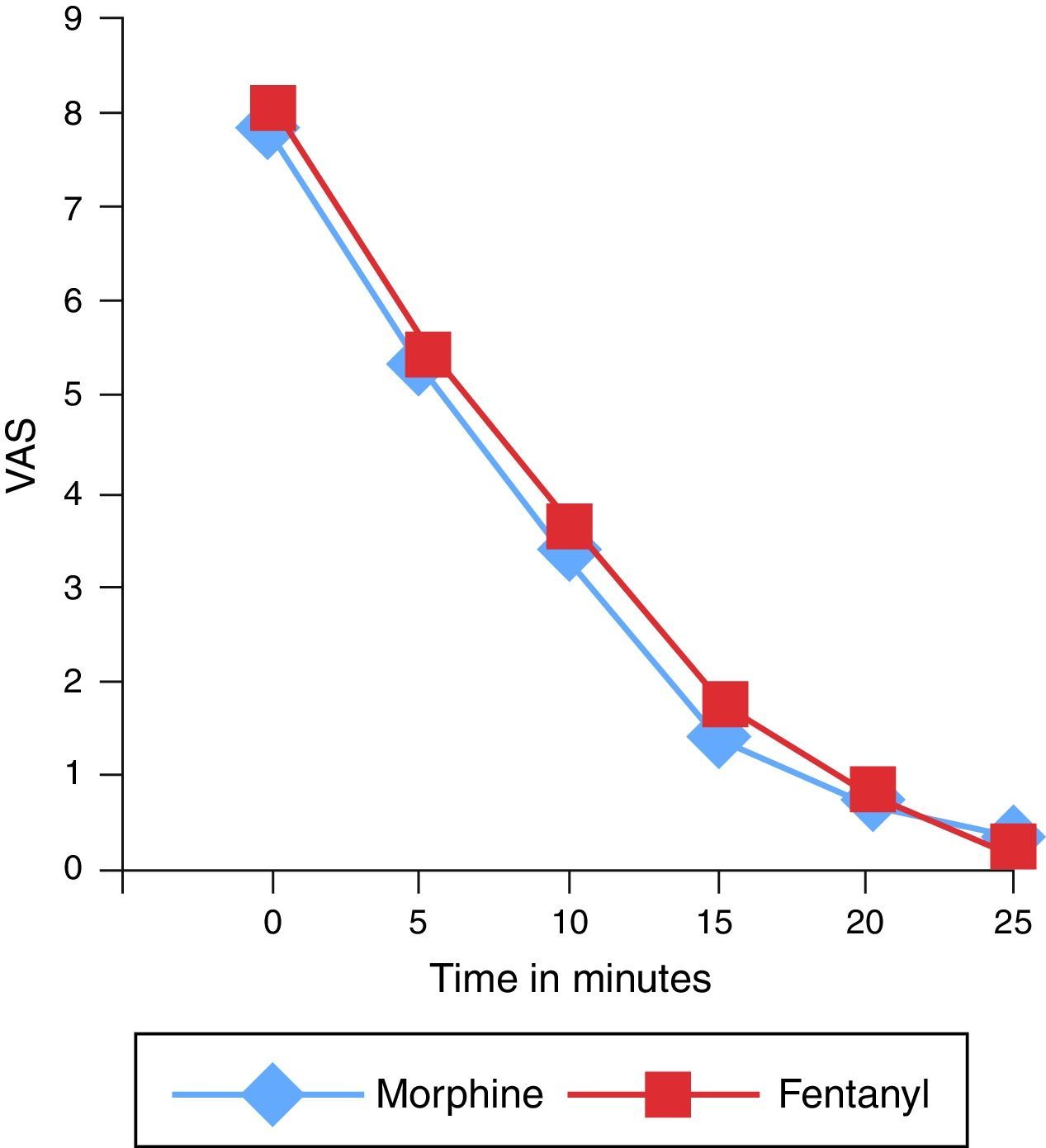
The mean (95% CI) value for initial pain on the VAS was 7.8±1.1 and 8.1±1 in the morphine and fentanyl groups respectively and 5.4±2 and 5.6±2.2 after 5min in both groups. After 10min, a mean VAS of 3.3±2.6 for the morphine group and 3.7±2.7 in the fentanyl group was obtained (p>0.05). In the following intervals, up to 25min after the initial titration, no significant differences in the mean VAS between the groups was observed (Fig. 3).
