To evaluate the effect of faster aspart over glycaemic variability in type 1 diabetes (T1D) patients treated with sensor-augmented pump (SAP) in a real-world scenario.
MethodsObservational study with SAP-treated adult T1D patients treated with faster aspart for three months. The primary endpoint was the mean amplitude of glucose excursions (MAGE).
ResultsFifty patients were treated with faster aspart. Eleven patients (23%) withdrew during the follow-up mainly due to worsening of diabetes control (9 patients). Mean age was 41.2 yrs. (range 21–59) and T1D duration 22.4±10.0 yrs. Mean SAP treatment duration was 3.6±3.1 yrs. We detected a reduction of -7.0 (95% CI −1.1, −12.9; p=0.021) in MAGE at the end of the study. Other glycemic variability indices were also improved: standard deviation of mean interstitial glucose (−3mg/dl; 95% CI, −1, −5; p=0.01), CONGA4 (−2.2; 95% CI −0.3, −4.2; p=0.029), CONGA6 (−2.6; 95% CI −0.6, −4.6; p=0.011), GRADE (−0.5; 95% CI −0.1, −0.9; p=0.022), HBGI (−0.7; 95% CI −0.2, −1.3; p=0.013), J-index (−2.9; 95% CI −0.7, −5.0; p=0.011) and MODD (−5.7; 95% CI −1.7, −9.7; p=0.006). A slight reduction in mean glucose management indicator was also detected (−0.14%; 95% CI, −0.02, −0.27; −1.4mmol/mol; 95% CI −0.1, −3.3; p=0.03).
ConclusionsIn SAP-treated T1D patients, faster aspart insulin was associated with reduced glycaemic variability, but also a high percentage of dropouts due to worsened glycaemic control. NCT04233203.
Evaluar el efecto de la insulina faster-aspart sobre la variabilidad glucémica en pacientes con diabetes mellitus tipo 1 (DM1) tratados con sistema integrado (bomba-sensor) en un escenario de vida real.
MétodoEstudio observacional en pacientes con DM1 tratados con sistema integrado (bomba-sensor), y tratados con insulina faster-aspart durante 3 meses. El objetivo primario fue la variación en la amplitud media de las excursiones glucémicas (Mean Amplitude of Glucose Excursions [MAGE]).
ResultadosCincuenta pacientes fueron tratados con insulina faster-aspart. Once pacientes (23%) suspendieron el tratamiento, fundamentalmente por empeoramiento del control glucémico (9 pacientes). La edad media de los pacientes fue de 41,2 años (rango: 21-59), con una duración media de la DM1 de 22,4±10,0 años. El tiempo medio de tratamiento con sistema integrado fue de 3,6±3,1 años. Detectamos al final del estudio una reducción en el MAGE de −7,0 (IC 95%: −1,1, −12,9; p=0,021). Observamos una mejora consistente en otros índices de variabilidad glucémica: desviación estándar de la glucosa intersticial (−3mg/dl; IC 95%: −1, −5; p=0,01), CONGA4 (−2,2; IC 95%: −0,3, −4,2; p=0,029), CONGA6 (−2,6; IC 95%: −0,6, −4,6; p=0,011), GRADE (−0,5; IC 95%: −0,1, −0,9; p=0,022), HBGI (−0,7; IC 95%: −0,2, −1,3; p=0,013), J-index (−2,9; IC 95%: −0,7, −5,0; p=0,011) y MODD (−5,7; IC 95%: −1,7, −9,7; p=0,006). Además, se encontró una discreta mejoría del indicador de manejo de la glucosa (−0,14%; IC 95%: −0,02, −0,27; −1,4mmol/mol; IC 95%: −0,1, −3,3; p−=0,03).
ConclusiónEl uso de faster-aspart en pacientes con DM1 tratados con sistema integrado (bomba-sensor) se relacionó con una reducción de la variabilidad glucémica en práctica clínica habitual, aunque se detectó un elevado porcentaje de abandonos no debidos a empeoramiento del control glucémico. NCT04233203.
