Obesity and gestational diabetes mellitus (GDM) are associated with an increased risk of perinatal complications and obesity in the offspring. However, the impact of gestational weight gain (GWG) on maternal and foetal outcomes is controversial.
Patients and methodsRetrospective study of 220 women with GDM and pre-pregnancy body mass index (BMI)>30kg/m2. Pregnant women were classified according to the Institute of Medicine (IOM) recommendations regarding their prior BMI and GWG. We evaluated the impact of GWG on perinatal and obstetric outcomes.
ResultsMean maternal age was 34.7±5.3 years. Pre-pregnancy obesity was classified as class I in 55.3% of the cases, class II in 32.0% and class III in 12.7%. GWG was adequate (5–9kg) in 24.2%, insufficient (<5kg) in 41.8% and excessive (>9kg) in 34.2%. Birth weight was within normal range in 81.9%, 3.6% were small for gestational age (microsomia) and 14.4% were large for gestational age (macrosomia). Insufficient GWG was associated with a higher rate of microsomal offspring, excessive GWG was associated to macrosomia and adequate GWG with normal birth weight.
ConclusionGWG in women with pre-pregnancy obesity and GDM impacts neonatal birthweight. Insufficient GWG is associated with microsomia and excessive GWG is associated with macrosomia. Women with adequate GWG according to the IOM guidelines obtained better perinatal outcomes.
La obesidad y la diabetes mellitus gestacional (DMG) se asocian a un mayor riesgo de complicaciones perinatales y obesidad en la descendencia. Sin embargo, el impacto de la ganancia de peso gestacional (GPG) en los resultados maternos-fetales es controvertido.
Pacientes y métodosEstudio retrospectivo de 220 mujeres con DMG e índice de masa corporal (IMC) previo al embarazo ≥30kg/m2. Las gestantes fueron clasificadas según las recomendaciones del Instituto de Medicina (IOM) según su IMC previo y su GPG. Se evaluó el impacto de la GPG en los resultados perinatales y obstétricos.
ResultadosLa edad materna media fue de 34,7±5,3 años. La obesidad pregestacional se clasificó como grado I en el 55,3% de los casos, grado II en el 32,0% y grado III en el 12,7%. La GPG fue adecuada (5-9kg) en el 24,2%, insuficiente (<5kg) en el 41,8% y excesiva (>9kg) en el 34,2%. El peso neonatal estaba dentro del rango normal en el 81,9%, el 3,6% fueron pequeños para la edad gestacional (microsomía) y el 14,4% grandes para la edad gestacional (macrosomía). La GPG insuficiente se asoció a una mayor tasa de neonatos microsómicos, mientras que la GPG excesiva se asoció a macrosomía y la GPG adecuada a normopeso neonatal.
ConclusiónLa GPG en mujeres con obesidad pregestacional y DMG impacta en el peso neonatal. Una GPG insuficiente se asocia a microsomía y una GPG excesiva a macrosomía. Las mujeres con una GPG adecuada según las directrices del IOM obtuvieron mejores resultados perinatales.
The increasing worldwide prevalence of obesity in women of child-bearing age constitutes a cause for concern.1,2 Data from the US National Health and Nutrition Examination Survey (NHANES) in women aged 20–39 years yielded an estimated prevalence of obesity of 31.8%, half of whom were classified as grade I obesity (body mass index, BMI, 30–34.9kg/m2).3 The impact of this burden relies on several complications, including reduced fertility, increased time taken to conceive, increased rate of obesity-related comorbidities and a higher risk of adverse outcomes for mother and offspring in the event of pregnancy. More specifically, women with obesity who become pregnant exhibit a higher likelihood of early miscarriage, increased risk of congenital fetal malformations, birth of macrosomal infants, premature birth, difficulties during labour and delivery and stillbirth. Pregnancy may also be complicated by associated comorbidities such as gestational diabetes mellitus (GDM), pregnancy-induced hypertension, pre-eclampsia, thromboembolism and mental disturbances, all of which may compromise the new mother's future health. Maternal obesity has also been associated with subsequent long-term post-partum morbidities, including difficulties in breastfeeding, weight retention and metabolic alterations in both mother and children.2 Effective and prompt interventions, particularly in weight management, should be implemented to minimise maternal and fetal consequences related to maternal obesity.
