To evaluate frequency of hypoglycaemia unawareness (HU) in patients with type 1 diabetes(T1D) transferred from Paediatrics following a specific therapeutic education programme (TEP) in an adult hospital.
Patients and methodYoung patients transferred from 2009-2011 were evaluated. The TEP included a coordinated transfer process, individual appointments and a group course. At baseline and at 12 months we evaluated glycated haemoglobin (HbA1c) frequency of severe (SH) hypoglycaemia/patient/year and non severe hypoglycaemia (NSH). The patients were classified into two groups and compared: hypoglycaemia awareness (HA) and HU according to the Clarke Test <3R or > 3R respectively.
ResultsFifty-six patients (age 18.1 ± 0.3 years, 46% females, HbA1c 8.0 ± 1.2%) underwent the TEP. In the baseline evaluation 16% presented HU. The number of SH was higher in the HU Group (0.33 ± 0.50 vs. 0.09 ± 0.28 p < 0.05). The percentage of patients with >2 NSH/week was higher, albeit not significantly, in the HU group (66% vs. 34%, p = 0.06). At 12 months 11% of the patients continued to present HU. The number of SH remained higher in the HU group (0.38 ± 1.06 vs.0.02 ± 0.15 p = 0.04).
ConclusionsThe percentage of young people with T1D with HU is quite high at transfer. Although the TEP improves hypoglycaemia awareness it does not solve this important problem. Patients with HU more frequently present SH. It is necessary to identify HU in order to reduce SH which continues to be a problem in people with T1D.
Evaluar la frecuencia de las hipoglucemias desapercibidas (HD) en pacientes con Diabetes Tipo 1 (DT1), trasladados de pediatría, que siguen programa específico de atención y educación terapéutica (PAET) en el hospital de adultos.
Pacientes y métodosJóvenes trasladados entre 2009-2011. El PAET incluyó proceso de traslado coordinado, visitas individuales y en grupo. Al inicio y 12 meses se valora: HbA1c, frecuencia de hipoglucemias graves paciente /año (HG) y no graves (HNG). Los pacientes fueron clasificados y comparados en 2 grupos: hipoglucemia percibida (HP) e HD, según los resultados del Test de Clarke <3R o >3R respectivamente.
ResultadosRealizaron PAET 56 pacientes (edad 18,1 ± 0,3 años, 46% chicas, HbA1c 8,0 ± 1,2%). En la valoración inicial 16% presentaban HD. El número de episodios de HG fue superior en el grupo HD (0,33 ± 0,50 vs. 0,09 ± 0,28 p < 0,05). El % de pacientes con > 2 HNG /semana fue superior en el grupo HD aunque sin significación estadística (66 vs 34%, p = 0,06). A los 12 meses todavía un 11 % de pacientes presentaba HD. El número de HG siguió siendo superior en el grupo con HD (0,38 ± 1,06 vs.0,02 ± 0,15 p = 0,04).
ConclusionesEl porcentaje de jóvenes con DT1 e HD es considerable en el momento del traslado. El PAET mejora su pronóstico pero no lo soluciona a medio plazo. Los pacientes con HD presentan mayor frecuencia de HG. La detección de HD es necesaria para reducir las HG que todavía son una asignatura pendiente.
Type 1 diabetes mellitus (T1DM) requires insulin use for glycemic control from the time of diagnosis. Patients and their families usually learn to integrate this type of treatment into their daily life through specific therapeutic education programs.1 This type of treatment has evolved over the years with the introduction of rapid- and slow-acting insulin analogues, the use of continuous subcutaneous insulin infusion systems, and more recently with the possibility of interstitial glucose monitoring.2 Intensive insulin therapy has contributed to reducing the microvascular and macrovascular complications of the disease, and has become the standard of care for all patients with T1DM.3,4
Despite all these advances, however, metabolic control remains suboptimal in a large proportion of patients with T1DM, and is particularly difficult to secure in adolescent patients.5 In addition, the period of transition from puberty to adulthood coincides with the transfer of these young people from pediatric centers to adult units, and this makes it particularly challenging when it comes to securing glycemic targets. Ideally, this transition process should be a continuous, structured, supervised and coordinated process between the pediatric and adult care teams.6,7
Hypoglycemia is the most common adverse effect of insulin therapy, particularly when used intensively with multiple doses.8 In addition to its direct and immediate consequences, hypoglycemia has been related to the appearance of other cardiovascular complications.9 Repeated hypoglycemic episodes are associated with the progressive loss of symptoms and warning signs. This dysfunction in the normal physiological response to hypoglycemia is known as hypoglycemia unawareness (HU) syndrome, and affects 20-25% of all patients with T1DM, a figure that increases to 50% in patients with T1DM for more than 20 years.10 Hypoglycemia and HU are currently one of the main limiting factors for achieving optimum glycemic control, and prevent patients from obtaining its long-term benefits.11 Few studies have analyzed the proportion of young people with T1DM who have HU at the time of transfer to the adult unit.
