Children born small for gestational age (SGA) show higher risk of neurodevelopmental and cognitive abnormalities. The objective of this study is to determine in children born SGA the neurodevelopment during the first 2 years of life and to establish the influence of anthropometric data, gestational age, multiple gestation and perinatal factors.
Patients and methodObservational, prospective, descriptive and analytical study of the neurocognitive assessment performed, with Brunet-Lézine test, on SGA children (n = 91) from 3 to 24 months of age, comparing with own controls.
ResultsNinety-one SGA children, 47% girls, 83.5% single pregnancies; mean gestational age 37.7 weeks (standard deviation (SD) 2.1). Weight at birth 2053 g (SD 433.1), length 43.9 cm (SD 2.6) and head circumference 31.7 cm (SD 1.7). The SGA population shows significantly lower neurodevelopment than the control population, with a tendency to improve during the first 2 years of life. There are no differences by sex. SGA children born to multiple gestations have lower neurodevelopment only during the first year of life. There is a direct and positive correlation between weight, length and head circumference with neurocognitive development at 6, 9, 12 and 18 months. Gestational age correlated with better neurodevelopment at 3 and 6 months.
ConclusionsChildren born SGA present lower neurodevelopment than the control population. A greater impact on weight, length, and head circumference at birth is correlated with poorer neurocognitive development. Multiparity does not show significant influence on neurodevelopment evolution.
Los niños nacidos pequeños para la edad gestacional (PEG) tienen mayor riesgo de presentar anomalías en el neurodesarrollo y la capacidad cognitiva. El objetivo del estudio es determinar el neurodesarrollo de niños PEG durante los primeros 2 años de vida y establecer la influencia de datos antropométricos, edad gestacional, gemelaridad y factores perinatales.
Pacientes y métodoEstudio observacional, prospectivo, descriptivo y analítico de la valoración neurocognitiva realizada, con el test de Brunet-Lézine, a niños PEG (n = 91) desde los 3 a los 24 meses de edad, comparándola con controles propios.
Resultados91 niños PEG, 47% mujeres, 83,5% gestaciones únicas; edad gestacional media 37,7 semanas (desviación estándar (DS) 2,1). Peso al nacimiento 2053,3 gramos (DS 433,1), longitud 43,9 cm (DS 2,6), y perímetro cefálico (PC) 31,7 cm (DS 1,7). La población PEG presenta un neurodesarrollo significativamente inferior a la población control, con tendencia a mejorar durante los primeros 2 años de vida. No existen diferencias por sexos. Los niños PEG nacidos de gestaciones múltiples presentan resultados neurocognitivos inferiores únicamente durante el primer año de vida. Existe correlación directa y positiva entre el peso, longitud y perímetro cefálico con el desarrollo neurocognitivo a los 6, 9, 12 y 18 meses. La edad gestacional se correlaciona con mejor neurodesarrollo a los 3 y 6 meses.
ConclusionesLos niños nacidos PEG presentan un neurodesarrollo inferior al de la población control. Una mayor afectación del peso, la longitud y el PC al nacimiento se correlaciona con peores datos neurocognitivos. La multiparidad no influye significativamente en la evolución del neurodesarrollo.