GDM, defined as diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes prior to gestation,4 is the most clearly related obesity-derived metabolic complication and its risk increases as maternal BMI does. The prevalence of GDM may vary, depending on the variations in the criteria used to diagnose it, and there has been a major degree of controversy regarding the most optimal diagnostic approach.4,5 In any event, an intervention to control maternal glucose levels reduces adverse obstetric outcomes, serious perinatal morbidity and may also improve the woman's health-related quality of life, although the long-term impact on the offspring has not been clearly ascertained.6–8 The first-line management of GDM relies on lifestyle modifications, based on a stringent control of carbohydrates combined with light exercise.9,10 However, a recent systematic review advocates individualised modified nutritional manipulation from usual intake, including but not limited to carbohydrate restriction.11 Oral hypoglycaemic agents or insulin are reserved for women who cannot be adequately managed with a dietary approach.
Gestational weight gain (GWG) directly affects overall pregnancy outcomes. Thus, the Institute of Medicine (IOM) described what would be a desirable approach based on pre-gestational BMI exclusively.12 Several previous studies have evaluated GWG in obese women and in GDM, independently, although what a desirable weight management would be in the context of both obesity and GDM and the consequences on maternal and foetal outcomes has not been clearly evaluated. The aim of this study is to analyse the relationship between GWG in women with obesity and GDM who follow a lifestyle treatment and its effects on maternal and foetal outcomes.
Research design and methodsStudy population9114 women gave birth in our hospital between January 2016 and October 2019. Of these women, 747 were followed up at some point in our Endocrinology Department mainly due to a diagnosis of GDM. We retrospectively collected data on the 249 pregnant women who were diagnosed with GDM and who had a pre-gestational BMI>30kg/m2. We subsequently excluded 29 patients who were lost to follow-up and/or because important data for our objectives were missing, yielding a total sample of 220 pregnant women. Our Hospital's Ethics and Clinical Research Committee approved the study protocol, which was compliant with the Declaration of Helsinki. Patient anonymity was preserved. All the data were pseudonymised, guaranteeing technical and functional separation between the research team and the person tasked with pseudonymization.
Study designAccording to our Hospital's protocol, GDM screening was performed in obese pregnant women during the first trimester with the two-step approach4: an initial 50-g oral glucose tolerance test (OGTT) was performed, and if glucose values were >140mg/dl after 1h, a second OGTT was performed using 100g. The diagnosis of GDM was established according to Coustan and Carpenter if two of the following values were met or surpassed: fasting 95mg/dl, 1h 180mg/dl, 2h 155mg/dl and 3h 140mg/dl. If the diagnosis of GDM was initially ruled out, the same approach was repeated during the second trimester around weeks 24 and 28 of pregnancy. Additional work-up with OGTT was performed in cases of excessive amniotic fluid or large-for-gestational-age foetuses, regardless of prior negative GDM results. All patients with GDM were referred to the endocrinologist. In case women were not able to tolerate the OGTT, they were also referred to the endocrinologist and frequent glucose monitoring was performed until a clinical decision was made regarding the presence or absence of GDM.
Patients were individually counselled by the endocrinologist and a registered dietician on healthy lifestyle strategies (whole grains, vegetables, nuts, and extra-virgin olive oil), diet, exercise and glucose monitoring, according to each patient's energy requirements, and complemented with written information sheets. Treatment with insulin was initiated in cases of insufficient glucose control with diet alone. if more than three out of five glucose capillary values were above the optimal range (>95mg/dl in baseline conditions or >140mg/dl 1h after meals) and more intensive diet recommendations did not suffice for adequate glucose control.
