Acute adrenal insufficiency (AAI) is a potentially fatal medical emergency whose prevention and treatment should be known by all medical professionals.
AAI is an underdiagnosed condition because of its non-specific symptoms, but its diagnosis and early treatment with glucocorticoids is vital.
It may be triggered by a de novo deficiency in cortisol synthesis or occur secondarily to omission of hormone replacement therapy (corticosteroids) or inadequate adjustment of the dose required in stress situations in patients previously diagnosed with adrenal insufficiency.
AAI prevention significantly decreases death from cardiovascular diseases and infections in patients with adrenal insufficiency, and also improves their quality of life. Adequate education of patients, relatives, and all healthcare professionals is therefore essential.
Therefore, the Adrenal Disorders Group of the Neuroendocrinology Area of the Spanish Society of Endocrinology and Nutrition (SEEN) has prepared, at the proposal of the SEEN's board, a guideline for optimal management of acute adrenal insufficiency.
The guideline is intended to provide practical recommendations for all healthcare professionals who may be involved in the diagnosis, treatment, and prevention of AAI.
It is also intended to provide patients and their families with action guidelines for AAI management and prevention.
La insuficiencia suprarrenal aguda (ISA) es una urgencia médica potencialmente letal cuya prevención y tratamiento deberían ser conocidos por todos los profesionales médicos. La ISA es una condición infradiagnosticada debido a la inespecificidad de los síntomas de presentación, pero su diagnóstico y tratamiento con glucocorticoides es vital.
Puede ser desencadenada por una deficiencia de novo en la síntesis de cortisol o secundaria a la omisión del tratamiento hormonal sustitutivo con corticoides o al ajuste inadecuado de la dosis requerida en situaciones de estrés en el paciente ya diagnosticado.
La prevención de la ISA disminuye de forma significativa la mortalidad cardiovascular y por infecciones de los pacientes con insuficiencia renal y mejora su calidad de vida. Por ello, es fundamental la adecuada educación del paciente, sus familiares y del personal sanitario.
El Grupo de Trabajo de Patología Suprarrenal del Área de Conocimiento de Neuroendocrinología de la Sociedad Española de Endocrinología y Nutrición (SEEN) ha elaborado, a partir de una propuesta de la Junta Directiva de la SEEN, esta guía para el óptimo manejo de la insuficiencia suprarrenal en fase aguda. Esta guía tiene el objetivo de ser eminentemente práctica y dar recomendaciones orientadas a todos los profesionales sanitarios que pueden estar involucrados en el diagnóstico, tratamiento y la prevención de la ISA. Así mismo, pretende facilitar pautas de actuación para el paciente y sus familiares en su manejo y prevención.
Acute adrenal insufficiency (AAI), or adrenal crisis, is caused by corticosteroid deficiency due to “de novo” failure of the adrenal gland cortex or inadequate glucocorticoid (GC) replacement therapy dose adjustment in the face of an intercurrent stress event.
Acute adrenal insufficiency is a medical emergency, with a high mortality rate, and treatment should be started immediately at the slightest suspicion of AAI,1–7 without waiting for prior laboratory confirmation.1–8
Patients with primary adrenal insufficiency (secondary to direct adrenal gland failure) and secondary adrenal insufficiency (secondary to hypothalamic-pituitary axis failure) require lifelong replacement therapy with GCs and, in the case of primary insufficiency, also mineralocorticoids (MCs).
The overall prevalence of adrenal gland insufficiency in the European Union is 2.1–4.2 cases per 10,000 individuals, with an incidence of AAI of 5–10 cases per 100patients/year (higher in primary insufficiency) and a mortality rate of 0.5/100patients.1,2,8
Acute adrenal insufficiency manifests in 50% of all cases at disease onset.