Of the 60 patients analyzed, 59 reached the target pain level (VAS<4) with the analgesic titration. One patient of the morphine group did not reach the target level, since he always described the pain as severe (VAS>7) after 9 doses (once every 5min) of the medication.
With respect to the VAS after 5 and 10min, the assumptions of normality and variance homogeneity were evaluated, giving the result that the data in the two groups were homogeneous. These results contrasted with an ANOVA of repeated measures in which it was corroborated that no differences existed between the study groups. Nevertheless, the reduction of the average observed in the VAS scale after 5min and the VAS after 10min in the two groups is due solely to time and is independent of the type of opioid used (Fig. 3).
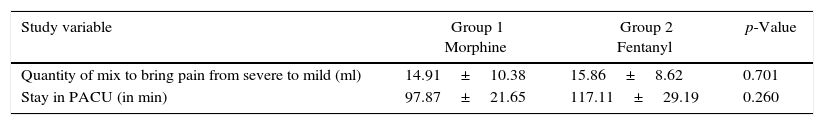
No significant difference was found in terms of the mean of the accumulated dose of opioids to bring the pain from severe to mild-moderate in the two groups (p=0.701) or in the duration in minutes of the patients’ stay in the PACU (p=0.260) (Table 2).
No complications like hypotension, bradycardia, respiratory depression, or chest-wall rigidity. The incidence of nausea and vomiting was 3.3% (1 patient) in the fentanyl group and pruritus was experienced by 3.3% (1 patient) in the morphine group, with no statistically significant differences between them (p=1).
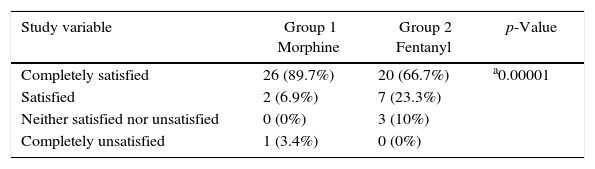
Satisfaction with the analgesia treatments used in the PACU was high, with no differences between the two groups (Table 3).
Degree of satisfaction with the analgesic agent used in the PACU.
| Study variable | Group 1 Morphine | Group 2 Fentanyl | p-Value |
|---|---|---|---|
| Completely satisfied | 26 (89.7%) | 20 (66.7%) | a0.00001 |
| Satisfied | 2 (6.9%) | 7 (23.3%) | |
| Neither satisfied nor unsatisfied | 0 (0%) | 3 (10%) | |
| Completely unsatisfied | 1 (3.4%) | 0 (0%) |
In this study, we found that fentanyl is comparable with morphine in equipotent doses for the reduction of POP from severe to mild-moderate levels as soon as 5min after titration. These results reiterate the utility of morphine, which continues to be recommended for the treatment of severe POP due to its high clinical evidence, greater experience of use, and pharmaceutical forms that allows for the establishment of a correct titration. It also has a low economic cost, as described in other studies.21,22
In the literature, other comparisons of morphine and fentanyl have been described previously for rescue analgesia. However, in these cases the titration was not completed by adjusting it to patient weight,23,24 which can affect the analgesic result if genetically determined variability of the opioid requirements among patients is considered.25
In our study, we observed that it was necessary to apply more than one bolus injection of analgesia after 5min in most of the patients to achieve a mild pain level in both groups of the study. It was only possible to lower pain levels to mild with the first dose of analgesia in 26% of the patients of the morphine group and 30% of the patients of the fentanyl group. These results are like the study of analgesic titration of Aubrun and cols,21 where, at similar doses of morphine, patients required 3 applications in 5min intervals to bring pain to mild levels in 98% of patients. It is possible that the residual anesthesia in recovery limits the response to a single analgesic doses, since there is a greater risk of sedation with increased doses. If this is the case, the process described by Aubrun—namely comparing different titrations intervals—in his study of 1600 patients may be necessary. While a better response rate would be expected after the first dose of analgesic, other titration studies have not shown benefits of increasing the morphine dose from 0.1mg/kg to 0.15mg/kg per bolus.26
Claxton and cols24 in a study performed at a hospital in Toronto, Canada, compared the management of severe POP with the use of morphine and fentanyl at fixed doses, without calculating the doses per the physical characteristics of the individuals. In our study, however, we performed the comparison of equipotent doses adjusted for patient weight, comparable to those used in the other studies.27,28 This provides for a more adequate comparison of the two medications and, as a result, a more clinically reproducible result.