The term “small for gestational age” (SGA) is used to describe newborns with a weight and/or length under two standard deviations (SDs) or the third percentile for their gestational age.1,2 It is a static concept based on weight and length at birth. This definition, however, does not distinguish constitutionally small newborns from newborns with intrauterine growth restriction (IUGR). “IUGR” describes fetuses with a fetal weight during pregnancy that is estimated, in this case by prenatal ultrasound, to be under the 10th percentile1,3 due to a harmful agent or an unfavourable intrauterine environment. It is therefore a dynamic concept that involves intrauterine ultrasound monitoring of growth rate, regardless of whether weight and length at birth fulfil the definition of SGA. Identification of SGA children is important because it shows a higher risk of perinatal and postnatal morbidity and mortality, with the possibility of shorter stature during childhood; adrenal and gonadal axis abnormalities; and cardiovascular disease, diabetes type 2 and metabolic syndrome in adulthood.2,4
Being born SGA could also have a negative impact on neurodevelopment, since life in utero and during the first year after birth are critical periods for the complex process of brain development.5,6 Some studies have found a link between SGA children and a decline in various cognitive and sensory functions, which in most cases is mild, and may be present in the neonatal period or become evident in later stages. These children have a decreased brain volume that could be related to cognitive decline7 and that, in morphological studies conducted by means of magnetic resonance imaging, is already apparent as of birth.8 Brain growth compromise affects the entire volume of the hippocampus and neuron density, thickness and myelination.9
These problems are observable as of childhood, since these children have a head circumference (HC) in low percentiles.10 They more commonly have neurological problems that affect functions regulated by the frontal cortex such as attention, creativity, language, memory and learning capacity, and many have attention deficit/hyperactivity disorder.10,11
Impaired intelligence quotient (IQ) could be among the consequences of being born SGA. These children show lower IQs than the normal population from three months to 14 years of age. Approximately 22% will have IQs under two standard deviations that gradually worsen with age, potentially leading to severe intellectual disability.12
These newborns could therefore be at increased risk of neurodevelopmental abnormalities; this should be taken into consideration with a view to early diagnosis and treatment of developmental delays.13
However, the evidence in this regard is weak due to the mixed nature of the studies, the different methods of evaluation used, the adjustments made for confounding factors, the small sample sizes in the studies and debate surrounding the definition of SGA. The objective of this study was to prospectively analyse the relationship between, on the one hand, anthropometric data at birth, gestational age, twinship and perinatal factors and, on the other hand, neurodevelopment during childhood in a cohort of children born SGA.
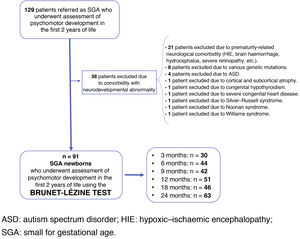