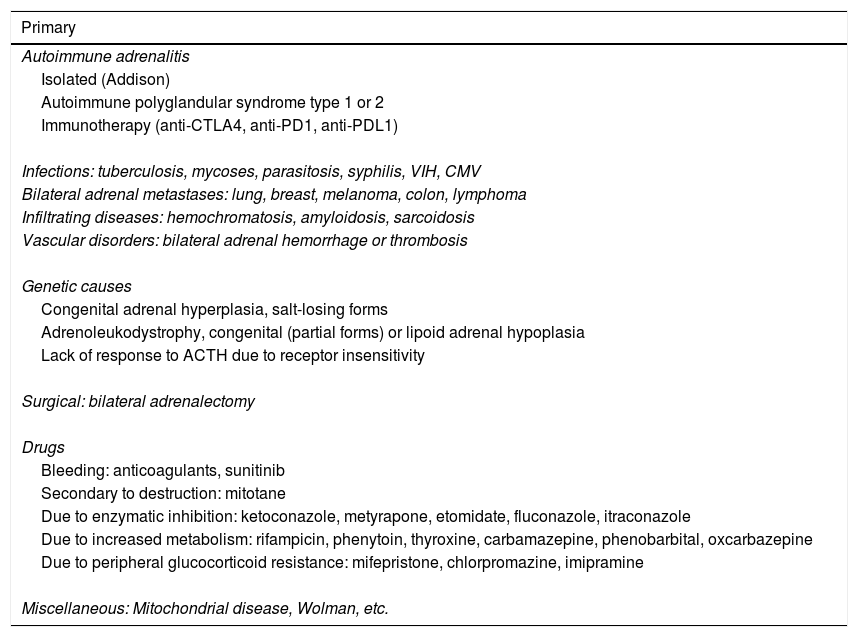
Table 1 describes the possible causes of adrenal insufficiency.2
Etiology of adrenal insufficiency.2,4–7,9
| Primary |
|---|
| Autoimmune adrenalitis |
| Isolated (Addison) |
| Autoimmune polyglandular syndrome type 1 or 2 |
| Immunotherapy (anti-CTLA4, anti-PD1, anti-PDL1) |
| Infections: tuberculosis, mycoses, parasitosis, syphilis, VIH, CMV |
| Bilateral adrenal metastases: lung, breast, melanoma, colon, lymphoma |
| Infiltrating diseases: hemochromatosis, amyloidosis, sarcoidosis |
| Vascular disorders: bilateral adrenal hemorrhage or thrombosis |
| Genetic causes |
| Congenital adrenal hyperplasia, salt-losing forms |
| Adrenoleukodystrophy, congenital (partial forms) or lipoid adrenal hypoplasia |
| Lack of response to ACTH due to receptor insensitivity |
| Surgical: bilateral adrenalectomy |
| Drugs |
| Bleeding: anticoagulants, sunitinib |
| Secondary to destruction: mitotane |
| Due to enzymatic inhibition: ketoconazole, metyrapone, etomidate, fluconazole, itraconazole |
| Due to increased metabolism: rifampicin, phenytoin, thyroxine, carbamazepine, phenobarbital, oxcarbazepine |
| Due to peripheral glucocorticoid resistance: mifepristone, chlorpromazine, imipramine |
| Miscellaneous: Mitochondrial disease, Wolman, etc. |
| Secondary |
|---|
| Due to suppression of the hypothalamic-pituitary axis: |
| Abrupt discontinuation of long-term treatment with glucocorticoids: systemic, topical, inhaled, intra-articular, and even eye drops. Chronic or repeated for over a total of 3 weeks or continuous nocturnal treatment for over 2 weeks; any dose that has induced a cushingoid phenotype |
| Other drugs: megestrol acetate, opioids, medroxyprogesterone, topiramate |
| After treatment for endogenous Cushing syndrome |
| Due to hypothalamic-pituitary involvement: |
| Primary (pituitary adenomas, craniopharyngiomas, gliomas, meningioma) or metastatic tumors (breast, lung, melanoma) |
| Infections: abscesses, tuberculosis, others |
| Infiltrating diseases: sarcoidosis, histiocytosis, hemochromatosis, Wegener |
| Hypophysitis: lymphocytic, granulomatous, others |
| Traumatic brain injuries |
| Postpartum hemorrhage (Sheehan) |
| Genetic diseases with isolated ACTH deficiency or panhypopituitarism, Prader-Willi syndrome |
| Iatrogenic: surgery, radiotherapy, immune therapy (anti-CTLA4, anti-PD1, anti-PDL1) |
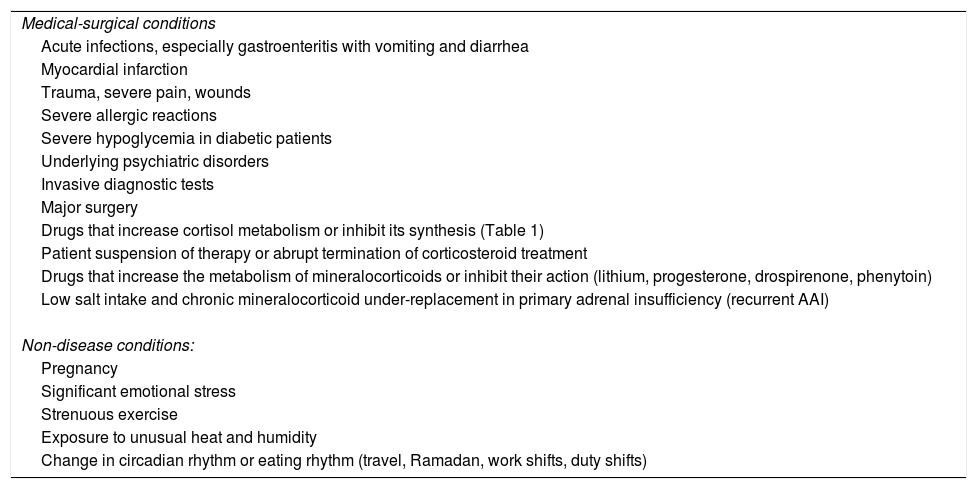