Tight glycaemic control achieved through intensive insulin treatment reduced the risk of developing chronic diabetes complications.1,2 The utility of glycated haemoglobin A1c (HbA1c) as a predictor of diabetes complications in type 1 diabetes (T1D) patients was well-established in the Diabetes Control and Complications Trial.3 Glucose variability (GV) appears to play an additional role in developing chronic complications T1D.4 Continuous subcutaneous insulin infusion (CSII) combined with real-time continuous glucose monitoring (RT-CGM), also called sensor-augmented pump (SAP), improved glycaemic control in T1D patients. Among other effects, SAP reduced HbA1c, GV and the risk of hypoglycaemia.5–8
Postprandial glycaemic control plays a substantial role in reaching recommended HbA1c goals in people living with diabetes.9 Rapid-acting insulin analogues (insulin aspart, insulin lispro and insulin glulisine) are typically administered via CSII to control both the basal and postprandial bolus insulin requirements of T1D patients.10 They have provided better postprandial glucose control compared with regular human insulin through an earlier and greater peak glucose-lowering effect.11 Faster-acting insulin aspart (faster aspart) is a newly formulated insulin aspart. When administered by subcutaneous injection had twice-as-fast onset of appearance, a 2-fold higher early exposure, and >50% greater early glucose-lowering effect compared with traditional insulin aspart.12 These pharmacological properties explained superior postprandial glycaemic control in CSII-treated T1D patients.13 However, the effect of faster aspart over main glycaemic variability metrics in SAP-treated T1D subjects remains unknown.
The present study aimed to evaluate the effect of faster aspart over glycaemic variability in SAP-treated T1D patients in a real-world scenario.
Material and methodsStudy designThe study was approved by the local ethic committee (Castilla-La Mancha Public Health Service, SESCAM, Spain) and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice, and publicly registered at ClinicalTrials.gov (NCT04233203). Participants provided written informed consent before study activities commenced. This single-centre 12-week observational retrospective study was conducted in the Department of Endocrinology and Nutrition, Ciudad Real General University Hospital, Spain. The trial was designed to evaluate the GV clinical effect of faster aspart in adult SAP-treated T1D patients in routine clinical practice. Therefore, we included all adult patients T1D subjects treated with SAP in our tertiary care hospital.
ParticipantsEligible participants were adults (≥18 years) with T1D (diagnosed clinically for ≥12 months) treated with SAP for ≥6 months and previously treated with insulin aspart during ≥3 months. Therapy with SAP was defined as the result of the combination of CSII plus RT-CGM with any kind of insulin infusion automatism (suspend on low or suspend before low functions). Patients suffering from other types of diabetes than T1D were excluded. No HbA1c, weight, or insulin dose limits were settled for this study.
ProceduresPatients initiated faster aspart according to the approved indication by the regional public health service (SESCAM) for T1D pump-treated patients. Pre-study insulin aspart and faster aspart (both 100U/ml) were administered by CSII using Medtronic Minimed 640G with SmartGuard (Medtronic Inc, MN, USA). Consumables included Paradigm Reservoir 3.0ml and Quick-set infusion set (6-mm or 9-mm cannula and 60-cm tube). Patients were reminded to subcutaneously insert their cannulas in the abdominal wall and change the complete systems every three days. Insulin pump settings were at the endocrinologist discretion according to a real-world scenario.
The same glucose targets were set for all the subjects: preprandial capillary plasma glucose 80–130mg/dl (4.4–7.2mmol/l), peak postprandial capillary plasma glucose<180mg/dl (<10.0mmol/l and nocturnal (00:00–06:00a.m.) capillary plasma glucose 100–140mg/dl (5.6–7.8mmol/l). Contour Next Link 2.4 (Ascensia Diabetes Care Holdings AG, Basel, Switzerland) was the glucometer used by the patients.
