Information regarding the postpartum period in women with type 1 diabetes (T1D) is scarce. We aim to evaluate the relation of impaired hypoglycaemia awareness (IAH) in early pregnancy and breastfeeding status (its presence and duration) with severe postpartum hypoglycaemia (SH).
Materials and methodsRetrospective cohort study of women with T1D followed during pregnancy between 2012 and 2019. Data on SH were recorded before and during pregnancy. IAH was evaluated at the first antenatal visit. Data on breastfeeding and the long-term postpartum period were collected by questionnaire and from medical records.
ResultsA total of 89 women with T1D were included with a median follow-up after pregnancy of 19.2 [8.7–30.5] months. Twenty-eight (32%) women had IAH at the first antenatal visit. At discharge, 74 (83%) started breastfeeding during a median of 8 [4.4–15] months. A total of 18 (22%) women experienced ≥1 SH during postpartum. The incidence of SH significantly increased from pregestational to the gestational and post-partum period (0.09, 0.15 and 0.25 episodes/patient-year, respectively). Postpartum SH rates were comparable in breastfeeding and non-breastfeeding women (21.4% vs. 25%, respectively, p>0.05). Clarke test score at the first antenatal visit was associated with postpartum SH (for each 1-point increase: OR 1.53; 95% CI, 1.06–2.21) adjusted for confounders. No other diabetes and pregnancy-related variables were identified as predictors of SH in this period.
ConclusionsSH are common in the long-term postpartum period independently of breastfeeding. Assessing IAH in early pregnancy could identify those at an increased risk of SH in the postpartum period.
Hay pocos datos sobre el periodo posparto en mujeres con diabetes tipo 1. Nuestro objetivo fue evaluar la relación entre la percepción alterada de las hipoglucemias (PAH) al inicio de la gestación y la lactancia (modalidad y duración) con las hipoglucemias graves (HG) posparto.
Materiales y métodosEstudio retrospectivo de cohortes de mujeres con diabetes tipo 1 seguidas durante la gestación durante 2012-2019. Se recogieron las HG antes y durante la gestación. Se evaluó la PAH en la primera visita obstétrica. Los datos sobre lactancia y el periodo posparto se recogieron mediante cuestionario y de la historia clínica.
ResultadosSe incluyeron 89 mujeres con diabetes tipo 1 con un seguimiento posparto de 19,2 (8,7-30,5) meses. Veintiocho (32%) tenían PAH en la primera visita obstétrica. Al alta, 74 (83%) iniciaron lactancia materna durante una mediana de 8 (4,4-15) meses. Dieciocho (22%) mujeres tuvieron≥1 HG en el posparto. La incidencia de HG aumentó significativamente del periodo pregestacional a la gestación y el posparto (0,09, 0,15 y 0,25 episodios/paciente-año, respectivamente). La tasa de HG posparto fue comparable en mujeres con lactancia materna y artificial (21,4 vs. 25%, respectivamente, p>0,05). La puntuación en el test de Clarke en la primera visita obstétrica se asoció independientemente a HG posparto (por incremento de un punto: OR 153; IC 95% 1,06-2,21). No se identificaron otros predictores de HG entre las variables relacionadas con la diabetes o la gestación durante este periodo.
ConclusionesLas HG son frecuentes en el largo plazo posparto, independientemente del modo de lactancia. Evaluar la PAH al inicio de la gestación podría identificar aquellas mujeres con un mayor riesgo de HG en el posparto.
In women with type 1 diabetes (T1D), strict glycaemic control during pregnancy is paramount to improve maternal and foetal adverse pregnancy outcomes.1 Glycaemic targets near normoglycaemia are recommended during pregnancy.2,3 Consequently, many women experience severe hypoglycaemia (SH) during pregnancy,4 especially those with a history of SH or impaired hypoglycaemia awareness (IAH).5 Nevertheless, data on SH on the postpartum period are scarce.
Strict glycaemic control, as well as diabetes duration, are risk factors for IAH in T1D. IAH leads to an increased risk of SH and its morbidity.6 Pregnant women with T1D are at increased risk for IAH, especially during early pregnancy. Also, IAH has been recently identified as a new risk factor for adverse pregnancy outcomes.7
Women with T1D also face extra challenges after childbirth because they have a double role: caring for the newborn and managing their diabetes. Breastfeeding could add an extra challenge in daily diabetes routines in the transition to motherhood.8 Immediately after delivery, insulin requirements decline to about 30%–50% of the pre-pregnancy dose due to lack of placental hormone influence. Together with a net increased energy demand of 500kcal/day related to breastfeeding, insulin must be adjusted carefully to avoid SH.9,10
Breastfeeding initiation rates in women with T1D compared to the general population are discrepant across the literature,11–14 although it is mostly suggested that breastfeeding duration is shorter in women with T1D11–15 largely explained by obstetric and sociodemographic variables.11,12 Long-term breastfeeding has also been associated with metabolic control and breastfeeding-related variables, such as pre-birth intention to breastfeed, early breastfeeding, number of feedings in the first 24h and breastfeeding at discharge.15–17 Glycaemic patterns of breastfeeding women and the impact of breastfeeding on glycaemia, particularly in hypoglycaemia, have been insufficiently described. Similarly, whether or not breastfeeding increases the risk of SH is still a matter for debate.8,18
Few studies have evaluated metabolic control in the postpartum period, and most of them were performed in the first weeks postpartum, ranging for up to 4–6 months, with contradictory findings. Some reported a similar incidence of hypoglycaemia and lower glucose variability in the first 6 months,19–21 while another small study (12 breastfeeding women followed for 8 weeks) described a higher rate of non-severe hypoglycaemia episodes in breastfeeding women at 2 weeks postpartum.22 SH have only been evaluated in two studies: one study identified 16 SH episodes in 11% of breastfeeding women at 4 months postpartum, this rate being similar in women who were feeding artificially; in another study, SH was reported by one (3%) mother and one (3%) control woman at 6 months post-partum.16,20 The role of IAH in the incidence of postpartum SH has not been previously studied.