The main cause of AAI being triggered in patients with chronic deficiency subjected to hormone replacement therapy is usually infection, particularly acute gastroenteritis.1–7,9Table 2 summarizes these circumstances.
Possible triggering causes of an adrenal crisis in patients with known adrenal insufficiency.
| Medical-surgical conditions |
| Acute infections, especially gastroenteritis with vomiting and diarrhea |
| Myocardial infarction |
| Trauma, severe pain, wounds |
| Severe allergic reactions |
| Severe hypoglycemia in diabetic patients |
| Underlying psychiatric disorders |
| Invasive diagnostic tests |
| Major surgery |
| Drugs that increase cortisol metabolism or inhibit its synthesis (Table 1) |
| Patient suspension of therapy or abrupt termination of corticosteroid treatment |
| Drugs that increase the metabolism of mineralocorticoids or inhibit their action (lithium, progesterone, drospirenone, phenytoin) |
| Low salt intake and chronic mineralocorticoid under-replacement in primary adrenal insufficiency (recurrent AAI) |
| Non-disease conditions: |
| Pregnancy |
| Significant emotional stress |
| Strenuous exercise |
| Exposure to unusual heat and humidity |
| Change in circadian rhythm or eating rhythm (travel, Ramadan, work shifts, duty shifts) |
The physiopathology of AAI is only partially understood. The absence of the enhancing effect of GCs upon α1-adrenergic receptor expression induces hypotension, while MC deficiency conditions volume depletion due to the lack of sodium and water reabsorption. Hypovolemia may be aggravated by vomiting, diarrhea and hyperhidrosis, etc. Acute adrenal insufficiency under conditions of strenuous physical or psychological-emotional stress, trauma, or major surgery appears to be secondary to the absence of the suppressive effect of GCs upon the toxicity of the inflammatory and innate immune hyper-response.1,2,9
Clinical manifestations and diagnosis of acute adrenal insufficiencyThe diagnosis of AAI is fundamentally clinical. If possible, blood sampling for cortisol and ACTH determination is advised before treatment is started, but it is not indispensable and should never be allowed to delay the start of patient management.1–7,9
The symptoms and signs of AAI depend on the degree and rate of onset of hormone deficiency, but are nonspecific, and patients characteristically suffer from severe and acute malaise.