OutcomesThe primary end-point was mean change in the amplitude of glucose excursion (MAGE) from the beginning to the end of the study. Secondary outcomes included changes during the follow-up in: (1) glucose standard deviation (SD) and variation coefficient (VC); (2) weighted average of glucose values at 100mg/dl (5.6mmol/l) (M100); (3) glycemia risk assessment diabetes equation (GRADE); (4) mean of daily differences (MODD); (5) J-index; (6) continuous overall net glycaemic action (CONGA); (7) high and low blood glucose index (HBGI and LBGI, respectively); (8) hypoglycaemia frequency; (9) time in range (TIR) (70–180mg/dl, 3.9–10.0mmol/l), time bellow range (TBR)<70mg/dl (<3.9mmol/l), TBR<54mg/dl (<3.0mmol/l), time above range (TAR)>180mg/dl (>10.0mmol/l) and TAR>250mg/dl (>13.9mmol/l) of interstitial glucose; (10) area under the curve (AUC)<70mg/dl (<3.9mmol/l), AUC<54mg/dl (<3.0mmol/l), AUC>180mg/dl (>10.0mmol/l) and AUC>250mg/dl (>13.9mmol/l) of interstitial glucose; (12) HbA1c; (13) mean capillary and interstitial glucose; (14) glucose management indicator (GMI); (15) daily self-monitoring of blood glucose (SMBG) frequency; (16) insulin requirements: basal and boluses; (17) local adverse events and safety: severe hypoglycaemia and serious insulin-related events including diabetic ketoacidosis (DKA), hospitalizations and death.
Data collection was conducted between 1 January and 31 December 2020 through chart reviews. It included the following data: date of birth, date of onset of diabetes, the age at which SAP began, pump and RT-CGM model, body mass index (BMI), insulin requirement (UI/kg/24h), basal and bolus insulin percentages, number of boluses, frequency of SMBG, carbohydrate daily intake, insulin pump settings, RT-CGM information, local and severe adverse effect related with insulin (severe hypoglycaemia, DKA, hospitalisation, or death). This information was obtained from electronic medical records and the specialised online webpage Medtronic CareLink (https://carelink.medtronic.eu). Continuous glucose monitoring data was gathered from the last two weeks before each visit. All glucose variability indexes were calculated through the standardised Glyculator 2.0 online website (https://apps.konsta.com.pl/app/glyculator/).14 Physical examination and blood analysis data were gathered from baseline (before faster aspart initiation) and subsequently at 12 weeks.
Statistical analysisAll statistical analyses were prespecified and performed using the full analysis set based on all participants receiving at least one dose of faster aspart. Results are presented based on data from all participants for the entire study period (intention to treat analysis), which includes data collected after participants prematurely discontinued faster aspart. Safety endpoints were collected from all participants who received at least 1 dose of faster aspart based on data collected up to and including 7 days after discontinuation of treatment. Results are presented as mean±SD values or percentages. Wilcoxon signed-rank test was used to analyse differences between the beginning and the study end. When this correction was necessary, comparisons between proportions were analysed using a chi-squared test and Fisher's test. A bivariate analysis was performed to determine which variables obtain the lowest p-values and could be candidates in a linear regression model with the variable MAGE as the dependent variable. Subsequently, change in MAGE was analysed using a linear regression model with these variables and those considered to have the highest biological plausibility (age, diabetes duration, SAP treatment duration, CGM adherence, BMI and insulin dose). A p-value<0.05 was considered statistically significant. Statistical analyses were conducted using IBM SPSS software version 25 for Windows (SPSS Inc., Chicago, Illinois, USA).