We evaluated diet adherence, self-monitoring of blood glucose, maternal weight and gestational outcomes (estimation of amniotic fluid and estimated foetus weight at each scheduled sonographic evaluation and maternal blood pressure) at the first visit, at weeks 20, 24, 32 and immediately before delivery. Pre-pregnancy weight was also recalled. Delivery data were collected and particular attention was paid to the type of delivery, birth weight, perinatal complications and hospital stay.
Categorisation of gestational weight gain and classification of birth weightAccording to the IOM recommendations,12 adequate GWG was defined according to pre-pregnancy BMI. For the particular case of women with obesity (BMI>30kg/m2), adequate GWG was considered to be between 5kg and 9kg. Thus, insufficient GWG was considered as <5kg and excessive GWG as >9kg.
For birth weight, newborns were regarded as LGA if their birth weight was >2.0 standard deviations (SD) or over the 90th percentile for sex and gestational age, and infants were regarded as SGA if their birth weight was <−2.0 SD or under the 10th percentile for sex and gestational age. Normal birth weight was defined as values between −2.0 and +2.0 SD for sex and gestational age (between the 10th and 90th percentile).
Statistical analysisThe statistical analysis was performed using SPSS version 25.0 (IBM SPSS Statistics Inc., Chicago, IL, USA). For descriptive data, results were expressed as mean±standard deviation for continuous variables and as frequency and percentages for categorical variables. Possible existing associations between gestational outcomes and GWG were evaluated using a two-way analysis of variance. The comparison between categorical groups was assessed with the Chi-squared test. Univariate and multivariate analyses were performed to evaluate further associations. Significance was considered at p <0.05.
ResultsBaseline characteristicsThe mean age of the women included in our study was 34.7±5.3 years and mean weight was 89.0±13.0kg (BMI 35.2±4.3kg/m2). Before pregnancy, 122 (55.5%) women were classified as having grade I obesity, 70 (31.8%) as grade 2, 26 (11.8%) as grade 3 and 2 (0.9%) as grade 4. For most women (120, 54.5%) this was the first pregnancy, for 76 (34.5%) the second, for 20 (9.1%) the third and for 4 women (1.8%) it was their fourth pregnancy. Only 7 (3.2%) pregnancies were multiple, and 17 (7.7%) were pregnancies after assisted reproductive techniques. Twenty-five patients (11.4%) had undergone a previous C-section.
Diagnosis and management of gestational diabetes mellitusThe diagnosis of GDM was made during the first trimester in 105 cases (47.7%), in 74 (33.6%) during the second trimester, as part of the universal screening at weeks 24–28 of gestation, and in 41 cases (18.6%) after the universal screening. In all cases, glycaemic status was routinely and strictly evaluated at each follow-up visit and was adequately controlled. Following evaluation during the second trimester, 33 patients (15.0%) required insulin, although this number increased to 46 (20.9%) during the third trimester.