Materials and methodsThe study was an analytical, descriptive, observational, prospective study of clinical and neurocognitive follow-up of SGA children born in the maternity ward of a tertiary hospital between 2000 and 2015. These children were referred from Neonatology and Paediatric Endocrinology units, at birth or while growing up, for voluntary assessment of their neurocognitive development from three to 24 months of age. They were referred from these units as they met the criteria for SGA, regardless of the course of their neurological development up to that point. This study was conducted based on the neurodevelopment data obtained in the first two years of life in this cohort.
Newborns with a length and/or weight at birth under two SDs for their gestational age and sex were included as SGA, according to graphs from the Estudio Español de Crecimiento de 2010 [2010 Spanish Growth Study].14 In the case of newborns from multiple pregnancies, special graphs were used for twin pregnancies (Estudio Español de Crecimiento de 2010), and those who fulfilled the definition of SGA were included.14
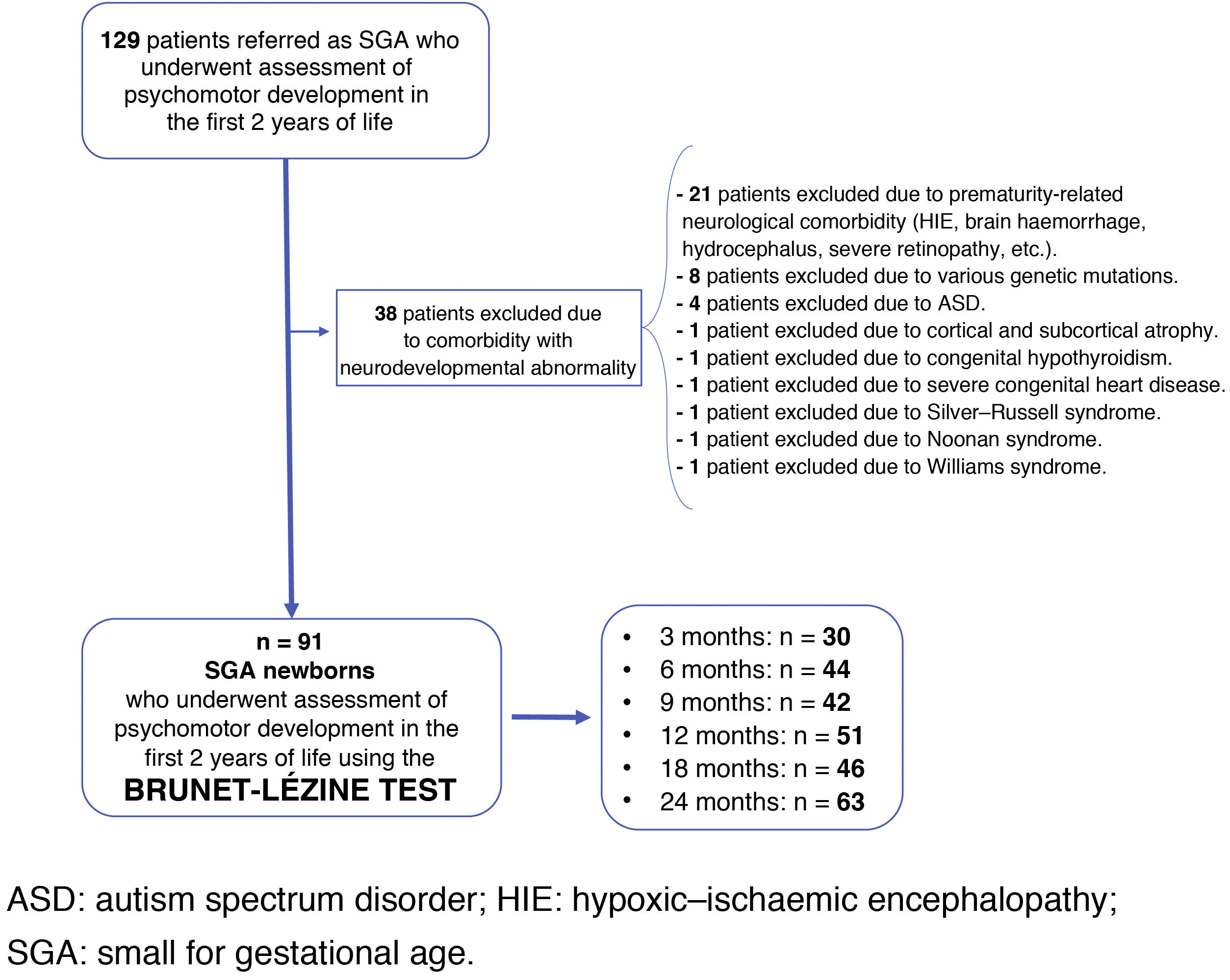
Newborns with diseases that had implications for their neurodevelopment during childhood—neonatal comorbidity essentially resulting from extreme prematurity, various genetic deletions and mutations, Silver–Russell syndrome, Noonan syndrome, Williams syndrome, or autism spectrum disorder—were excluded.
Fig. 1 shows the study population flow chart.
Neurocognitive assessment was conducted by the same psychologist at the chronological ages of three months (n = 30), six months (n = 44), nine months (n = 42), 12 months (n = 51), 18 months (n = 46) and 24 months (n = 63). The Brunet-Lézine Scale of Psychomotor Development of Children, a validated test to evaluate psychomotor and intellectual development during the first two years of life, was used. The results were standardised with those obtained from the Estudio Longitudinal del Crecimiento, Desarrollo y Maduración Intelectual [Longitudinal Study of Growth, Development and Intellectual Maturation],15 which served as a control group at three months (n = 233), six months (n = 290), nine months (n = 303), 12 months (n = 299), 18 months (n = 269) and 24 months (n = 260). Assessment of the psychomotor development of the control group was conducted by the same psychologist using the same test at the same centre. Data on screening, characteristics and outcomes for controls are listed in the Estudio Longitudinal del Crecimiento, Desarrollo y Maduración Intelectual.15
The test calculates an overall developmental quotient (DQ), which is used in place of IQ in childhood, through the results obtained in four areas: postural control and motor skills, eye–motor coordination, language/communication and sociability/autonomy.
The primary endpoint was the Z-score for DQ on the Brunet-Lézine test determined at each age of evaluation. The secondary endpoints were the Z-scores for weight (g), length (cm) and HC (cm) at birth, in addition to sex, gestational age (weeks), singleton or multiple pregnancy and Apgar test scores.
For purposes of analysis and presentation of the results, measures of central tendency and dispersion were calculated for quantitative variables. Qualitative variables were expressed in terms of percentages with their respective 95% confidence intervals. The mean difference and its significance were calculated using Student's t-test for independent data, once the normality of the distribution had been confirmed. To calculate correlation, Pearson's or Spearman's coefficient was used, depending on whether or not the criteria for normality of both distributions were fulfilled.
The threshold for statistical significance was set at P ≤ .05.
