Anaplastic thyroid cancer (ATC) is an uncommon and extremely aggressive undifferentiated tumour derived from the follicular epithelium. It has an annual incidence of one to two cases per million inhabitants and represents 1%–2% of all thyroid cancers.1 It generally manifests in a person's fifties and sixties, with a higher incidence in women. The risk factors for the diagnosis of ATC are, on the one hand, low educational level and previous goitre (both factors probably related to iodine deficiency), blood group B and obesity.2,3
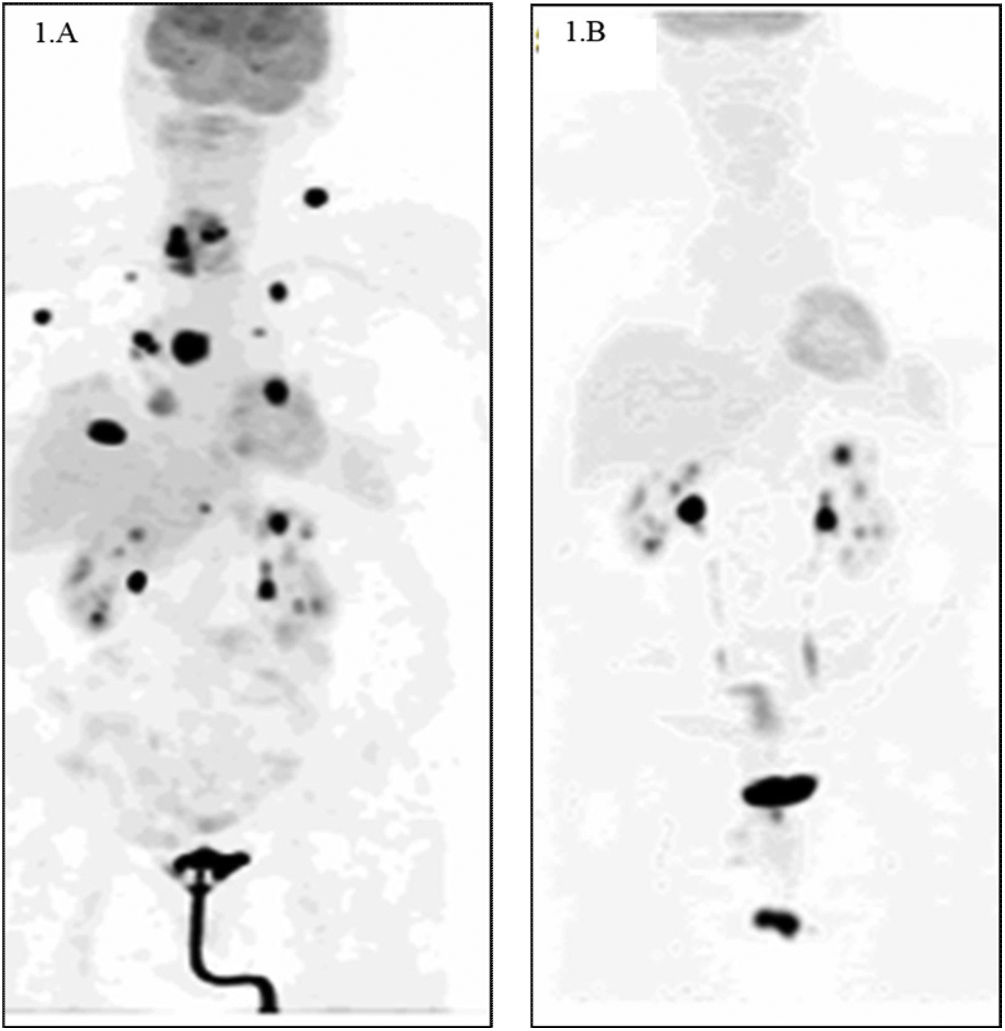
We present the case of a 76-year-old woman with personal history of interest of a sliding hiatal hernia and gastro-oesophageal reflux, who was admitted to the intensive care unit in June 2020 due to a 15-day history of progressive dyspnoea associated with a non-productive cough and dysphagia for solids. The patient's vital signs at the time of admission were blood pressure 190/120mmHg, heart rate 135 bpm and basal oxygen saturation 65%, for which she required orotracheal intubation and connection to a mechanical ventilation system. After ruling out SARS-CoV-2 infection, computed tomography angiography was performed, which excluded pulmonary thromboembolism, revealing the presence of a hypodense mass that seemed to be attached to the right thyroid lobe, suggestive of a neoformative process. The study was completed with computed tomography (CT) of the neck, where a solid, hypodense, heterogeneous mass was observed with calcifications measuring 43×34×44mm that caused invasion of the tracheal lumen by infiltration of the right posterolateral wall of the trachea without lateral cervical lymphadenopathy of significant size. In view of the clinical suspicion of ATC, it was decided to perform a core needle biopsy (CNB), with tissue fragments composed predominantly of avascular necrosis obtained, with small foci of plasmacytoid-type tumour cells, with abundant cytoplasm and nucleus displaced to the periphery. In the immunohistochemical study, weak nuclear positive expression was observed for PAX-8 and TTF 1 and weak expression for CD56, with absence of expression for thyroglobulin, calcitonin, synaptophysin and chromogranin. The Ki-67 proliferation index score was low, but the interpretation was doubtful due to the presence of intense necrosis in the sample. With these findings, ATC was diagnosed. An 18F-fluorodeoxyglucose positron emission tomography (FDG PET) scan was performed, revealing a right cervical mass with increased metabolic activity with an SUVmax of 28.5 and images suggestive of multiple bilateral pulmonary, lymph node (in the right supraclavicular and left axillary area), muscular and right atrium metastases (Fig. 1A).