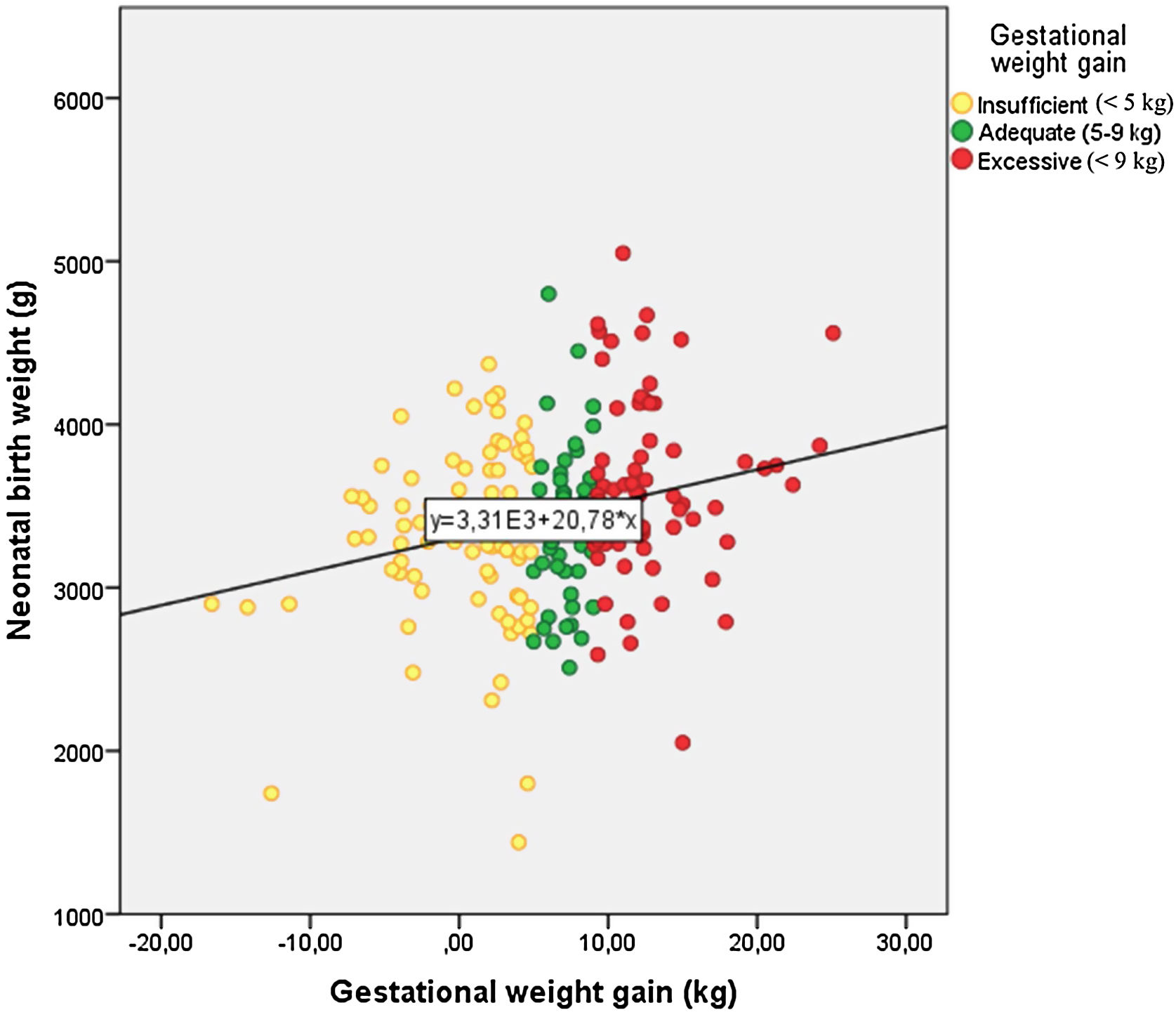
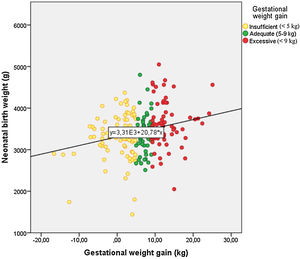
Gestational weight gain and obstetric outcomesMean maternal weight increased progressively to 93.2±12.4kg, 95.5±13.0kg, 97.8±13.4kg at 20, 32 and at the last visit before delivery, respectively. The average gestational week of the last visit in the Endocrinology Department before delivery was 32.1±7.0. Mean total GWG was 6.3±6.7 (interquartile range 2.6–11.0) kg. According to the IOM guidelines, 41.8% had an insufficient GWG, 24.2% an adequate GWG and 34.0% an excessive GWG. We did not find a significant correlation between GWG and age (R=0.012, p=0.866). There was a higher proportion of women diagnosed with GDM during the first trimester (before universal screening) who reached insufficient GWG (52.2%), while there was a higher number of women with excessive GWG for those who were diagnosed after universal screening (3rd trimester) (56.1%) (p=0.004). There was a significant correlation between the overall GWG and the time when GDM was diagnosed (R=0.290, p=0.000). However, the sub-analysis of the correlation between GWG in each trimester showed that GWG during the last weeks of pregnancy (from the visit in the third trimester to before delivery) was not statistically significant (R=−0.016, p=0.821). Weight gain at the time of diagnosis of GDM was significantly different between the categories of pregestational BMI, with women with obesity grade 1 having a higher GWG up until that moment than women with grade 4 obesity (p=0.015).