ResultsSubjectsOverall, 50 adult subjects (70% female) on SAP therapy received at least one dose of faster aspar and were included in the study. Eleven patients (23%) withdrew during the follow-up mainly due to worsening of diabetes control (9 patients), local side effects (local itchiness) and obstructions (one patient each). The patients reported the deterioration of diabetes control as the greatest glycaemic fluctuations (4 patients) and tendency toward high glucose excursions (5 patients). Mean duration of faster aspart treatment among patients who dropped out was 22±15 days (range 4–40). All of them returned to previous insulin aspart. Patients who withdrew from faster aspart and those treated with faster aspart until the end of the study showed similar baseline characteristics (Supplementary material). No patient was included during the coronavirus-19 lockdown period in Spain (15 March 2020–21 June 2020). The mean age was 41.2 yrs. (range 21–59) and T1D duration 22.4±10.0 yrs. Thirty-six per cent of the patients suffered from chronic diabetes complications (microvascular 30%, macrovascular 8%). Mean SAP treatment duration was 3.6±3.1 yrs.
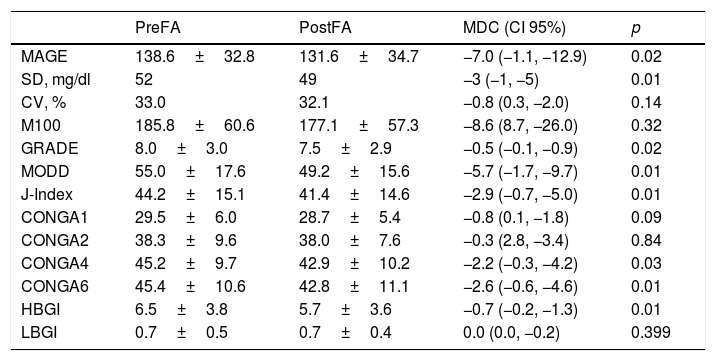
Glycaemic variabilityWe detected a reduction of −7.0 (95% CI −1.1, −12.9; p=0.021) in MAGE at the end of the study. A descend of SD of mean interstitial glucose (−3mg/dl; 95% CI, −1, −5; p=0.01) was also observed. This improvement in glycaemic variability was confirmed in other index such as CONGA4 (−2.2; 95% CI −0.3, −4.2; p=0.029), CONGA6 (−2.6; 95% CI −0.6, −4.6; p=0.011), GRADE (−0.5; 95% CI −0.1, −0.9; p=0.022), HBGI (−0.7; 95% CI −0.2, −1.3; p=0.013), J-index (−2.9; 95% CI −0.7, −5.0; p=0.011) and MODD (−5.7; 95% CI −1.7, −9.7; p=0.006). Rest of glycaemic variability index are shown in Table 1.
Glycaemic variability outcomes.
| PreFA | PostFA | MDC (CI 95%) | p | |
|---|---|---|---|---|
| MAGE | 138.6±32.8 | 131.6±34.7 | −7.0 (−1.1, −12.9) | 0.02 |
| SD, mg/dl | 52 | 49 | −3 (−1, −5) | 0.01 |
| CV, % | 33.0 | 32.1 | −0.8 (0.3, −2.0) | 0.14 |
| M100 | 185.8±60.6 | 177.1±57.3 | −8.6 (8.7, −26.0) | 0.32 |
| GRADE | 8.0±3.0 | 7.5±2.9 | −0.5 (−0.1, −0.9) | 0.02 |
| MODD | 55.0±17.6 | 49.2±15.6 | −5.7 (−1.7, −9.7) | 0.01 |
| J-Index | 44.2±15.1 | 41.4±14.6 | −2.9 (−0.7, −5.0) | 0.01 |
| CONGA1 | 29.5±6.0 | 28.7±5.4 | −0.8 (0.1, −1.8) | 0.09 |
| CONGA2 | 38.3±9.6 | 38.0±7.6 | −0.3 (2.8, −3.4) | 0.84 |
| CONGA4 | 45.2±9.7 | 42.9±10.2 | −2.2 (−0.3, −4.2) | 0.03 |
| CONGA6 | 45.4±10.6 | 42.8±11.1 | −2.6 (−0.6, −4.6) | 0.01 |
| HBGI | 6.5±3.8 | 5.7±3.6 | −0.7 (−0.2, −1.3) | 0.01 |
| LBGI | 0.7±0.5 | 0.7±0.4 | 0.0 (0.0, −0.2) | 0.399 |
Data are expressed in mean±standard deviation and percentages. CI, confidence interval; CONGA, continuous overall net glycaemic action; FA, faster aspart insulin; GRADE, glycemia risk assessment diabetes equation; HBGI, high blood glucose index; LBGI, low blood glucose index; MAGE, mean amplitude of glucose excursion; MDC, mean difference in change; MODD, mean of daily differences; M100, weighted average of glucose values at 100mg/dl; SD, standard deviation; CV, variation coefficient.