Given the lack of information in this patient population, the present study was carried out to assess the short- to middle-term frequency of HU and its impact upon patients with T1DM transferred from pediatric centers and who are enrolled in a specific therapeutic care and education program (TEP) at the adult unit.
Patients and methodsAn retrospective observational study was carried out in patients with T1DM aged > 18 years and consecutively transferred from the Endocrinology Department of Sant Joan de Déu Pediatric Hospital (Barcelona, Spain) to the Adult Diabetes Unit of Hospital Clínic de Barcelona (Barcelona, Spain), during the period 2009-2011. Both hospitals are reference centers in T1DM care. The exclusion criteria were: less than one year since the onset of T1DM; a transfer performed outside the established program; and the absence of multiple dose insulin therapy (MDI).
The therapeutic care and education program (TEP)12 included a transfer process coordinated from both units, and individual, group and telematics visits. The program has four phases:
Phase 1: patient preparation for pediatric discharge, a clinical-educational report and an appointment.
Phase 2: the first clinical-educational visit to collect information on age, gender, the body mass index (BMI), years since diabetes onset, the insulin regimen, frequency of capillary blood glucose self-monitoring and metabolic control: glycosylated hemoglobin (HbA1c) and the frequency of non-severe hypoglycemia ([NSH] capillary blood glucose < 70 mg/dl in the previous 2 weeks, > 2 episodes/week, > 5 episodes /week) and severe hypoglycemia (SH) in the previous year (defined as an episode requiring the help of another person and/or glucagon administration). Evaluation is also made of the level of knowledge (the Diabetes Knowledge Questionnaire 2-DKQ2,13 consisting of 16 multiple-choice questions. Maximum score: 35 points). Hypoglycemia perception is assessed based on the Clarke test,14,15 which comprises 8 questions with answers classified as A (a good awareness of symptoms) and R (a reduced awareness of symptoms), a score < 2 R indicating normal hypoglycemia awareness (HA), 3 indeterminate awareness, and > 4 anomalous awareness. This study considered HU when the Clarke test score was ≥ 3, and the patients were classified and compared in two groups: those with HA and those with HU. Quality of life is assessed using two questionnaires. The first questionnaire is the Diabetes Quality of Life instrument (DQoL),16 specific for T1DM, and consisting of four scales: satisfaction (a score ranging from 15-75), impact (a score ranging from 17-85), social concern (a score ranging from 7-35) and concern about diabetes (a score ranging from 4-20). In the DQoL questionnaire, the lower the score, the better perceived the quality of life. The second questionnaire is the SF-12 test, which assesses the overall quality of life,17 and consists of 12 questions (a score ranging from 12-47). In the SF-12 questionnaire, the higher the score, the better the patient quality of life. Following this initial assessment, learning objectives and changes in treatment regimen are agreed, if needed, and the patients are informed about the program activities in this first year.
Phase 3: the patients complete a group therapy education course (8 hours) in which knowledge and skills for the daily self-management of treatment are worked upon.
Phase 4: individual follow-up with quarterly visits. The results of all the analyzed variables are assessed at the 12-month visit. Throughout the process, the patients have a telephone number (24 hours, 7 days a week) for emergency consultations (if needed) with the endocrinologist on duty.
Statistical analysisThe results are presented as the mean ± standard deviation (SD) and percentages. The comparisons of quantitative variables between the patients with HA and HU, and of the variables at the start and end of the program, were based on the use of tests for independent or paired data and of a parametric or nonparametric nature, depending on the characteristics of the variables. The chi-squared test was used to compare qualitative variables.
Statistical significance was considered for p < 0.05. The SPSS version 20.0 statistical package (SPSS Inc., Chicago, IL, USA) was used throughout.