With this background, our aim was to describe the metabolic changes in the postpartum period over a longer time frame compared to previous studies, focusing on SH. More specifically, we seek to identify the relationship of IAH in early pregnancy and breastfeeding status (either its presence or duration) with postpartum SH.
Material and methodsStudy designThis is a retrospective, single-centre, observational cohort study of women with T1D followed during their pregnancy at a reference diabetes and pregnancy unit in a tertiary university hospital in Spain from 2012 to 2019. The study protocol was conducted according to the principles of the Declaration of Helsinki and was approved by the institution's Ethics Committee. All participants provided their written informed consent.
During pregnancy, patients were followed every 1–3 weeks at joint visits with the endocrinologist and obstetrician until delivery. The insulin dose was reduced by 50% after delivery and the patients were seen again at 6 weeks postpartum. Subsequently, the patients resumed follow-up with their reference endocrinologist at 3–4 months postpartum. Patients were followed according to the recommendations of the Guidelines of the Spanish Group of Diabetes and Pregnancy. HbA1c goals during pregnancy were <6.5%.2
Data collectionThe demographic and anthropometrical (weight and body mass index [BMI]) data, smoking status and clinical characteristics of the patients, as well as type of insulin treatment and insulin doses, were collected pre-pregnancy (last visit before the day of the last menstrual period), at the first antenatal visit and during the pregnancy follow-up visits. Data on other comorbidities such as hypertension (defined as taking antihypertensive treatment or repeated systolic blood pressure ≥140mmHg and/or diastolic blood pressure ≥90mmHg) and dyslipidaemia (defined as taking lipid-lowering drugs or LDL-cholesterol>160mg/dL) were also reported. Microvascular complications were screened at the first visit: Diabetic retinopathy was defined by fundus oculi performed by a specialised ophthalmologist, diabetic nephropathy was considered if urinary albumin excretion was ≥30mg/g confirmed in at least two out of three consecutive determinations and diabetic neuropathy when symptoms were present. Macrovascular complications were defined as a history of ischaemic heart disease, ischaemic stroke, transient ischaemic attack (TIA) or peripheral artery disease. Glycaemic control was assessed through HbA1c values (National Glycohemoglobin Standardization Program Diabetes Control and Complication Trial (DCCT) Tosoh G8 Automated high-performance liquid chromatography; Tosoh Bioscience Inc, South San Francisco, California; normal range 4.0%–6.0% [20–42mmol/mol]) prior to conception (<3 months) and at each trimester during pregnancy: in weeks 8–10 (1st trimester), weeks 22–24 (2nd trimester) and weeks 32–34 (3rd trimester). Postpartum HbA1c was the HbA1c obtained closest to the postpartum evaluation date at 17.9 (9.3–28) months postpartum.
The women received structured education and special dietary advice on carbohydrate intake and energy intake during pregnancy.
Pregnancy outcomesThe maternal and neonatal outcomes evaluated were primary caesarean delivery, induced or dystocic deliveries, gestational hypertensive disease (including gestational hypertension and preeclampsia), large for gestational age defined as birth weight above the 90th centile for gestational age, small for gestational age (SGA) defined as birth weight below the 10th centile for gestational age, macrosomia defined as neonatal weight at or above 4000g, prematurity (delivery before week 37.0), severe prematurity (delivery before week 34.0) and neonatal severe adverse outcomes (defined as any of the following: neonatal hypoglycaemia [defined as plasma glucose levels of <45mg/dl in the first 24h of life and <50mg/dl thereafter], obstetric trauma, neonatal hyperbilirubinaemia, neonatal respiratory distress [clinical diagnosis by the neonatologist], neonatal intensive care unit admission [for treatment or observation] or perinatal death). Gestational age was defined as weeks completed based on the earliest ultrasound assessment.
Postpartum periodData on breastfeeding (BF) initiation were collected from the obstetrics discharge documents. Specific data on the postpartum period were collected retrospectively through a questionnaire emailed to the patients at the time of data collection. It included questions on breastfeeding status, kind of breastfeeding (exclusive or combined), duration and reasons leading to breastfeeding discontinuation or non-initiation. It also included questions on glycaemic control during the long-term postpartum period, such as a record of SH episodes, weight, insulin doses and type of insulin treatment, data complemented from clinical records.
Hypoglycaemia evaluationSH were defined as hypoglycaemia requiring assistance from a third party. The validated Spanish version of the Clarke test was used to evaluate hypoglycaemia awareness.23 A score equal to or above three points was considered as IAH.24,25 The Clarke test score and the number of SH episodes in the previous 2 years were recorded at the first antenatal visit. SH events during pregnancy were recorded prospectively at every visit. Information on SH in the postpartum period was obtained retrospectively through a questionnaire.
Statistical analysesResults are presented as mean (standard deviation [SD]) in quantitative variables with normal distribution, median [interquartile range (IQR)] in those with non-normal distribution or percentages, in categorical variables. Normal distribution was tested for each variable using the Kolmogorov test.