The bivariate analysis determined the previous HbA1c, mean interstitial glucose, LGBI, TBR, TAR and AUC as candidates (lowest p-values) in a linear regression model with MAGE as the dependent variable. These variables and those considered to have the highest biological plausibility were evaluated in the simple linear regression models for the MAGE increase. Only LGBI before faster aspart was found to exert a significant effect on the MAGE, obtaining a significant model (p=0.038) in which the increase of each unit of the variable LGBI before faster aspart causes an increase of 13.5 (0.78–26.29) units in the difference in MAGE. The explanability of the model was R2=0.103.
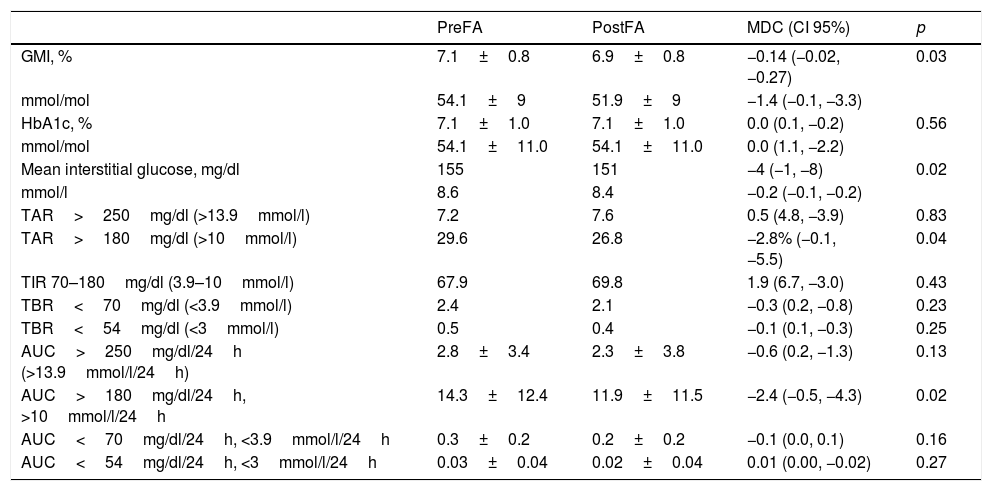
Other glycaemic controlWe detected a slight reduction in mean GMI (−0.14%; 95% CI, −0.02, −0.27; −1.4mmol/mol; 95% CI −0.1, −3.3; p=0.03) and mean interstitial glucose (−4mg/dl; 95% CI, −1, −8; −0.2mmol/l; 95% CI, −0.1, −0.2; p=0.02) among faster aspart users at the study end. This change was associated with a decrease in TAR>180mg/dl (>10.0mmol/l) (−2.8%; 95% CI, −0.1, −5.5; p=0.043) and AUC>180mg/dl/24h (>10.0mmol/l/24h) (−2.4mg/dl/24h; 95% CI, −0.5, −4.3; −0.1mmol/l/24h; 95% CI, −0.2, 0; p=0.02) during the follow-up. No differences in the rest of glycaemic variables analysed were found during the study. Complementary secondary glycaemic outcomes can be observed in Table 2.