ResultsFifty-six patients diagnosed with T1DM were transferred between 2009 and 2011 and were included in the study. The mean age was 18.1 ± 0.3 years, 46% were females, and the duration of T1DM was 8.0 ± 4.0 years. All the patients were treated with multiple dose insulin. During this period, none of them were subjected to continuous subcutaneous insulin infusion (CSII) or continuous glucose monitoring. The initial HbA1c concentration after transfer was 8.0 ± 1.2%. Of the 56 patients, 9 presented HU (16%), and the number of severe hypoglycemia (SH) episodes during the final year was 0.14 ± 0.30 episodes/patient/year (Table 1).
Baseline characteristics of the patients included in the study.
| Number of patients | 56 |
|---|---|
| Age (years) | 18.1 ± 0.3 |
| Gender (female/male) | 26/30 |
| Duration of T1DM (years) | 8.0 ± 4.0 |
| MDI therapy (%) | 100 % |
| BMI (kg/m2) | 23.1 ± 2.4 |
| HbA1c (%) | 8.0 ± 1.2 |
| Hypoglycemia unawareness (%) Clarke test > 3 | 9/56 (16) |
| SH episodes/patient/year | 0.14 ± 0.30 |
T1DM: type 1 diabetes; HbA1c: glycosylated hemoglobin; SH: severe hypoglycemia; BMI: body mass index; MDI: multiple dose insulin.
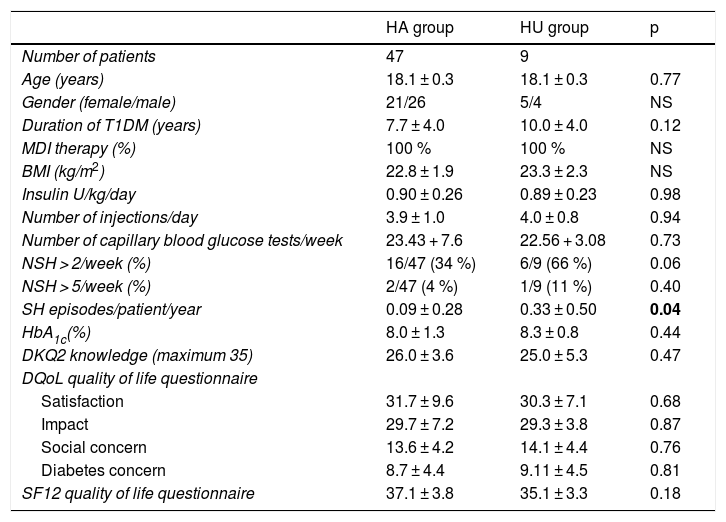
At the start of the TEP, no significant differences were seen in age, gender, disease duration, the BMI, the type of treatment, the dose and number of daily insulin injections, the weekly frequency of capillary blood glucose tests, HbA1c, the degree of knowledge of the disease, or the perceived quality of life between the patients with HA and those with HU. The percentage of patients with > 2 episodes of NSH/week was higher in the HU group, though without reaching statistical significance (HU 66% [6 of 9 patients] vs. HA 34% [16 of 47 patients]; p = 0.06). Likewise, there was no significant difference in the percentage of patients with > 5 episodes of NSH/week between the two groups (HU 11% [1 of 9 patients] vs. HA 4% [2 of 47 patients]; p = 0.40). It is important to note that the frequency of severe hypoglycemia episodes/patient/year was significantly higher in the HU group (HU 0.33 + 0.50 vs. HA 0.09 + 0.28; p = 0.04) (Table 2).
Baseline clinical characteristics of the patients with normal hypoglycemia awareness (HA) and with hypoglycemia unawareness (HU).