The research project was approved by the Independent Ethics Committee of the Autonomous Community of Aragón (CP-CI PI20/293), as well as the hospital medical board.
ResultsA total of 91 children were followed up until two years of age. Of them, 47% were female and 83.5% were from singleton pregnancies. Mean birth weight was 2,053.3 ± 433.1 g (−2.3 SD), mean birth length was 43.9 ± 2.6 cm (−2.6 SD) and mean birth HC was 31.7 ± 1.7 cm (−1.4 SD). Mean gestational age was 37.8 ± 2.1 weeks. Mean Apgar score was 8.2 ± 1.5 at one minute and 9.5 ± 0.8 at five minutes.
By subgroup, among males, mean birth weight (n = 48) was 2,096.3 ± 447.6 g (−2.3 SD), mean birth length was 44.3 ± 2.5 cm (−2.7 SD) and mean birth HC was 31.9 ± 1.8 cm (−1.2 SD). In females (n = 43), mean birth weight was 2,005.2 ± 416.3 g (−2.4 SD), mean birth length was 43.5 ± 2.7 cm (−2.7 SD) and mean birth HC was 31.5 ± 1.5 cm (−1.5 SD). A total of 15 patients were from multiple pregnancies, of which 12 were twin pregnancies. Mean birth weight in SGA children from multiple pregnancies was 1,692.4 ± 355.4 g (−2.6 SD), mean birth length was 42.2 ± 2.3 cm (−2.8 SD), mean birth HC was 30.8 ± 2.0 cm (−1.3 SD) and mean gestational age at birth was 36.1 ± 1.7 weeks.
In the entire sample, 22 SGA children were preterm newborns, of whom 18 were late preterm (gestational age ≥34 weeks), with a mean gestational age at birth of 35.2 ± 1.8 weeks, a mean birth weight of 1,553.5 ± 375.1 g (−2.4 SD), a mean birth length of 40.7 ± 2.7 cm (−2.8 SD) and a mean birth HC of 29.7 ± 1.6 cm (−1.6 SD).
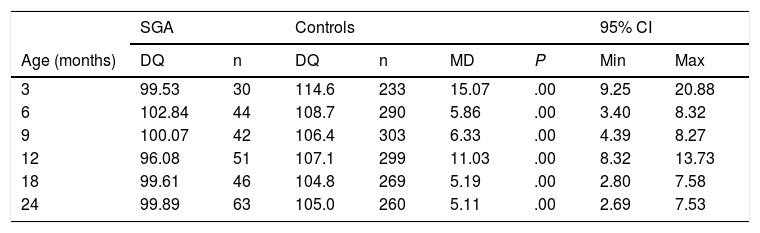
Table 1 shows the overall DQ scores obtained in both SGA children and the control group,15 along with the mean difference between them. The DQ of the SGA population was found to be significantly lower than that of the controls.
Comparison of results for developmental quotient as a total score for all areas of the Brunet-Lézine test compared to the control group at the different ages of the study.
| SGA | Controls | 95% CI | ||||||
|---|---|---|---|---|---|---|---|---|
| Age (months) | DQ | n | DQ | n | MD | P | Min | Max |
| 3 | 99.53 | 30 | 114.6 | 233 | 15.07 | .00 | 9.25 | 20.88 |
| 6 | 102.84 | 44 | 108.7 | 290 | 5.86 | .00 | 3.40 | 8.32 |
| 9 | 100.07 | 42 | 106.4 | 303 | 6.33 | .00 | 4.39 | 8.27 |
| 12 | 96.08 | 51 | 107.1 | 299 | 11.03 | .00 | 8.32 | 13.73 |
| 18 | 99.61 | 46 | 104.8 | 269 | 5.19 | .00 | 2.80 | 7.58 |
| 24 | 99.89 | 63 | 105.0 | 260 | 5.11 | .00 | 2.69 | 7.53 |
95% CI: 95% confidence interval; DQ: developmental quotient; MD: mean difference; SGA: small for gestational age.
Source: Ferrández Longás et al.15
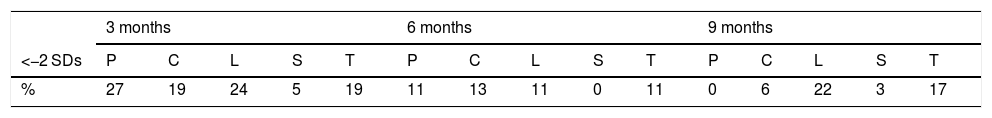
Table 2 lists the percentage of children with Z-scores under 2 SDs in each follow-up visit, for each test area and overall.