Rest of glycaemic control outcomes.
| PreFA | PostFA | MDC (CI 95%) | p | |
|---|---|---|---|---|
| GMI, % | 7.1±0.8 | 6.9±0.8 | −0.14 (−0.02, −0.27) | 0.03 |
| mmol/mol | 54.1±9 | 51.9±9 | −1.4 (−0.1, −3.3) | |
| HbA1c, % | 7.1±1.0 | 7.1±1.0 | 0.0 (0.1, −0.2) | 0.56 |
| mmol/mol | 54.1±11.0 | 54.1±11.0 | 0.0 (1.1, −2.2) | |
| Mean interstitial glucose, mg/dl | 155 | 151 | −4 (−1, −8) | 0.02 |
| mmol/l | 8.6 | 8.4 | −0.2 (−0.1, −0.2) | |
| TAR>250mg/dl (>13.9mmol/l) | 7.2 | 7.6 | 0.5 (4.8, −3.9) | 0.83 |
| TAR>180mg/dl (>10mmol/l) | 29.6 | 26.8 | −2.8% (−0.1, −5.5) | 0.04 |
| TIR 70–180mg/dl (3.9–10mmol/l) | 67.9 | 69.8 | 1.9 (6.7, −3.0) | 0.43 |
| TBR<70mg/dl (<3.9mmol/l) | 2.4 | 2.1 | −0.3 (0.2, −0.8) | 0.23 |
| TBR<54mg/dl (<3mmol/l) | 0.5 | 0.4 | −0.1 (0.1, −0.3) | 0.25 |
| AUC>250mg/dl/24h (>13.9mmol/l/24h) | 2.8±3.4 | 2.3±3.8 | −0.6 (0.2, −1.3) | 0.13 |
| AUC>180mg/dl/24h, >10mmol/l/24h | 14.3±12.4 | 11.9±11.5 | −2.4 (−0.5, −4.3) | 0.02 |
| AUC<70mg/dl/24h, <3.9mmol/l/24h | 0.3±0.2 | 0.2±0.2 | −0.1 (0.0, 0.1) | 0.16 |
| AUC<54mg/dl/24h, <3mmol/l/24h | 0.03±0.04 | 0.02±0.04 | 0.01 (0.00, −0.02) | 0.27 |
Data are expressed in mean±standard deviation and percentages. FA, faster aspart insulin; GMI, glucose management indicator; HbA1c, glycated haemoglobin A1c; TAR, time above range; TBR, time bellow range; TIR, time in range; AUC, area under the curve; MDC, mean difference in change; CI, confidence interval.
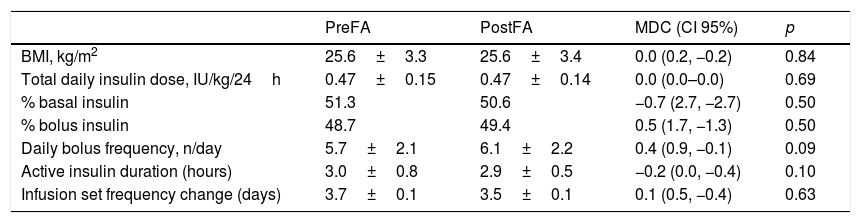
Insulin requirement maintained stable during the study with no differences in daily insulin requirement, basal and bolus insulin percentages, or daily bolus frequency. SOL and SBL functions were enabled at the beginning of the study and at the end of the follow-up in all patients. Infusion system change frequency did not change with faster aspart. The rest of the insulin outcomes are shown in Table 3.