Comparisons between groups (BF vs. non-BF, SH vs. non-SH) were performed using the Student t test for normally distributed variables or the Mann–Whitney U test for non-normally distributed variables. The McNemar test was used for paired categorical variables. Proportions were compared using a Pearson's chi-square or Fisher exact test, as appropriate.
Logistic regression models were performed to estimate the odds ratio (OR) of (1) breastfeeding at discharge and (2) postpartum SH. The first model included pre-gestational HbA1c and BMI, age, diabetes duration, IAH and gestational SH. The second model included the Clarke test score, pregnancy SH, diabetes duration, continuous subcutaneous insulin infusion (CSII) therapy, pre-gestational HbA1c and pre-gestational BMI.
Furthermore, the cohort was divided according to breastfeeding duration (non-BF, BF<6 or ≥6 months). For this analysis, only women who at 6 months post-partum had either completed or were still breastfeeding were included.
The significance level was defined as a p-value <0.05. All statistical calculations were performed using the IBM SPSS Statistics 22.0 (SPSS Inc.; Chicago, IL, USA) programme.
ResultsParticipants’ characteristicsA total of 89 singleton pregnancies were included in the study. Data on the postpartum period, including postpartum SH, were available for 82 patients.
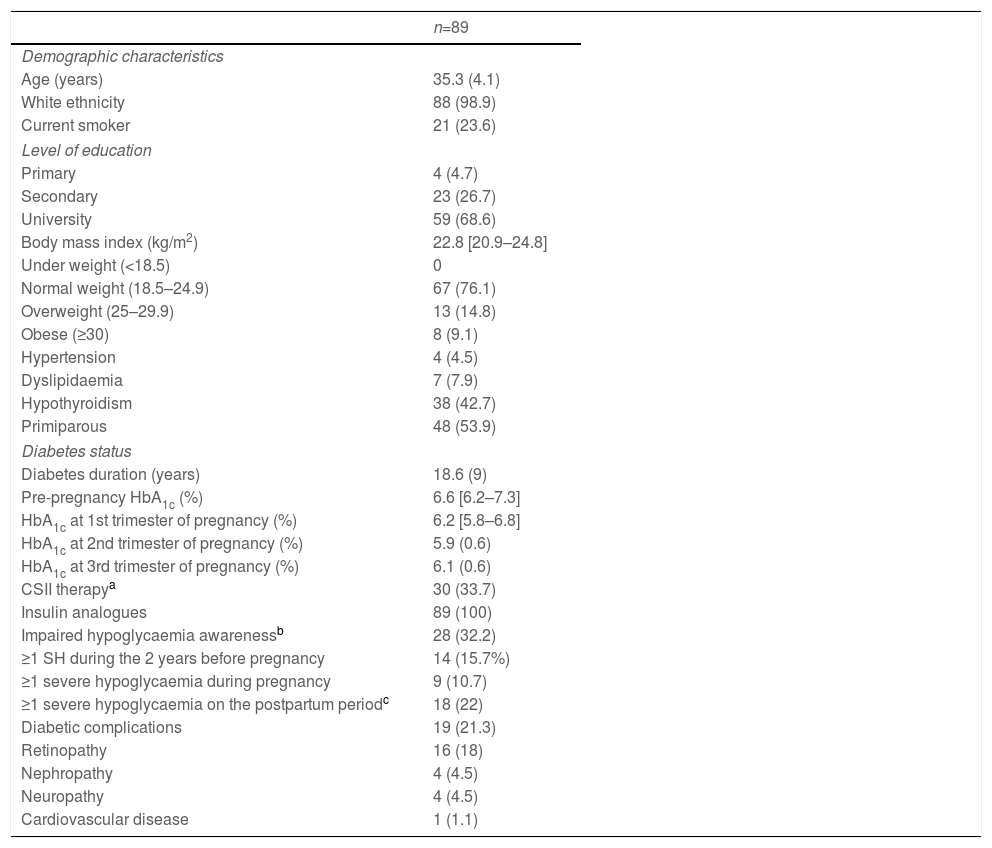
The women had a mean age of 35.3 (4.1) years and a diabetes duration of 18.6 (9) years. One third (28 [32.2%]) had IAH at the beginning of pregnancy (evaluated in the first antenatal visit at 7.9 (6.9–8.5) weeks of gestation), 30 (33.7%) were using CSII and 10 (11.2%) were using continuous glucose monitoring (CGM): 6 (6.7%) were using i-MCG and 4 (4.5%), rt-MCG within the context of sensor-augmented pump (SAP) systems. All women were on insulin analogues. They were on the following basal insulins: 83.3% glargine U-100, 8.3% glargine U-300, 6.3% detemir and 2.1% degludec; and prandrial insulins: 32% lispro, 66% aspart and 2% glulisine. Of the women using CSII, 58% were on lispro and 42% aspart. The baseline data of the women included are shown in Table 1.