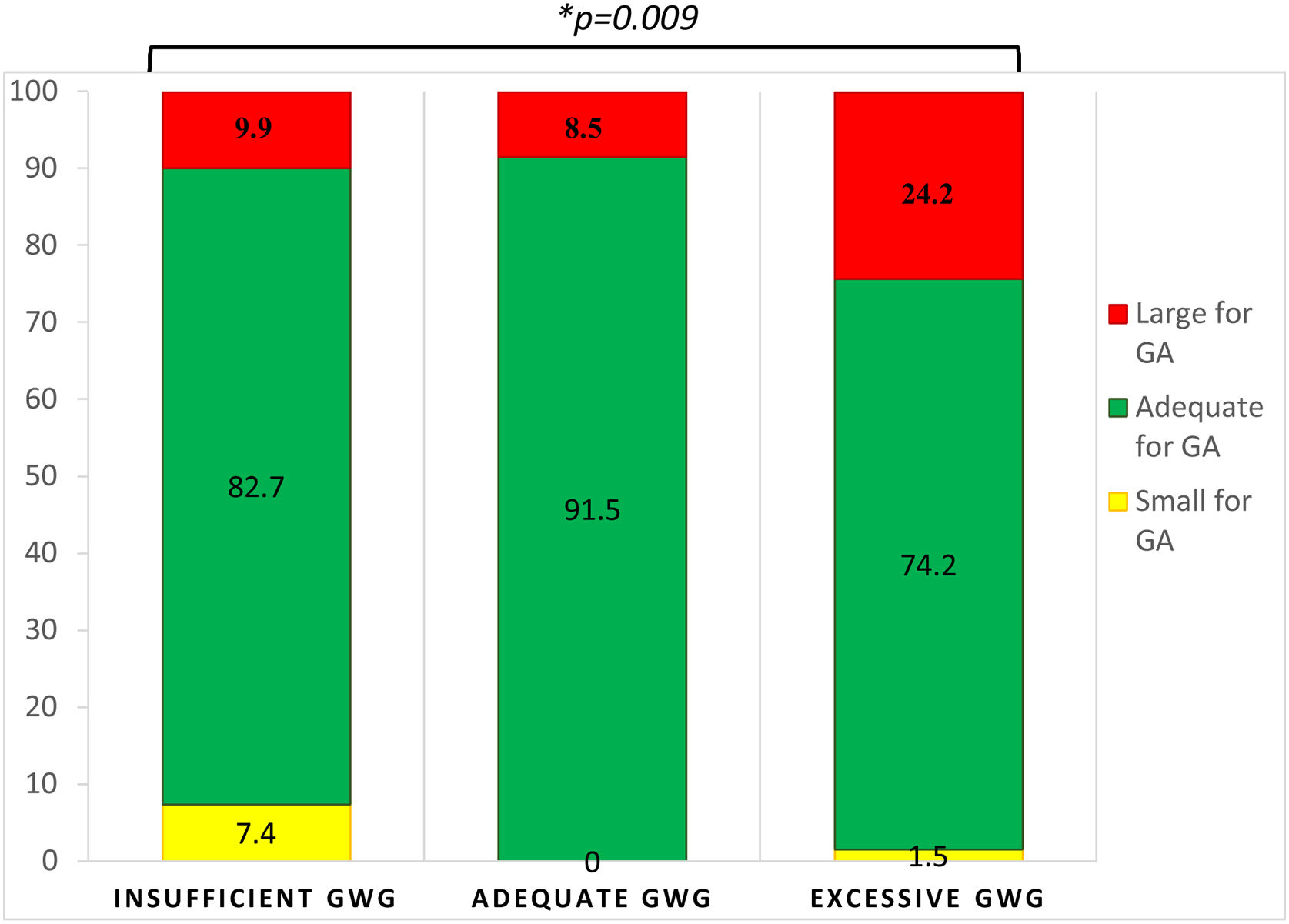
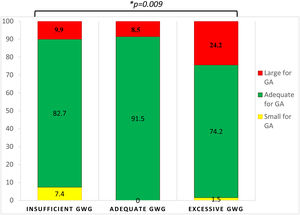
Birth weight and obstetric outcomesMean neonatal birth weight was 3421.0±575.9g, with 82.0% being considered as normal, 14.4% as LGA and 3.6% as SGA. There was no correlation between neonatal birth weight and GDM diagnosis time (R=0.077, p=0.285). GWG was significantly correlated with neonatal birth weight (R=0.228, p=0.001) (Fig. 1). Women with insufficient GWG (<5kg) had a higher rate of SGA infants (7.4%) compared to those with an adequate GWG (0.0%) or an excessive GWG (1.5%). Moreover, women with excessive GWG (>9kg) had a higher rate of LGA infants (24.2%) compared to women with adequate GWG (8.5%) or insufficient GWG (9.9%). An adequate GWG was associated with a higher rate of adequate infant birth weight (91.5%) compared to cases with insufficient or excessive GWG (82.7% and 74.2%, respectively). The comparisons yielded statistical significance (p=0.009) (Fig. 2). GWG was not different between women with or without insulin treatment (p=0.784).
Thirty women (13.4%) developed pre-eclampsia. There were 7 cases of miscarriage before week 20 and 1 case of intra-uterus death at 38 weeks of gestation. Delivery occurred at median week 40 (interquartile range 38–40), whereby the majority were regarded as full-term deliveries (78.4%), although there were 29 cases (13.2%) of pre-term deliveries (before week 37). Delivery was induced in 60 cases (27.3%), required instrumental devices in 32 cases (14.6%) and 71 women (32.3%) underwent a C-section. We did not find any statistically significant associations between GWG and any other maternal or perinatal adverse outcomes, including admission to the neonatal intensive care unit, neonatal glycaemia or hyperbilirubinaemia (Table 1).
Demographic data and obstetric complications according to the IOM category of gestational weight gain (GWG) recommendations. Values show mean±standard deviation or number of patients and percentage (%). p-Values are shown for Chi-squared test (categorical values) and analysis of variance (continuous variables).
| GWG according to the IOM guidelines | |||||
|---|---|---|---|---|---|
| Total | Insufficient GWG | Adequate GWG | Excessive GWG | p | |
| Age (years) | 35±5 | 35±5 | 35±6 | 35±5 | 0.595 |
| Grade of obesity | |||||