Distribution expressed as a percentage of the ranges of standard deviation of the Z-score under 2 at the different follow-up visits by area of the Brunet-Lézine test and in total.
| 3 months | 6 months | 9 months | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <−2 SDs | P | C | L | S | T | P | C | L | S | T | P | C | L | S | T |
| % | 27 | 19 | 24 | 5 | 19 | 11 | 13 | 11 | 0 | 11 | 0 | 6 | 22 | 3 | 17 |
| 12 months | 18 months | 24 months | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <−2 SDs | P | C | L | S | T | P | C | L | S | T | P | C | L | S | T |
| % | 13 | 11 | 26 | 7 | 28 | 13 | 4 | 25 | 9 | 11 | 17 | 5 | 8 | 3 | 10 |
C: coordination; L: language; P: postural control; S: sociability; SD: standard deviation; T: total Z-score.
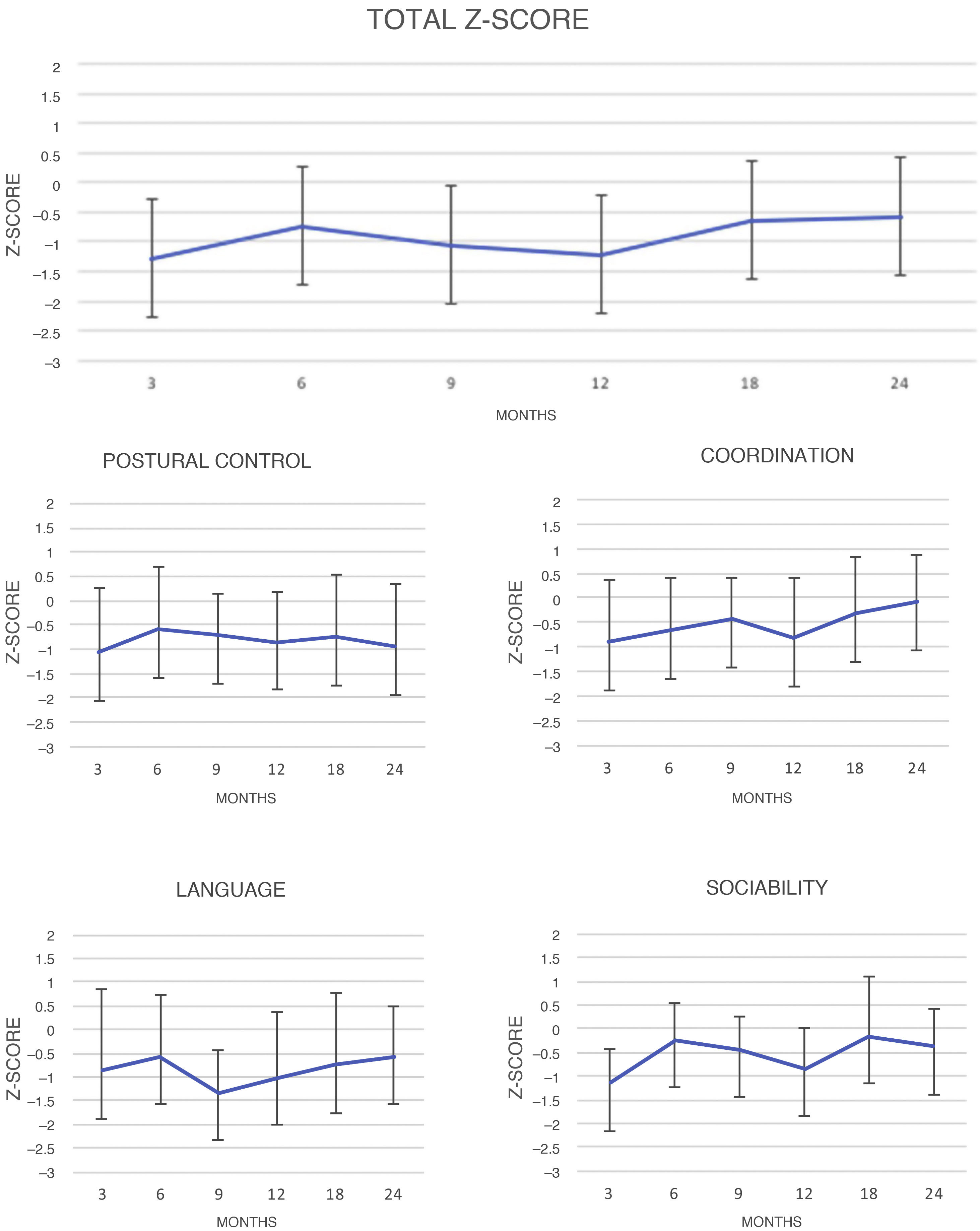
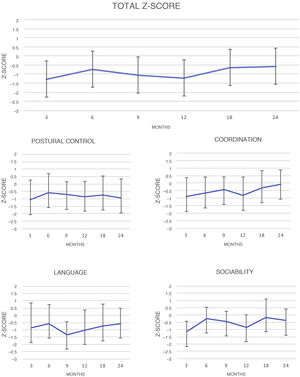
Fig. 2 shows changes over time in results for DQ scores, both overall and for each area of the test.