Other outcomes.
| PreFA | PostFA | MDC (CI 95%) | p | |
|---|---|---|---|---|
| BMI, kg/m2 | 25.6±3.3 | 25.6±3.4 | 0.0 (0.2, −0.2) | 0.84 |
| Total daily insulin dose, IU/kg/24h | 0.47±0.15 | 0.47±0.14 | 0.0 (0.0–0.0) | 0.69 |
| % basal insulin | 51.3 | 50.6 | −0.7 (2.7, −2.7) | 0.50 |
| % bolus insulin | 48.7 | 49.4 | 0.5 (1.7, −1.3) | 0.50 |
| Daily bolus frequency, n/day | 5.7±2.1 | 6.1±2.2 | 0.4 (0.9, −0.1) | 0.09 |
| Active insulin duration (hours) | 3.0±0.8 | 2.9±0.5 | −0.2 (0.0, −0.4) | 0.10 |
| Infusion set frequency change (days) | 3.7±0.1 | 3.5±0.1 | 0.1 (0.5, −0.4) | 0.63 |
Data are expressed in mean±standard deviation and percentages. FA, faster aspart insulin; MDC, mean difference in change; CI, confidence interval.
Two additional patients reported local side effects (one local pain and the other non-itching papule). However, faster aspart treatment was not suspended. One patient treated with faster aspart suffered a DKA requiring hospitalisation due to a febrile syndrome with genitourinary infection and was judged to be unlikely related to the trial product. No severe hypoglycaemia was detected during the study. No other episodes of serious adverse events occurred. No patient died during the follow-up.
DiscussionThis real-world study demonstrates that the use of faster aspart in SAP-treated T1D adult patients may reduce glycaemic variability, among other glycaemic control benefits. We detected a significant 5% reduction in MAGE (−7.0 [95% CI −1.1, −12.9; p=0.021]) added to other glucose variability index improvements.
An initial exploratory crossover trial confirmed a superior glucose-lowering effect after meals of faster aspart over insulin aspart.15 Subsequently, faster aspart provided an effective and safe option for CSII treatment in the double-blind randomised Onset 5 trial.13 In this study, faster aspart was superior to insulin aspart in the control of postprandial glucose increments. Neither of these controlled studies evaluated the impact of faster aspart over glycaemic variability in CSII-treated T1D patients. Nevertheless, a small case series retrospective study conducted in 2019 showed the benefits of faster aspart reducing intra-day fluctuations in glucose levels as evaluated by MAGE in type 2 diabetes patients.16 Moreover, a recent real-world study, the GoBolus Study, detected a similar reduction in MAGE (−7.5; p=0.03) in patients switching to faster aspart in multiple-dose insulin-injection treated T1D patients.17
We also reliably detected improvements in other within-day glucose variability indexes such as SD, CONGA4 and CONGA6. An SD improvement was described in the GoBolus study, although this improvement was not detected in other studies.16–19 Unfortunately, no previous studies have thoroughly investigated the effect of faster aspart in a broader range of glucose variability parameters.
The International Consensus on Time in Range established VC as the key CGM glycaemic variability metric.20 Before this publication, MAGE was widely used as an essential glycaemic variability index. The present study's design was performed before the publication of the consensus, and, at that point, we could not foresee its future widespread use. We did not detect a reduction in the VC along the study, although an improvement in MAGE and aforementioned glycaemic variability metrics was observed. Therefore, we propose faster aspart as useful insulin to improve glycaemic variability in SAP-treated adult T1D patients.
Several of the major interrelationships among measures of glucose variability control has been described.21 These measures can be grouped to indicate whether they are predominantly assessing hypoglycaemia (e.g., Hypoglycaemia Index, LBGI, %GRADEhypoglycemia), hyperglycaemia (e.g., Hyperglycaemia Index, HBGI, %GRADEhyperglycemia), euglycemia (e.g., %GRADEeuglycemia), or a combination of all three facets of glycaemic control (e.g., M100, GRADE). We detected a reduction in GRADE, J-index, MODD and HGBI, indicating an improvement over different aspects of glucose control variability.