| 1 | 115 | 38 (33.0) | 27 (23.5%) | 50 (43.5%) | 0.039 |
| 2 | 65 | 35 (53.8) | 15 (23.1) | 15 (23.1) | |
| 3 | 25 | 11 (44.0) | 9 (36.0) | 5 (20.0) | |
| 4 | 2 | 1 (50.0) | 0 (0.0) | 1 (50.0) | |
| Gestational age at first endocrine visit | 22±8 | 20±8 | 23±8 | 25±8 | 0.001 |
| Time at diagnosis of GDM | |||||
| Before universal screening (<24wk) | 92 | 48 (52.2) | 22 (23.9) | 22 (23.9) | 0.004 |
| Universal screening (24–28 wk) | 74 | 27 (36.5) | 21 (28.4) | 26 (35.1) | |
| After universal screening (>28 wk) | 41 | 10 (24.4) | 8 (19.5) | 23 (56.1) | |
| Insulin treatment | 35 | 14 (40.0) | 6 (17.1) | 15 (42.9) | 0.755 |
| Birth weight (g) | 3436±554 | 3309±548 | 3383±485 | 3632±562 | 0.003 |
| Days hospitalisation | 4±2 | 3±2 | 4±3 | 4±2 | 0.186 |
| Gestational age at delivery | 39±2 | 39±2 | 39±2 | 39±2 | 0.664 |
| C-section | 71 | 23 (32.4) | 19 (26.8) | 29 (40.8) | 0.464 |
| Induction for delivery | 59 | 24 (40.7) | 15 (25.4) | 20 (33.9) | 0.995 |
| Instrumental delivery | 31 | 16 (51.6) | 2 (6.5) | 13 (41.9) | 0.152 |
| Obstetric complications | 47 | 16 (34.0) | 11 (23.4) | 20 (42.6) | 0.710 |
| Birthweight category | |||||
| Below p10 (SGA) | 2 | 1 (50.0) | 1 (50.0) | 0 (0.0) | 0.707 |
| Adequate (p10-p89.99) | 157 | 65 (41.4) | 37 (23.6) | 55 (35.0) | |
| Above p90 (LGA) | 35 | 13 (37.1) | 11 (31.4) | 11 (31.4) | |
| Birthweight category in grams | |||||
| <2500 (low birth weight) | 7 | 6 (85.7) | 0 (0.0) | 1 (14.3) | 0.009 |
| 2500–3900 (normal) | 159 | 67 (42.1) | 43 (27.0) | 49 (30.8) | |
| >4000 (macrosomal) | 28 | 8 (28.6) | 4 (14.3) | 16 (57.1) | |
| Pre-eclampsia | 30 | 10 (33.3) | 7 (23.3) | 13 (43.3) | 0.721 |
| Premature labour (<37 wk) | 27 | 12 (44.4) | 7 (25.9) | 8 (29.6) | 0.946 |
In this study, we found that GWG in pregnant women with obesity and GDM determines neonatal birth weight, that a higher percentage of women with excessive GWG deliver LGA infants and those with insufficient GWG deliver more SGA infants. We observed that women with GDM and adequate GWG according to the IOM recommendations regarding their prior BMI had a higher percentage of infants with normal birth weight.