The results for overall DQ and for DQ in each area at each visit were compared to one another. Significant differences in overall DQ means were found between the following times: three versus 24 months (P = .00), nine versus 24 months (P = .02) and 12 versus 24 months (P = .02). By area, notable differences were found in the following areas: coordination at 3 versus 24 months (P = .00), six versus 24 months (P = .00) and 12 versus 24 months (P = .00); language at nine versus 24 months (P = .00); and sociability at three versus 24 months (P = .00) and 12 versus 24 months (P = .00). No significant differences were found in postural control.
When the results were compared by sex, no significant differences were found, and only the mean difference was statistically significant in coordination at 12 months, in favour of girls (P = .01).
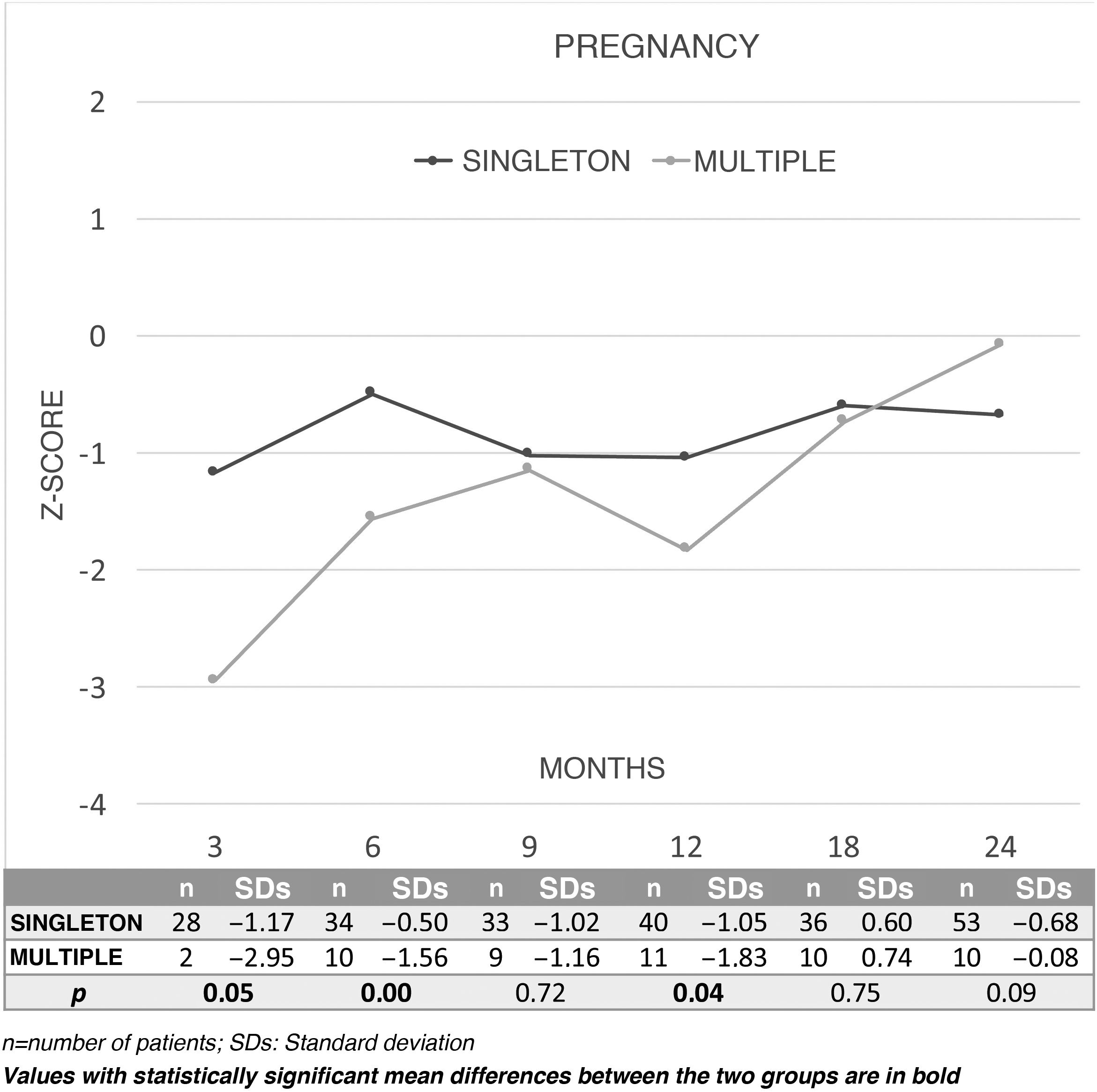
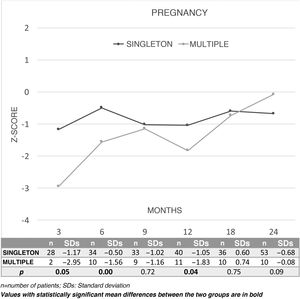
Fig. 3 shows changes over time in the results for overall DQ in terms of Z-scores during the first two years of life between children from a singleton pregnancy (n = 76) or a multiple pregnancy (n = 15) and the comparison between the two. Children born SGA from singleton pregnancies showed a better DQ at three, six and 12 months than those born from multiple pregnancies. By area, better outcomes were seen in singleton pregnancies at three months in coordination (P = .03) and language (P = .04), and at six months in coordination (P = .00). By contrast, the multiple-pregnancy group showed better outcomes in coordination at 24 months (P = .03).
The correlation between the test results and the Z-scores for birth weight, length and HC was calculated. A positive correlation was found between the Z-score for weight and language at six months (r = 0.33; P = .01) and postural control at nine months (r = 0.31; P = .02). Such a correlation was also found between the Z-score for length and sociability at nine months (r = 0.27; P = .04) and language at 12 months (r = 0.24; P = .05). A positive correlation was found between HC and coordination (r = 0.32; P = .02) and total Z-score at 18 months (r = 0.36; P = .01).