Maternal characteristics during pregnancy.
| n=89 | |
|---|---|
| Demographic characteristics | |
| Age (years) | 35.3 (4.1) |
| White ethnicity | 88 (98.9) |
| Current smoker | 21 (23.6) |
| Level of education | |
| Primary | 4 (4.7) |
| Secondary | 23 (26.7) |
| University | 59 (68.6) |
| Body mass index (kg/m2) | 22.8 [20.9–24.8] |
| Under weight (<18.5) | 0 |
| Normal weight (18.5–24.9) | 67 (76.1) |
| Overweight (25–29.9) | 13 (14.8) |
| Obese (≥30) | 8 (9.1) |
| Hypertension | 4 (4.5) |
| Dyslipidaemia | 7 (7.9) |
| Hypothyroidism | 38 (42.7) |
| Primiparous | 48 (53.9) |
| Diabetes status | |
| Diabetes duration (years) | 18.6 (9) |
| Pre-pregnancy HbA1c (%) | 6.6 [6.2–7.3] |
| HbA1c at 1st trimester of pregnancy (%) | 6.2 [5.8–6.8] |
| HbA1c at 2nd trimester of pregnancy (%) | 5.9 (0.6) |
| HbA1c at 3rd trimester of pregnancy (%) | 6.1 (0.6) |
| CSII therapya | 30 (33.7) |
| Insulin analogues | 89 (100) |
| Impaired hypoglycaemia awarenessb | 28 (32.2) |
| ≥1 SH during the 2 years before pregnancy | 14 (15.7%) |
| ≥1 severe hypoglycaemia during pregnancy | 9 (10.7) |
| ≥1 severe hypoglycaemia on the postpartum periodc | 18 (22) |
| Diabetic complications | 19 (21.3) |
| Retinopathy | 16 (18) |
| Nephropathy | 4 (4.5) |
| Neuropathy | 4 (4.5) |
| Cardiovascular disease | 1 (1.1) |
CSII: continuous subcutaneous insulin infusion.
Data are expressed as mean (standard deviation) or median [interquartile range] and n (percentage), as appropriate.
Questionnaire information was obtained for 71 (79.8%) pregnancies at 19.2 [8.7–30.5] months postpartum. No differences in birth weight, gestational age, type of delivery or obstetric complications were observed between responders and non-responders to the questionnaire.
Breastfeeding characteristicsA total of 74 (83.1%) women were breastfeeding at discharge, with a median duration of 8 [4.4–15] months. At the time of data collection (19.2 [8.7–30.5] months postpartum), 25 (33.8%) women were still breastfeeding (median duration of breastfeeding 12 [5–23] months). Most patients (57 [95%]) breastfed on demand, while only 3 (5%) did so on a fixed timetable basis. The reasons for non-initiation of breastfeeding were: women's preference (n=4, 26.7%), maternal illness (n=2, 13.3%), neonatal illness (n=1, 6.7%), hypoglycaemia (n=1, 6.7%), unknown (n=7, 46.7%).
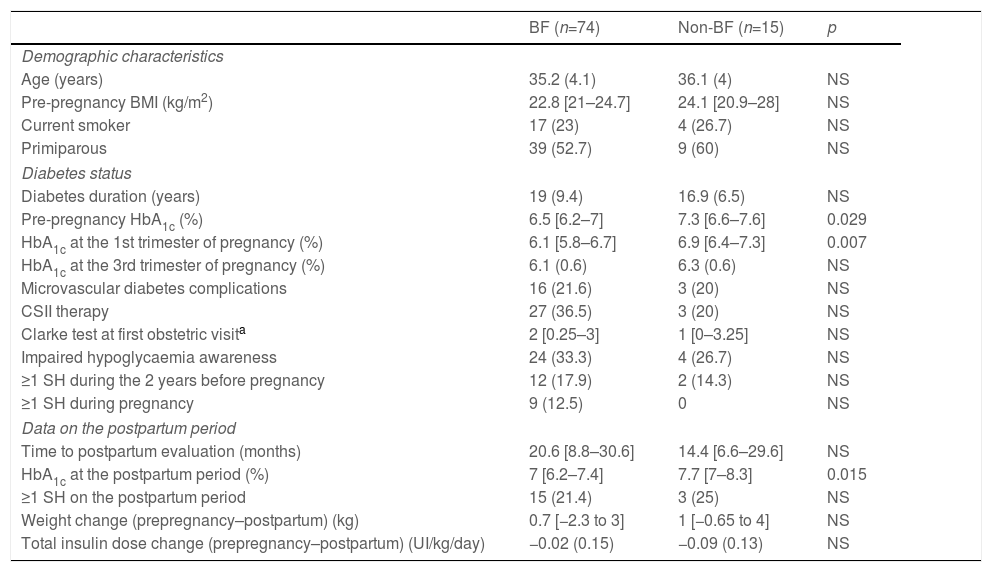
Compared to the non-BF group, women breastfeeding at discharge had a lower pre-pregnancy HbA1c, a lower HbA1c in the first trimester and a lower HbA1c in the long-term postpartum period (p<0.05 for all comparisons). No other differences in metabolic control during pregnancy or the postpartum period were observed (Table 2). Regarding obstetric and neonatal outcomes, the BF-group had higher birth weight (3391.6 [491.4]g vs. 2988.9 [756.8]g; p=0.010), but this was not significant following adjustment for gestational age. No other differences regarding pregnancy outcomes were observed (data not shown). No predictors of breastfeeding at discharge were identified in the logistic regression model.