To our knowledge, this is the first time that the IOM GWG recommendations have been assessed in women with both obesity and GDM and not only based on pre-pregnancy BMI. The impact of obesity and GDM on birth weight and pregnancy outcomes are well known, although the potential influence of GWG has not been studied as extensively when both comorbidities coexist. Obese women are more prone to excessive GWG and its derived complications,13,14 although a strict dietary approach, to which patients may adhere more frequently in cases of GDM, may lead to insufficient GWG and SGA. In fact, our patients with insufficient GWG presented a higher rate of SGA infants, despite their previous obesity and GDM, which are well-known risk factors for LGA. Thus, optimal management is crucial for optimal outcomes, although this is not always easy to achieve. In our cohort, many patients met the IOM criteria for insufficient or excessive GWG, and patients with an adequate IOM GWG were in the minority. These findings concur with a recent publication15 and a recent metanalysis which found that women have difficulties in adhering to IOM GWG recommendations, even in clinical trials, despite the fact that these recommendations apparently do help to achieve better pregnancy outcomes.16 GWG recommendations in obese women and their appropriateness in women with GDM is a controversial issue. In this study, a control group of normal-weight pregnant women was not available to compare the results in terms of birth weight. However, the category that gained weight below the IOM recommendations shows a birth weight distribution similar to what was expected in the general population. Approximately 10% of the infants were born with a birth weight above the 90th percentile, and just under 10% were SGA. On the other hand, the women who gained excessive weight had an increased proportion of LGA infants.
It is true that the timing of the last weight measurement varied, the average being 32 weeks, and GWG would have continued to increase up until delivery around week 40. However, we may assume that the trend in GWG would have been similar, despite not having the exact data for these last weeks. Therefore, overall, our results suggest that GWG does in fact determine neonatal birth weight, as has been previously described17 and should therefore be addressed carefully; healthcare providers should strive to find efficient strategies to achieve a healthier GWG as defined by the IOM recommendations.