A) 18F-fluorodeoxyglucose positron emission tomography (FDG PET) on diagnosis. A right cervical mass with increased metabolic activity with an SUVmax of 28.5 and images suggestive of multiple bilateral pulmonary, lymph node (in the right supraclavicular and left axillary area), muscular and right atrium metastases. B) FDG PET after 11 months of treatment with BRAF/MEK inhibitors (dabrafenib-trametinib). Morpho-metabolic involution of the right cervical mass with disappearance of the metastatic lesions at all levels is observed.
After the diagnosis of ATC and awaiting the molecular study to detect the BRAFV600gene mutation, which was positive, in June 2020 the patient received two cycles of systemic chemotherapy with carboplatin and paclitaxel and two cycles of palliative radiotherapy (RT) on the thyroid lesion, adding a margin of 1cm for active bleeding from the tracheostomy, with a total dose of 16Gy. She also received nutritional support with percutaneous endoscopic gastrostomy (PEG). In July 2020, treatment was started with dabrafenib 300mg daily+trametinib 20mg daily in a PEG-adapted liquid formula, with a favourable clinical response. After 11 months of treatment with BRAF/MEK inhibitors, the control FDG PET showed a morpho-metabolic involution of the right cervical mass with disappearance of the metastatic lesions at all levels (Fig. 1B). In August 2021, a total thyroidectomy was performed without complications, with a pathology result of a total thyroidectomy specimen with areas of fibrosis without evidence of residual neoplasia. At the present time, the patient is stable, with no evidence of recurrence of her underlying process and she does not require PEG or tracheostomy.
ATC is a tumour that has classically had a very poor prognosis, with an overall survival of 20% one year after diagnosis,4 and that used to cause more than half of the deaths associated with thyroid cancer despite its low incidence. Survival two years after diagnosis is very rare, although there are case series published prior to the use of BRAF/MEK inhibitors that describe 10-year survival ranging from 3% to 10% in patients without metastatic disease, unlike our case.5,6
The great advances made in our understanding of ATC have led to a paradigm shift in the management of this disease that has been reflected in the guidelines published in recent years.7,8 Firstly, in stages IVa and IVb, if the tumour is considered resectable, a surgical approach should be taken, with a total thyroidectomy performed with prophylactic or therapeutic central-lateral lymphadenectomy,7,8 taking into account that more aggressive surgeries including laryngectomy or tracheal or oesophageal resections are not routinely recommended.8 In stage IVc ATC, palliative surgery can be considered in selected cases such as preventive procedures in which there is an imminent compromise of the airway, or resections of locoregional disease due to symptomatic metastatic disease or in cases with few distant metastases.8 Finally, in those cases with unresectable ATC during the initial evaluation, if, after the administration of RT, systemic chemotherapy or BRAF/MEK inhibitors, there is a possibility of surgery, surgical treatment should be reconsidered,8 as happened in our case, in which the tumour was initially considered unresectable due to extensive locoregional and metastatic involvement, with a total thyroidectomy performed after 13 months of systemic treatment due to the patient's excellent clinical response. This therapeutic regimen of starting systemic therapy with BRAF/MEK inhibitors and subsequent surgery has been associated with an increase in overall survival.9
In stage IVc ATC, systemic chemotherapy with doxorubicin, taxanes (including paclitaxel) and platinum derivatives has been considered the treatment of choice, either alone or in combination with intensity-modulated RT.8 In our case, pending the results of the genetic study, treatment with systemic chemotherapy was started and palliative RT was administered due to tumour bleeding. Before starting systemic treatment, all patients with ATC should be evaluated for the BRAFV600 mutation, which is present in more than 70% of cases,10 in order for targeted therapy with BRAF/MEK inhibitors (dabrafenib and trametinib), approved since 2018 by the US Food and Drug Administration (FDA), to be considered for the treatment of BRAFV600-mutated ATC patients. As stated in the Clinical Practice Guidelines published in 2021 by the American Thyroid Association,8 this treatment is indicated as first choice in BRAFV600-mutated patients with unresectable stage IVb and stage IVc ATC, rejecting RT with a strong strength of recommendation.
In conclusion, the multidisciplinary approach and the therapeutic advances in recent years in the management of patients with ATC have contributed to improving overall survival in these cases, even in patients with advanced stages of the disease.