Clinical characteristics, metabolic control and severe hypoglycaemia during and after pregnancy according to breastfeeding status at discharge.
| BF (n=74) | Non-BF (n=15) | p | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 35.2 (4.1) | 36.1 (4) | NS |
| Pre-pregnancy BMI (kg/m2) | 22.8 [21–24.7] | 24.1 [20.9–28] | NS |
| Current smoker | 17 (23) | 4 (26.7) | NS |
| Primiparous | 39 (52.7) | 9 (60) | NS |
| Diabetes status | |||
| Diabetes duration (years) | 19 (9.4) | 16.9 (6.5) | NS |
| Pre-pregnancy HbA1c (%) | 6.5 [6.2–7] | 7.3 [6.6–7.6] | 0.029 |
| HbA1c at the 1st trimester of pregnancy (%) | 6.1 [5.8–6.7] | 6.9 [6.4–7.3] | 0.007 |
| HbA1c at the 3rd trimester of pregnancy (%) | 6.1 (0.6) | 6.3 (0.6) | NS |
| Microvascular diabetes complications | 16 (21.6) | 3 (20) | NS |
| CSII therapy | 27 (36.5) | 3 (20) | NS |
| Clarke test at first obstetric visita | 2 [0.25–3] | 1 [0–3.25] | NS |
| Impaired hypoglycaemia awareness | 24 (33.3) | 4 (26.7) | NS |
| ≥1 SH during the 2 years before pregnancy | 12 (17.9) | 2 (14.3) | NS |
| ≥1 SH during pregnancy | 9 (12.5) | 0 | NS |
| Data on the postpartum period | |||
| Time to postpartum evaluation (months) | 20.6 [8.8–30.6] | 14.4 [6.6–29.6] | NS |
| HbA1c at the postpartum period (%) | 7 [6.2–7.4] | 7.7 [7–8.3] | 0.015 |
| ≥1 SH on the postpartum period | 15 (21.4) | 3 (25) | NS |
| Weight change (prepregnancy–postpartum) (kg) | 0.7 [−2.3 to 3] | 1 [−0.65 to 4] | NS |
| Total insulin dose change (prepregnancy–postpartum) (UI/kg/day) | −0.02 (0.15) | −0.09 (0.13) | NS |
BF: breastfeeding, CSII: continuous subcutaneous insulin infusion, SH: severe hypoglycaemia, WHO: World Health Organization.
Data presented as n (%), mean (SD) or median [interquartile range].
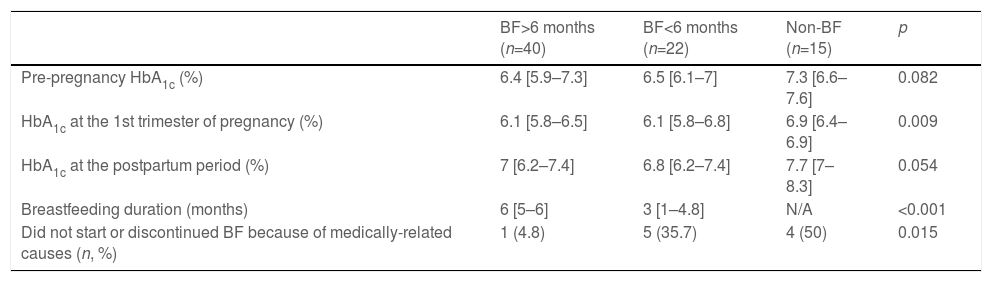
Finally, the cohort was divided according to breastfeeding duration (Table 3). No significant differences on glycaemic control were found between women breastfeeding for <6 or ≥6 months. Nevertheless, women who did not initiate or stopped breastfeeding before 6 months reported a medically-related cause more frequently than those breastfeeding for ≥6 months.
Glycaemic control, BF duration and reasons leading to BF non-initiation or discontinuation according to BF duration.
| BF>6 months (n=40) | BF<6 months (n=22) | Non-BF (n=15) | p | |
|---|---|---|---|---|
| Pre-pregnancy HbA1c (%) | 6.4 [5.9–7.3] | 6.5 [6.1–7] | 7.3 [6.6–7.6] | 0.082 |
| HbA1c at the 1st trimester of pregnancy (%) | 6.1 [5.8–6.5] | 6.1 [5.8–6.8] | 6.9 [6.4–6.9] | 0.009 |
| HbA1c at the postpartum period (%) | 7 [6.2–7.4] | 6.8 [6.2–7.4] | 7.7 [7–8.3] | 0.054 |
| Breastfeeding duration (months) | 6 [5–6] | 3 [1–4.8] | N/A | <0.001 |
| Did not start or discontinued BF because of medically-related causes (n, %) | 1 (4.8) | 5 (35.7) | 4 (50) | 0.015 |
The analysis only includes women who at 6 months post-partum either had completed or were still breastfeeding. N/A: non-applicable.
A total of 35 SH episodes were recorded, 18 (22%) women experienced one or more SH during the long-term postpartum period (data obtained at 19.2 [8.7–30.5] months postpartum) with an overall incidence of 0.25 episodes/patient-year. This incidence was significantly higher than that which was reported in the pregestational and gestational period in the same cohort (0.09 episodes/patient-year and 0.15 episodes/patient-year, respectively).