Care for GWG is becoming increasingly important in view of the upward prevalence of obesity and GDM in women of child-bearing age the world over.18 Indeed, GDM was observed in around 8% of the pregnant women in our hospital and obesity was identified in 30.7% of them. Obesity entails a higher risk of associated comorbidities, which may also increase the rate of adverse obstetric outcomes.2,19 For instance, a recent Scottish study20 identified a prevalence of obesity of almost 20% from the total cohort of pregnant women, and an odds ratio of 8.25 of developing GDM. In addition, maternal age has been identified as another adverse factor, since its increase in the last few years in high-income countries has been associated with a higher risk of GDM. In this regard, the mean age in our cohort was almost 35 years, slightly higher than the figures reported by some previous studies,21–23 which may have influenced the total prevalence of obesity and GDM in our cohort.
The difference between true GDM and existing unidentified intolerance to carbohydrates is not always clear-cut in pregnant women with obesity. In fact, since routine screening is not extensively performed in non-pregnant women of reproductive age, evaluation during pregnancy may reveal existing hyperglycaemia and not a mere increase in insulin resistance inherent to the second and third trimesters of pregnancy.4,24 Universal screening before pregnancy or during the first trimester is still controversial,25 and early screening protocols in our cohort could have overestimated the resulting prevalence of GDM.4,26,27 In fact, GDM diagnosis time may determine different GWG, as we observed in our cohort. However, in any event, an intervention to keep glucose levels under control is deemed necessary for better pregnancy outcomes, and this is frequently associated with a strict control of GWG. For instance, a previous study that evaluated weight changes in women with GDM observed that an excessive GWG was associated with adverse obstetric outcomes, including a higher rate of LGA infants; and insufficient GWG, or even weight loss, could be associated with a lower frequency of the need for hypoglycaemic medications, C-sections and macrosomia, without increasing the prevalence of SGA infants.28
Our study presents certain limitations related to its retrospective design and bias in nutritional interventions for obese women with GDM, since these patients usually have more frequent follow-up check-ups than patients with no metabolic background. However, the aim of our study was precisely to evaluate the outcomes of women with these two issues regarding GWG and their outcome. We do not have long-term data on post-delivery maternal weight and carbohydrate status or on their infants’ long-term metabolic outcome. This was beyond the objectives of our retrospective observational study. Although our cohort of patients is limited to a single centre, it does reflect real-world clinical practice, since our Hospital is a reference facility for a broad territory in our region. More long-term and interventional studies are required to evaluate postpartum consequences for mother and offspring and to evaluate whether GWG recommendations should be universal, based solely on prior BMI, regardless of coexisting GDM or any other form of previous glucose intolerance.
In conclusion, we would draw attention to the importance of controlling GWG during pregnancy with an intensive intervention and follow-up, particularly in obese women with coexisting GDM, in whom background metabolic and behavioural issues may involve opposing weight trends. The IOM guidelines on GWG also appear to be useful in the concomitant GDM setting. GWG may really affect birth weight and pregnancy outcome and should therefore be managed appropriately.
Authors’ contributionsMGRC, CGF, EGV, ACB, PBM and RVT contributed to the conception and design of the study, followed up patients, researched, analysed and interpreted the data and reviewed and edited the manuscript. ARL analysed and interpreted the data and wrote the manuscript. All authors critically reviewed the final version of the manuscript and approved it for submission and subsequent potential publication.
Ethics approvalOur Hospital's Ethics and Clinical Research Committee approved the study protocol, which was compliant with the Declaration of Helsinki. Patient anonymity was preserved. All the data were pseudonymised, guaranteeing ensuring technical and functional separation between the research team and the person tasked with pseudonymization.
Compliance with ethical standardsThe study was approved by the Ethics Committee of our Hospital and complied with the Declaration of Helsinki.
Conflict of interestThe authors declare that there is no conflict of interest that could be perceived as directly or indirectly compromising the impartiality of the research reported.