| HA group | HU group | p | |
|---|---|---|---|
| Number of patients | 47 | 9 | |
| Age (years) | 18.1 ± 0.3 | 18.1 ± 0.3 | 0.77 |
| Gender (female/male) | 21/26 | 5/4 | NS |
| Duration of T1DM (years) | 7.7 ± 4.0 | 10.0 ± 4.0 | 0.12 |
| MDI therapy (%) | 100 % | 100 % | NS |
| BMI (kg/m2) | 22.8 ± 1.9 | 23.3 ± 2.3 | NS |
| Insulin U/kg/day | 0.90 ± 0.26 | 0.89 ± 0.23 | 0.98 |
| Number of injections/day | 3.9 ± 1.0 | 4.0 ± 0.8 | 0.94 |
| Number of capillary blood glucose tests/week | 23.43 + 7.6 | 22.56 + 3.08 | 0.73 |
| NSH > 2/week (%) | 16/47 (34 %) | 6/9 (66 %) | 0.06 |
| NSH > 5/week (%) | 2/47 (4 %) | 1/9 (11 %) | 0.40 |
| SH episodes/patient/year | 0.09 ± 0.28 | 0.33 ± 0.50 | 0.04 |
| HbA1c(%) | 8.0 ± 1.3 | 8.3 ± 0.8 | 0.44 |
| DKQ2 knowledge (maximum 35) | 26.0 ± 3.6 | 25.0 ± 5.3 | 0.47 |
| DQoL quality of life questionnaire | |||
| Satisfaction | 31.7 ± 9.6 | 30.3 ± 7.1 | 0.68 |
| Impact | 29.7 ± 7.2 | 29.3 ± 3.8 | 0.87 |
| Social concern | 13.6 ± 4.2 | 14.1 ± 4.4 | 0.76 |
| Diabetes concern | 8.7 ± 4.4 | 9.11 ± 4.5 | 0.81 |
| SF12 quality of life questionnaire | 37.1 ± 3.8 | 35.1 ± 3.3 | 0.18 |
DKQ2: knowledge questionnaire: Diabetes Knowledge Questionnaire 2; DQoL: T1DM specific quality of life questionnaire; T1DM: type 1 diabetes mellitus; HbA1c: glycosylated hemoglobin; SH: severe hypoglycemia; NSH: non-severe hypoglycemia; BMI: body mass index; MDI: multiple dose insulin; SF12: general health-related quality of life questionnaire.
Data in bold are statistically significant.
At the end of the TEP in the global patient series, nonsignificant reductions were observed in: HbA1c (from 8.01 ± 1.22 to 7.70 ± 0.99%) (p = 0.18); the percentage of patients with HU (from 16% at baseline to 11% [2 patients in the HA group converted to HU and 4 patients in the HU group converted to HA]); and the number of SH episodes/patient/year (from 0.14 + 0.30 to 0.08 + 0.44) (p = 0.32).
Likewise, no differences were seen at the end of the TEP in relation to the BMI, the type of treatment, the dose and number of daily insulin injections, the weekly frequency of capillary blood glucose tests, HbA1c, the degree of knowledge of the disease, or the perceived quality of life between the patients with HA and those with HU.
It is notable that 44% of the patients in the HU group at the start of the program improved their awareness of hypoglycemic symptoms with a Clarke score < 3R after 12 months. However, in the group of patients initially classified as presenting HA, a 4.2% increase was observed in the number of patients with a Clarke score ≥ 3R.
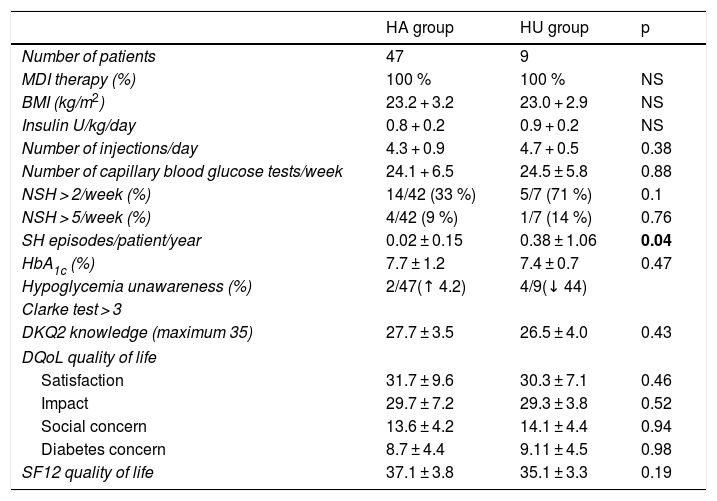
No statistically significant differences were seen in the percentage of patients with > 2 episodes of NSH/week in the group with HU as compared to the group with HA (HA 33% vs. HU 71%; p = 0.1), or in the percentage of patients with > 5 episodes of NSH/week (HA 9% vs. HU 14%; p = 0.76). It should be noted that the number of SH episodes remained significantly higher in the group of patients with HU (HA 0.02 + 0.15 vs. HU 0.38 + 1.06; p = 0.04) (Table 3).