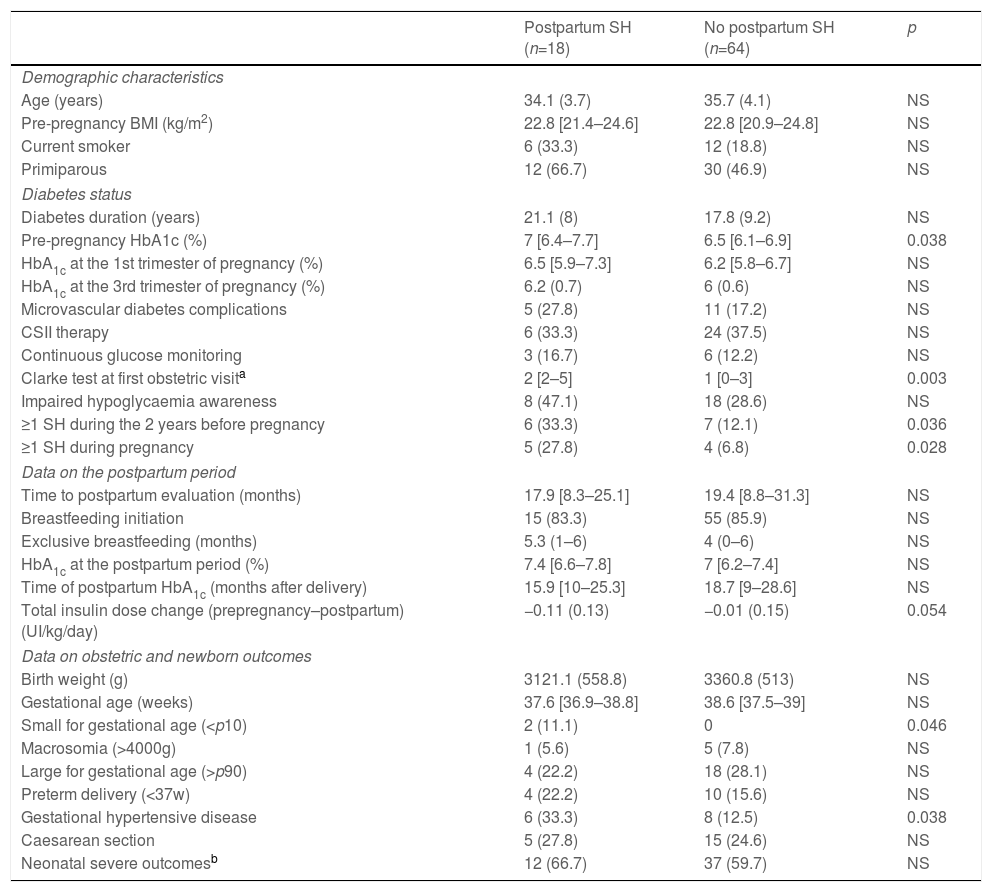
Compared to women who did not suffer SH in the postpartum period, women with SH had higher pre-pregnancy HbA1c levels, higher Clarke test score in the first antenatal visit and a higher prevalence of pre-pregnancy and pregnancy SH. No differences in metabolic control during gestation and the postpartum period were observed (Table 4).
Clinical characteristics, metabolic control, severe hypoglycaemia during and after pregnancy and pregnancy outcomes according to postpartum SH.
| Postpartum SH (n=18) | No postpartum SH (n=64) | p | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 34.1 (3.7) | 35.7 (4.1) | NS |
| Pre-pregnancy BMI (kg/m2) | 22.8 [21.4–24.6] | 22.8 [20.9–24.8] | NS |
| Current smoker | 6 (33.3) | 12 (18.8) | NS |
| Primiparous | 12 (66.7) | 30 (46.9) | NS |
| Diabetes status | |||
| Diabetes duration (years) | 21.1 (8) | 17.8 (9.2) | NS |
| Pre-pregnancy HbA1c (%) | 7 [6.4–7.7] | 6.5 [6.1–6.9] | 0.038 |
| HbA1c at the 1st trimester of pregnancy (%) | 6.5 [5.9–7.3] | 6.2 [5.8–6.7] | NS |
| HbA1c at the 3rd trimester of pregnancy (%) | 6.2 (0.7) | 6 (0.6) | NS |
| Microvascular diabetes complications | 5 (27.8) | 11 (17.2) | NS |
| CSII therapy | 6 (33.3) | 24 (37.5) | NS |
| Continuous glucose monitoring | 3 (16.7) | 6 (12.2) | NS |
| Clarke test at first obstetric visita | 2 [2–5] | 1 [0–3] | 0.003 |
| Impaired hypoglycaemia awareness | 8 (47.1) | 18 (28.6) | NS |
| ≥1 SH during the 2 years before pregnancy | 6 (33.3) | 7 (12.1) | 0.036 |
| ≥1 SH during pregnancy | 5 (27.8) | 4 (6.8) | 0.028 |
| Data on the postpartum period | |||
| Time to postpartum evaluation (months) | 17.9 [8.3–25.1] | 19.4 [8.8–31.3] | NS |
| Breastfeeding initiation | 15 (83.3) | 55 (85.9) | NS |
| Exclusive breastfeeding (months) | 5.3 (1–6) | 4 (0–6) | NS |
| HbA1c at the postpartum period (%) | 7.4 [6.6–7.8] | 7 [6.2–7.4] | NS |
| Time of postpartum HbA1c (months after delivery) | 15.9 [10–25.3] | 18.7 [9–28.6] | NS |
| Total insulin dose change (prepregnancy–postpartum) (UI/kg/day) | −0.11 (0.13) | −0.01 (0.15) | 0.054 |
| Data on obstetric and newborn outcomes | |||
| Birth weight (g) | 3121.1 (558.8) | 3360.8 (513) | NS |
| Gestational age (weeks) | 37.6 [36.9–38.8] | 38.6 [37.5–39] | NS |
| Small for gestational age (<p10) | 2 (11.1) | 0 | 0.046 |
| Macrosomia (>4000g) | 1 (5.6) | 5 (7.8) | NS |
| Large for gestational age (>p90) | 4 (22.2) | 18 (28.1) | NS |
| Preterm delivery (<37w) | 4 (22.2) | 10 (15.6) | NS |
| Gestational hypertensive disease | 6 (33.3) | 8 (12.5) | 0.038 |
| Caesarean section | 5 (27.8) | 15 (24.6) | NS |
| Neonatal severe outcomesb | 12 (66.7) | 37 (59.7) | NS |
BMI: body mass index, CSII: continuous subcutaneous insulin infusion, SH: severe hypoglycaemia.