We also detected a significant effect of LBGI over MAGE. So far, the LGBI has not been directly related to MAGE.22,23 The LBGI reflects the frequency and extent of hypoglycaemic episodes and presents the results in “risk space”. Thus, the LBGI is a weighted average of the number of hypoglycaemic readings, with progressively increasing weights as interstitial glucose levels go down. Therefore, the LBGI has been associated with the risk for hypoglycaemia and prediction of severe hypoglycaemic episodes. Therefore, the connection between LGBI and MAGE seems plausible.
We observed a reduction in GMI and mean interstitial glucose. Faster aspart was non-inferior to insulin aspart regarding the change in HbA1c.13 T1D treated with aspart achieved lower HbA1c than those treated with faster aspart in the Onset 5 trial with an estimated treatment difference of 0.09% (1.0mmol/mol). A recent meta-analysis of randomised controlled trials estimated that faster aspart was associated with small but significant improvement in HbA1c (−0.08%, −0.9mmol/mol).24 We did not observe a reduction in HbA1c but an improvement in GMI (−0.14%, −1.4mmol/mol). Glycated haemoglobin is currently the primary measure guiding glucose management and a valuable marker of the risk of developing diabetes complications.25 However, GMI has been proposed by some authors to provide a useful measure for connecting RT-CGM metrics to laboratory HbA1c.26 Our similar results support the beneficial effect of switching to a faster aspart in long-term diabetes control.
The last set of analyses included the safety assessment. No microscopically confirmed episodes of infusion set occlusions were observed in the Onset 4 trial.10 Subsequently, faster aspart was confirmed as a safe option for CSII treatment in T1D patients in the Onset 5 trial, although a numerically higher number of infusion-site reactions (a cited reason for non-routine changes) was reported.13 We did not observe an increase in the frequency of infusion-set changes in our safety analysis set. However, one patient prematurely withdrew because of this, and two more reported local side effects during the follow-up. Besides, we detected one severe adverse event (DKA due to genitourinary infection). However, the overall safety profiles for faster aspart and aspart insulins were broadly similar in the Onset 5 trial.13 Therefore, further prospective studies may be needed to get a real sense of the true effect of continuous subcutaneous administration of faster aspart insulin.
There were a few limitations to this study. Firstly, the study was conducted as a retrospective, one-arm observation study where the interventions were known to participants and investigators given the nature of the study and medical devices. Besides, the study size was not calculated according to the primary endpoint due to the retrospective characteristic of the study. In addition, we did not have any comparison group; however, patients were compared as per a pre-switch and post-switch basis. Finally, we observed a high number of dropouts (23%) which hinder the interpretation of the study results. The intention to treat analysis, including all patients receiving at least one dose of faster aspart and the safety analysis sets were designed to avoid this last limitation.
In conclusion, we suggest that faster aspart may have additional benefits over glycaemic variability in SAP-treated adult T1D patients. However, the described high dropout rate should make us consider our results with caution. Since clinical studies about glucose variability of new molecules for diabetes treatment are scarce, more studies are needed to further elucidate the impact of new treatments for T1D patients over other aspects of the traditional glucose targets.
Authors’ contributionJMF and FJGR designed the study. JMF wrote the study protocol and published it in a public registry. RVC and AMS gathered data. JRMR contributed to the interpretation of the results. JMF took the lead in writing the manuscript. JRMR performed statistical regression analysis and reviewed statistical analysis. All authors provided critical feedback and helped shape the final manuscript.
FundingThis study received no funding.
Conflict of interestThe authors declare that they have not received research support or compensation from NovoNordisk. JMF reports consultant fees and speaker honoraria from NovoNordisk, Lilly, Sanofi, Medtronic, Tandem, Dexcom, Abbott, Roche and Ypsomed. The rest of the authors declare that they have no conflicts of interest concerning this article.
The authors are very grateful to Dr. Konrad Pagacz for his advice and assistance during the analysis of the glucose variability index through the Glyculator 2.0 tool.