Clinical characteristics of the patients with normal hypoglycemia awareness (HA) and with hypoglycemia unawareness (HU) after 12 months.
| HA group | HU group | p | |
|---|---|---|---|
| Number of patients | 47 | 9 | |
| MDI therapy (%) | 100 % | 100 % | NS |
| BMI (kg/m2) | 23.2 + 3.2 | 23.0 + 2.9 | NS |
| Insulin U/kg/day | 0.8 + 0.2 | 0.9 + 0.2 | NS |
| Number of injections/day | 4.3 + 0.9 | 4.7 + 0.5 | 0.38 |
| Number of capillary blood glucose tests/week | 24.1 + 6.5 | 24.5 ± 5.8 | 0.88 |
| NSH > 2/week (%) | 14/42 (33 %) | 5/7 (71 %) | 0.1 |
| NSH > 5/week (%) | 4/42 (9 %) | 1/7 (14 %) | 0.76 |
| SH episodes/patient/year | 0.02 ± 0.15 | 0.38 ± 1.06 | 0.04 |
| HbA1c (%) | 7.7 ± 1.2 | 7.4 ± 0.7 | 0.47 |
| Hypoglycemia unawareness (%) | 2/47(↑ 4.2) | 4/9(↓ 44) | |
| Clarke test > 3 | |||
| DKQ2 knowledge (maximum 35) | 27.7 ± 3.5 | 26.5 ± 4.0 | 0.43 |
| DQoL quality of life | |||
| Satisfaction | 31.7 ± 9.6 | 30.3 ± 7.1 | 0.46 |
| Impact | 29.7 ± 7.2 | 29.3 ± 3.8 | 0.52 |
| Social concern | 13.6 ± 4.2 | 14.1 ± 4.4 | 0.94 |
| Diabetes concern | 8.7 ± 4.4 | 9.11 ± 4.5 | 0.98 |
| SF12 quality of life | 37.1 ± 3.8 | 35.1 ± 3.3 | 0.19 |
DKQ2: knowledge questionnaire: Diabetes Knowledge Questionnaire 2; DQoL: T1DM specific quality of life questionnaire; HbA1c: glycosylated hemoglobin; SH: severe hypoglycemia; NSH: non-severe hypoglycemia; BMI: body mass index; MDI: multiple dose insulin; SF12: general health-related quality of life questionnaire
Data in bold are statistically significant.
The results of our study suggest that the proportion of patients with T1DM and HU is not negligible among young people transferring from pediatric centers to adult units. While the adoption of a specific consensus-based patient transfer program can help reduce this proportion, it is unable to eradicate the problem.
It should be noted that patients with HU at both initial evaluation and after 12 months of follow-up within the program showed a significantly higher incidence of severe hypoglycemic episodes/patient/year. Likewise, the frequency of > 2 episodes of NSH/week and of > 5 episodes of NSH/week was higher in the group of patients with HU, both at the start and at the end of the program.
On making comparisons with other studies, Abraham et al.,18 using the same Clarke questionnaire, found the prevalence of HU to be 33% in 390 young people with T1DM and > 12 years of age in 2002, and 21% in 402 young people in 2015. The authors emphasized that the presence of HU increased the risk of severe hypoglycemia (SH) in both of the studied cohorts.
In another study conducted in our geographical area by Conget et al.19 in the adult population (418 patients aged 36-55 years) and using the same questionnaire, 25% of the subjects presented HU, though the cut-off point used in that study was 4R. In this same study, the years of disease were related to a higher score in the questionnaire. In addition, 14% of the patients with HU had experienced at least one SH episode during the previous year.
With regard to the degree of glycemic control and the number of SH episodes, the results in our study are similar to those reported by Haynes et al.20 in a registry of three pediatric patient databases. In a population of 7102 individuals (Type 1 Diabetes Exchange) under 18 years of age and with HbA1c 8.6 + 1.4%, the incidence of SH was 0.07 episodes/patient/year; in the Diabetes Patienten Verlausfsdokumentation involving 18,787 patients with HbA1c 8.0 ± 1.4%, the incidence was 0.03 episodes/patient/year; and in the 865 patients of the Western Australian Children Diabetes Database with HbA1c 8.2 ± 1.3%, the reported incidence was 0.06 episodes/patient/year.
The incidence of SH in our patients with HU was 10 times higher than that reported in the aforementioned databases, which gives an idea of the risk associated with the presence of this condition.21 In addition to the direct and indirect risks associated with SH, some studies have associated the presence of HU with a three-fold greater risk of death.22 These findings have not been corroborated by other studies, however.23
The DCCT/EDIC study24 showed that being an adolescent increased the associated risk of SH. It also found having SH to be the most potent predictor of new episodes of SH later in life.