One explanation of the comparable response in the percentage of patients with pain relief from morphine or fentanyl that we observe in our study is to be found in previous studies of the pharmacokinetic properties of morphine. In experiments, when morphine was injected intravenously into dogs, certain levels were detected in the cerebrospinal fluid after 2–3min, and concentrations were also detected in the cerebral tissue after 5min.29 In clinical practice, Aubrun and collaborators22 compared the best interval for the titration of morphine between 5min or 10min in the PACU, reporting a higher percentage of pain relief, with no corresponding increase in adverse effects, when morphine is applied in 4min intervals. It is believed that the greater lipophilicity of fentanyl and its potential of more rapidly crossing the blood–brain barrier, does not offer any advantage in POP analgesia measured after 5min in comparison with equipotent doses of morphine.
After reviewing the potential danger of adverse effects, some studies argue that nausea and vomiting appear to be less frequent with the use of fentanyl.30–34 However, in other ones these differences have not been evidenced,35 in our study, no statistically significant differences were observed in the opioids in terms of nausea and vomiting. That said, the only case that presented this complication received fentanyl. A case of pruritus was suffered by a patient from the morphine group. Other adverse events associated with the use of opioids in PACU were not reported.
Gender differences in the pain perception and in response to treatment are well described in different studies.36,37 By contrast, in our study, no important differences in the VAS results were observed between the two intervention groups.
Our study does have limitations. One of them was that the measurement of pain relief was not done continuously but in 5min intervals. While the pain relief after the application of the rescue dosage is continuous, and it is possible that differences could be seen before the 5-min mark in the opioids subject to study, this measurement may also be affected by the sedation that is frequent in patients in the PACU and is even a result of the analgesia from the opioid administered. It is estimated that 5min after the application of the first dose of analgesic, it is clinically significant to assess the analgesic effectivity of an opioid and its potential advantage compared to other options. In this trial, only two of the medications used commonly against pain were study, but it would be possible to choose other potent opioids.
ConclusionsPostoperative analgesic rescue with fentanyl or morphine in equipotent doses is effective for reducing the time to reduce pain from severe to mild, without important adverse effects in either of the medications. In accordance with the findings of this study, morphine—because of its safety profile—should continue to be the drug of choice in PACUs for the treatment of severe postoperative pain.
Ethical disclosureProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki)
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingUniversidad de Antioquia and Hospital Universitario San Vicente Fundación.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cadavid-Puentes A, Bermúdez-Guerrero FJ, Giraldo-Salazar O, Muñoz-Zapata F, Otálvaro-Henao J, Ruíz-Sierra J, et al. Comparación de la efectividad del fentanilo versus morfina, en dolor severo postoperatorio. Ensayo clínico aleatorizado, doble ciego. Rev Colomb Anestesiol. 2017;45:100–107.