The correlation between gestational age and the different areas of the test was positive at three months in coordination (rho = 0.49; P = .01) and total Z-score (rho = 0.43; P = .02), and at six months in postural control (rho = 0.41; P = .01), coordination (rho = 0.35; P = .02) and total Z-score (rho = 0.44; P = .01).
DiscussionSGA newborns exhibit worse outcomes in neurocognitive development over the course of childhood.7,16–19 Most studies have focused on neurodevelopment in older children or adults; the evidence in newborns, the subject of this research study, is limited.
This study, focused on assessment of early neurodevelopment, confirmed test scores in SGA children to be significantly lower than those in the control population born appropriate for gestational age.
Historically, different definitions of SGA have been used. The term “IUGR” has sometimes even been used interchangeably with the term “SGA”.20 Von Beckerath et al.21 compared long-term differences in neurodevelopment between SGA children and children with IUGR and found a heightened risk of neurodevelopmental abnormalities in both groups. A recent systematic review22 made a distinction between outcomes in children with IUGR and SGA children, finding worse outcomes compared to appropriate-for-gestational-age children. This study considered only newborns who met the criteria for being SGA, regardless of growth restriction during pregnancy.
Assessment of changes over time during the two years of follow-up revealed an apparent trend towards improvement, since values under 1 SD were obtained initially, at three, nine and 12 months, and improved −0.53 and −0.58 SDs at 18 and 24 months, respectively. Negative Z-scores for both overall DQ and DQ for the specific areas of the test persisted over time, although this pattern was not the same across all areas. Results for postural control did not vary significantly in any of the follow-up assessments, but in the area of coordination there was indeed a positive course, with near-achievement of the population mean at 24 months (−0.08 SDs). In language, there was a decline up to nine months and a statistically significant improvement up to 24 months (from −1.36 SD at nine months to −0.58 at 24 months).