Data available for 82 patients, 7 patients were excluded from the analysis because of missing or discordant data on severe hypoglycaemia.
Neonatal severe outcomes defined as any of the following: neonatal hypoglycaemia [defined as plasma glucose levels of <45mg/dL in the first 24h of life and <50mg/dL thereafter], obstetric trauma, neonatal hyperbilirubinaemia, neonatal respiratory distress [clinical diagnosis by the neonatologist], neonatal intensive care unit admission [for treatment or observation] or perinatal death.
Interestingly, among the women who at 6 months post-partum had either completed or were still breastfeeding, those who suffered SH episodes were more frequently still breastfeeding at the time of the postpartum evaluation (at 19.2 [8.7–30.5] months postpartum): 60% compared to 22.5%, p=0.021.
With regard to pregnancy outcomes, women with postpartum SH had SGA offspring more frequently (n=2 [11.1%] vs.n=0, p=0.046) and a higher prevalence of gestational hypertensive disease (33.3% vs. 12.5% p=0.038) compared to the non-SH group. No other differences were observed (Table 4).
After adjustment for pregnancy SH, diabetes duration, CSII therapy, pre-gestational HbA1c and pre-gestational BMI, the Clarke test score at the first antenatal visit remained independently associated with postpartum SH (for each 1-point increase: OR 1.53; 95% CI, 1.06–2.21, p=0.024).
DiscussionIn our cohort of pregnant women with T1D, both a high breastfeeding initiation rate and a long duration of breastfeeding were observed. The incidence of SH increased significantly in the long-term postpartum period compared to before and during pregnancy, although no differences were found according to breastfeeding status. Interestingly, this is the first study to identify IAH in early pregnancy as a risk factor of SH in the postpartum period in women with T1D.
Exclusive breastfeeding is recommended for at least 4–5 months for women with diabetes, as it provides many short- and long-term benefits for mothers and infants, for instance reducing childhood obesity, maternal body weight and protecting mothers from cardiovascular disease.18,26 In our cohort of women with T1D, breastfeeding rates at discharge were 83.1%, slightly higher than the general population rate in our country (77%).27 Comparative rates of breastfeeding initiation in women with T1D are discrepant.11,12,14 However, most studies suggest that breastfeeding duration is shorter in women with T1D,11,15 largely explained by more frequent caesarean sections, earlier delivery and lower age and education.11,12 Long-term breastfeeding is also positively related to lower pre-pregnancy HbA1c and BMI, previous breastfeeding experience and early initiation of breastfeeding.15,16
In our study, women breastfeeding at discharge had a lower HbA1c before pregnancy in the first trimester and in the long-term postpartum period. Nevertheless, when adjusted for covariates, neither HbA1c at any time nor other diabetes-related variables predicted breastfeeding at discharge in our cohort. This concurs with a previous study, which identified lower pre-pregnancy HbA1c with long-term breastfeeding in T1D.16 Our findings are consistent with available evidence that suggests that variables unrelated to diabetes are the main determinants of breastfeeding initiation and duration in women with T1D.14–16,28 Still, a significantly better glycaemic control before, during and after pregnancy is observed in women who breastfeed. Breastfeeding would appear to identify a group of women with better diabetes management. One possible explanation is that women who are more aware of the importance of glycaemic control are also more conscious of breastfeeding benefits.
One interesting finding in our study was that medically-related causes were more frequently reported as the cause of non-initiation or cessation of breastfeeding by women not breastfeeding at discharge or who stopped <6 months compared to those weaning after 6 months, although no statistical differences were detected in adverse maternal or neonatal outcomes according to breastfeeding status. Previous studies have identified high-risk pregnancies, assisted and preterm deliveries, more frequent caesarean sections and longer hospital stays as negative predictive factors of breastfeeding initiation and duration in women with diabetes.12,18
In this study, we identified a higher incidence of SH in the long-term postpartum period compared to the gestational and pregestational periods: SH incidence increased nearly 2-fold and 3-fold, respectively. Nevertheless, no relation to breastfeeding status was found in our cohort. Data on the incidence and risk of SH in the postpartum period are scarce, contradictory and are limited to a short period of time after delivery. A recent study described that around two-thirds of the mothers with T1D self-reported more unstable blood glucose and one quarter had experienced hypoglycaemic episodes at 2 and 6 months after delivery. Additionally, a weak association was found between lower general well-being and more unstable blood glucose at 2 months.8 Another short study suggested a higher rate of hypoglycaemic episodes, particularly overnight and fasting, within 2 weeks postpartum in 12 breastfeeding women compared to the non-breastfeeding group.22 On the other hand, a study showed that while an acute reduction in maternal glucose was observed after suckling, this was insufficient to cause hypoglycaemia in most episodes. A lower glucose variability and a similar incidence of hypoglycaemia was observed in breastfeeding women compared to women who fed artificially.19 In another study, self-reported hypoglycaemic episodes were comparable in both breastfeeding and artificially-feeding women during the first 2 months postpartum despite lower mean glucose levels during the first week postpartum in the breastfeeding group.21 None of these studies evaluated SH episodes or IAH. Another study showed mild and severe hypoglycaemia to be comparable between women breastfeeding and artificially feeding at 4 months’ postpartum.16 Finally, a recent study using CGM at 1, 2 and 6 months postpartum concluded that time in hypoglycaemia after breastfeeding at night was low and did not differ from those who fed artificially. Furthermore, breastfeeding mothers spent a higher proportion of night-time in the target range. The SH rates were low and comparable across groups.20
Our results contribute to the evidence that SH risk in the postpartum period is not driven by breastfeeding at discharge. Indeed, SH in this period was related to a higher Clarke test score at the first antenatal visit in our cohort. No previous studies have evaluated the role of IAH in postpartum SH. Only one previous study, performed in the first 4 weeks postpartum and with only 6 women completing the study, showed that several episodes of asymptomatic hypoglycaemia were detected through CGM, although conclusions could not be reached on account of the small sample size.29 Our group has previously described that a pre-pregnancy care programme improves glycaemic control and glycaemic variability without increasing SH events.24,30 Thus, these findings underscore the importance of close follow-up to tackle metabolic control and hypoglycaemia awareness starting before pregnancy.