Other studies have shown that hypoglycemia with or without symptoms is an unpleasant situation that affects patient quality of life and results in fear of hypoglycemia, particularly in children, adolescents and/or parents. This situation in turn may limit much desired good metabolic control25.
As regards the prevention of SH, many authors stress that it is important for patients and/or their families to follow structured educational programs.24 Among the few randomized studies conducted in the patient transition period,26 we find that certain clinical parameters, such as HbA1c, remain similar in both groups (control and intervention) despite increased satisfaction and adherence to visits, with a reduction of stress over the 18 months, in the intervention group. Such improvements are not maintained in the subsequent 12 months. These results confirm that the approach to management in this period is not easy.
Our TEP12,27 allows for the individualization of treatment self-management, the therapeutic scheme, and the agreement of objectives based on individual patient assessment, taking into account the patient’s level of knowledge, quality of life and awareness of hypoglycemia, all of which are important factors for decision-making and for increased patient empowerment. With regard to HU, the program is able to reduce the percentage of patients with HU, but cannot solve the problem.
The introduction of therapeutic alternatives such as insulin analogues and technological devices such as CSII, continuous glucose monitoring using flash or real time systems, allows for gradual improvements in the prevention of hypoglycemia.28,29
The use of CSII systems may reduce HU and improve symptoms awareness, as was shown by Giménez et al.30 in a study involving a cohort of 20 patients with T1DM in which, based on the Clarke test, HU was detected in 95% of the cases. The patients had also experienced ≥ 2 episodes of SH in the previous two years. Severe hypoglycemia decreased from 1.25 episodes/patient/year to only 0.05 after 24 months. The awareness of hypoglycemia symptoms progressively improved at 6 and 12 months, though it was not until after 24 months that 17 of the 20 patients had recovered awareness. Although the patients in the present study did not use these devices, the results obtained might have been better if they could have been used.
Our study has limitations attributable to the number of patients involved and to the evaluation of results after 12 months, this being too short an interval for assessing changes in hypoglycemia awareness and/or SH.30 Another limitation is the fact that this was a single-center study, establishing before-and-after evaluations of an educational program, with no control group. Consequently, no cause-effect relationship could be established.
The strengths of the study include the fact that it was conducted in line with standard clinical practice as part of a structured and specific educational program targeted at young people transferred from pediatric centers to the adult hospital unit, where HU was systematically detected based on the Clarke test. This questionnaire is easy to use and has good psychometric characteristics, both in its original version in English and in its validated versions in Spanish and Catalan.15
In sum, the transfer of young adults with T1DM from pediatric centers to adult units is always a critical time for patients, their environment, and for control of the disease. The presence of HU may make this process more difficult, and our study shows that neither is its prevalence negligible nor its resolution guaranteed over the middle term. All this suggests that the detection of HU should be incorporated into the patient transfer process, and that its resolution should form part of the objectives of any specific educational program focusing on these individuals.
AuthorshipMercè Vidal and Ignacio Conget conceived the present study. Carmen Yoldi and Roque Cardona monitored the patients before their transfer to the adult hospital. Marga Giménez, Daria Roca, Marga Jansà and Mercè Vidal monitored the patients at the visits following transfer to the adult hospital. Ignacio Conget and Marga Jansà supervised the project methodology and performed the statistical analysis. Mercè Vidal coordinated and wrote the first draft, which was reviewed by all the authors. Mercè Vidal, Marga Jansà and Ignacio Conget wrote the final version of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Thanks are due to all the professionals from both the Pediatrics Team of Hospital Sant Joan de Déu and the Adults Unit of the Hospital Clínic, for their dedication and professionalism in the setting of the therapeutic care and education program (TEP) aimed at young people with type 1 diabetes during the transfer period. Thanks are also due to Dr. Sabina Ruiz and Dr. Antonio Amor for their support in the statistical analysis.
We also thank the young people and/or their relatives or caregivers who completed the therapeutic care and education program.
Please cite this article as: Vidal M, Jansà M, Roca D, Yoldi C, Cardona-Hernández R, Giménez M, et al. Hipoglucemia desapercibida en jóvenes con diabetes tipo 1 trasladados a un centro de adultos. Endocrinol Diabetes Nutr. 2020;67:394–400.