Table 3 summarizes the main symptoms and signs of AAI.7
Major symptoms and signs of acute adrenal insufficiency.7
| Severe hemodynamic instability with signs of hypovolemia, dehydration and hypotensionAsthenia with proximal muscle weakness, myalgia and crampsAnorexia, nausea, vomiting, abdominal pain (sometimes with signs of peritoneal irritation) and altered bowel transit (diarrhea versus constipation)Febricula/fever (idiopathic or associated with infection)Weight lossCognitive impairment with headache or altered level of consciousness (from drowsiness to coma in cases of severe or prolonged alteration)Cardiomyopathy with reversible acute myocardial failurePrevious psychiatric diagnosis of anorexia nervosa, depression |
In cases of primary adrenal insufficiency, mucocutaneous hyperpigmentation (predominant in scars, the breast areolar region, photoexposed areas), as well as diminished pubic and axillary hair, is noted in women.
In both the personal and the family history, and in the physical examination, it is important to look for signs or symptoms of other autoimmune disorders (goiter, vitiligo, hypothyroidism, celiac disease, etc.).1–5
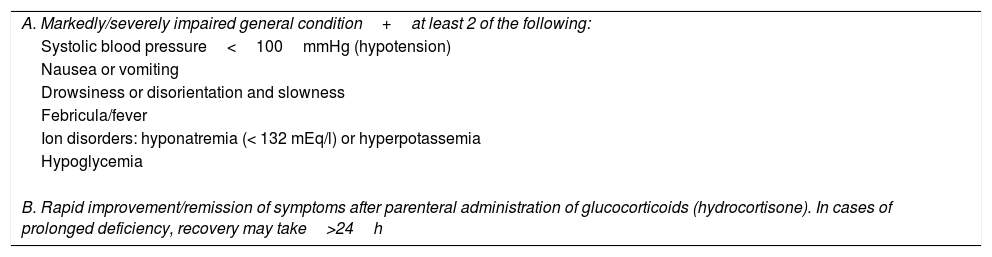
Some groups propose the use of a diagnostic algorithm for AAI (Table 4).1
Diagnostic criteria of acute adrenal insufficiency.a
| A. Markedly/severely impaired general condition+at least 2 of the following: |
| Systolic blood pressure<100mmHg (hypotension) |
| Nausea or vomiting |
| Drowsiness or disorientation and slowness |
| Febricula/fever |
| Ion disorders: hyponatremia (< 132 mEq/l) or hyperpotassemia |
| Hypoglycemia |
| B. Rapid improvement/remission of symptoms after parenteral administration of glucocorticoids (hydrocortisone). In cases of prolonged deficiency, recovery may take>24h |
Four severity degrees of AAI are defined according to the clinical scenario in which patient care is required, and to the final outcome1:
- •
Grade 1: outpatient care;
- •
Grade 2: in-hospital care;
- •
Grade 3: Intensive Care Unit (ICU);
- •
Grade 4: death due to adrenal crisis.
The vast majority of episodes of AAI in which medical care is sought correspond to grade 1, except in cases of trauma or accidents.1
Complementary testsThe laboratory tests in particular reveal electrolyte alterations in the form of hyponatremia, in primary adrenal insufficiency, and hyperpotassemia and azotemia (secondary to prerenal failure), normocytic anemia, lymphocytosis with neutropenia, eosinophilia, hypoglycemia (especially in children) and hypercalcemia.
From the hormonal perspective, decreased cortisol levels are observed, with ACTH levels that are elevated in primary adrenal insufficiency, and low or inappropriately normal in secondary adrenal insufficiency. Cortisol values should be interpreted in relation to situations of stress and, if malnutrition or sepsis is suspected, correction for total albumin/protein should be made. However, a level below 3.6μg/dl (<100nmol/l) is strongly suggestive of adrenal insufficiency, while concentrations above 15μg/dl rule out the diagnosis, and levels between 5 and 15g/dl require correlation with the clinical condition of the patient.
The electrocardiogram in turn can reveal bradycardia or signs of acute myocardial dysfunction.
Treatment of acute adrenal insufficiencyAll healthcare professionals, and in particular emergency service professionals, should be aware of the management protocol applicable to AAI, since it is a potentially fatal condition if not identified and treated adequately and quickly.