Importantly, in our study, SH occurred even although the recommendation of a 50% decrease in insulin dose after pregnancy was followed as per current guidelines.2,3,31 Our data suggest that further decreases in insulin doses after delivery could be necessary, particularly in patients at risk of SH. This is in line with recent studies that show that insulin requirements remain up to 21% lower than before pregnancy during the first four months postpartum.10 Thus, an appropriate adjustment of the insulin dose and carbohydrate intake is essential to prevent SH during breastfeeding. Various factors could explain the lower insulin requirements in the first 4 months postpartum. Firstly, independently of feeding modality, the effect of the placental hormones disappears in the immediate postpartum period, which results in a sharp decrease in insulin requirements. Secondly, exclusive breastfeeding is associated with an extra energy expenditure of 450–500kcal/day, which is subsequently reduced when complementary feeding is started. Finally, other factors such as changes in the mother's sleep pattern could play a role. This all leads to decreased insulin requirements that should be acknowledged when treatment adjustments after delivery are planned.
In our study, 19 months after delivery, the women were close to pre-pregnancy weight and insulin dose regardless of breastfeeding status. This is in line with data at 12 months postpartum.10 A qualitative study showed that women with T1D have an insufficient linkage between health care during maternity and postpartum child and diabetes care despite having a greater need for support during this period.15 Overall, this suggests that a closer follow-up in the first weeks and months postpartum should be pursued in the care of women with T1D. It is vital to increase diabetes professionals’ knowledge and awareness of the singularity of the postpartum period in terms of changes in metabolic control, particularly the high risk of SH, in order to offer a more detailed follow-up, develop specific educational interventions and improve the continuity between pregnancy and usual diabetes care.
Interestingly, we described a higher prevalence of gestational hypertensive disease and SGA offspring in women with postpartum SH. Women at risk of SH in the postpartum period were those with a pathological Clarke test in the first antenatal visit. Previous data from our group have shown that pregnant women with IAH had a more atherogenic lipidic profile during pregnancy, which in turn was associated with an increased risk of preeclampsia.25 Our hypothesis is that the low-grade inflammation could promote an insulin-resistance state. On the other hand, the small number of SGA (n=2) precludes reaching firm conclusions and should be confirmed in a larger cohort.
The main strengths of our study are: Firstly, it was conducted in a single centre, a reference diabetes and pregnancy unit with a homogeneous protocol and follow-up. Secondly, to our knowledge, this is the first study with a long-term postpartum follow-up (almost 2 years). Third, Clarke test was prospectively collected to all pregnant women in the first antenatal visit, minimising memory bias. In addition, the Clarke questionnaire is a safe, easy and quick test to identify IAH and has been validated in several previous studies.7,32,33 Nevertheless, our study has also some limitations. The relatively small sample size could preclude the detection of differences in infrequent outcomes. Still, studies on the postpartum have shown a difficulty in recruiting and retaining participants.29 Moreover, although hypoglycaemia awareness was only assessed at the first obstetric visit and could lead to bias, no changes in IAH during pregnancy have been observed previously.5 No changes in hypoglycaemia awareness were observed in the first 6 months of breastfeeding in one study, although this data was self-reported.20 Additionally, very few patients were using CGM since, at the time the study was performed, CGM was not a part of routine clinical practice. Data on the percentage of time in range as well as glycaemic variability measures such as standard deviation or coefficient of variation are missing. The evaluation of metabolic control was only performed by HbA1c levels, although despite its limitation in pregnancy34 it is still a robust predictor of adverse pregnancy outcomes.35 Given the greater use of CGM and combined systems in recent years, a better detection of non-severe hypoglycaemia will probably reduce the incidence of SH in the immediate and late postpartum period. Finally, we describe a higher incidence of post-delivery hypoglycaemia compared to previous studies. This can be explained by the observation of a longer period after delivery, although bias because of retrospective data collection and recall bias of SH during this period cannot be excluded.
In conclusion, breastfeeding rates are high in this cohort of women with T1D and SH incidence is increased after delivery irrespective of feeding modality. In the light of our findings, hypoglycaemia awareness should be routinely assessed in early pregnancy to identify women at risk of postpartum SH. Strategies in clinical practice should be developed to improve diabetes care on the transition to motherhood in women with T1D, particularly a closer follow-up during the postpartum period, specific diabetes educational support and an improved and progressive continuity to resuming usual diabetes care.
Authors’ contributionL.B and V.P. acquired and processed all clinical data. V.P. and I.V. contributed to the study concept and design. All authors participated in data analysis and interpretation and critically reviewed the final version of the manuscript. L.B, V.P. and I.V. wrote the manuscript, designed the figures and take the final responsibility for the decision to submit for publication. I.V. and V.P. are the guarantors of this work and, for instance, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interestThe authors declare no conflict of interest.
There was no specific funding for this article.