Along these dimensions, Arcangeli et al.23 conducted a systematic review of neurodevelopment in SGA children, distinguishing them from children with IUGR. The results of the meta-analysis showed that overall Z-scores were significantly lower (−0.32 SD; 95% confidence interval: −0.38 to −0.25; P = .00) in SGA children compared to controls, as in our series.
In the subgroup analysis, sex did not appear to be a determining factor in neurodevelopment; statistically significant differences were found only in coordination at 12 months, in favour of girls.
When twinship was taken into account, SGA children from a multiple pregnancy were seen to have significantly lower scores in the first year (three, six and 12 months), and these differences were seen to disappear in the second year. These differences could probably be explained by the need to share placental nutrition in a multiple pregnancy.
A correlation was found between neonatal anthropometrics and neurocognitive development. A significant positive correlation was found between weight and language at six months and between weight and postural control at nine months. Birth length showed a significant positive correlation with sociability at nine months and with language at 12 months. HC showed a significant positive correlation at 18 months with overall DQ and with the coordination area, even though the mean HC in the sample was above −2 SDs (HC 31.7 ± 1.7 cm, −1.4 SDs). We believe this might be explained by the more pronounced cases of SGA children with lower anthropometric values. Many studies have consistently found both a low birth weight or length and a decrease in brain size to be associated with poor intrauterine growth and, on a secondary basis, more severe cognitive deficits. A 2013 study by Lee and Houk20 established brain growth as a significant predictive factor associated with a reduction in cognitive ability. Barre et al.24 conducted a 2011 meta-analysis linking worse language development to very low birth weight (<1500 g). De Bie et al.25 found that a reduction in total brain volume was correlated with HC, as well as cognitive function in relation to attention.
Gestational age demonstrated a moderate influence on neurodevelopment, since a significant correlation was found between worse test scores and younger gestational age at three and six months only. This could be accounted for by the fact that the tests were administered at the subjects' real versus corrected age. We decided to jointly analyse preterm and full-term newborns as the vast majority were late preterm (18 out of the 22 preterm newborns with a mean gestational age of 35.2 ± 1.8 weeks), and prior studies have confirmed a similar pattern in the two groups.18
However, De Bie et al.'s review25 confirmed IQ in preterm children to be lower in both children born appropriate for gestational age and in SGA children, being lower in preterm children born SGA. Graz et al.,26 for their part, concluded that being born SGA and preterm appears to have a mild impact on neurodevelopment at the age studied, predominantly in the form of symptoms of hyperactivity, and emphasised that the most powerful predictors of neurodevelopment seem to be gestational age, severe brain lesions, socioeconomic status, perinatal asphyxia and tobacco exposure.
Our study had limitations related to possible confounding factors that may extrinsically influence development, such as family socioeconomic status and social class. The sample of patients was not randomly selected; rather, the patients' parents voluntarily chose to participate in this study. This meant that not all the controls had the same number of patients. The strengths of the study were its longitudinal follow-up and the fact that neurocognitive development was assessed by the same psychologist and by standardisation thereof with particular values of normality.15
ConclusionsThis study found that neurocognitive development during the first two years of life in children born SGA, though within normal limits, was significantly below that of the control population. The factors that appeared to have the strongest impact on this development were perinatal auxology and, to a lesser extent, gestational age. The influence of multiparity was only notable in the first year of life. All things considered, a perinatal history of being born SGA should be considered a possible risk factor for the course of neurocognitive development in these children. It is therefore advisable to more closely monitor such children for purposes of early detection of neurodevelopmental abnormalities.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: García Ventura M, de Arriba Muñoz A, Puga González B, Abenia Usón P, Sánchez Malo MJ, Labarta Aizpún JI. Influencia de factores perinatales en el desarrollo neurocognitivo de niños nacidos pequeños para la edad gestacional durante los primeros 2 años de vida. Endocrinol Diabetes Nutr. 2022;69:271–278.
The preliminary results included in this article were presented as an oral presentation at the 41st conference of the Sociedad Española de Endocrinología Pediátrica [Spanish Society of Paediatric Endocrinology] (SEEP), Madrid, 22–24 May 2019.