In the emergency room, in the event of a strong clinical suspicion of adrenal insufficiency in a patient with no previous diagnosis of the disorder, or in situations of severe intercurrent episodes in a patient with confirmed adrenal insufficiency, urgent treatment is required and, if possible, peripheral blood sampling should be performed for hormone determination (cortisol and ACTH) before treatment is started.
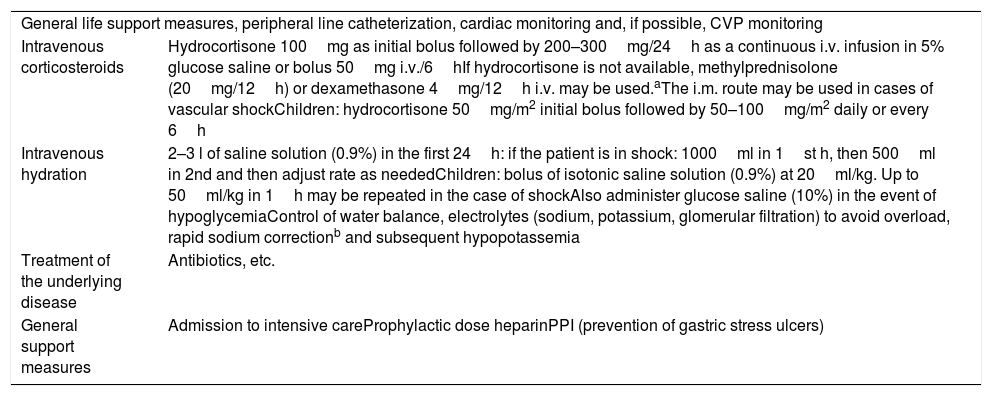
Acute emergency treatment (24h)The basic key to management is immediate treatment with intravenous hydrocortisone at stress doses and the rapid correction of hypovolemia and water and electrolyte alterations (Table 5).
Acute treatment of adrenal crisis.
| General life support measures, peripheral line catheterization, cardiac monitoring and, if possible, CVP monitoring | |
| Intravenous corticosteroids | Hydrocortisone 100mg as initial bolus followed by 200–300mg/24h as a continuous i.v. infusion in 5% glucose saline or bolus 50mg i.v./6hIf hydrocortisone is not available, methylprednisolone (20mg/12h) or dexamethasone 4mg/12h i.v. may be used.aThe i.m. route may be used in cases of vascular shockChildren: hydrocortisone 50mg/m2 initial bolus followed by 50–100mg/m2 daily or every 6h |
| Intravenous hydration | 2–3 l of saline solution (0.9%) in the first 24h: if the patient is in shock: 1000ml in 1st h, then 500ml in 2nd and then adjust rate as neededChildren: bolus of isotonic saline solution (0.9%) at 20ml/kg. Up to 50ml/kg in 1h may be repeated in the case of shockAlso administer glucose saline (10%) in the event of hypoglycemiaControl of water balance, electrolytes (sodium, potassium, glomerular filtration) to avoid overload, rapid sodium correctionb and subsequent hypopotassemia |
| Treatment of the underlying disease | Antibiotics, etc. |
| General support measures | Admission to intensive careProphylactic dose heparinPPI (prevention of gastric stress ulcers) |
HR: heart rate; PPI: proton pump inhibitor; i.m.: intramuscular; i.v.: intravenous; BP: blood pressure; CVP: central venous pressure.
Avoid dexamethasone in cases of K>6mEq/l in primary adrenal insufficiency, due to the lack of a mineralocorticoid effect. Although an advantage of dexamethasone use is that it allows for the diagnosis of adrenal insufficiency even after administration, because it does not interfere with the method for measuring endogenous cortisol, its suppressive effect upon the hypothalamic-pituitary-adrenal axis may result in falsely low cortisol values.
The correction of hyponatremia should be made with caution: a corrective rate of no more than 10mmol/l is recommended in the first 24h, and 18 in the first 48h. In cases of an increased risk of brain edema (women, elderly subjects, children and malnourished patients), it is advisable not to exceed 6–8mmol/l in the first 24h and 15mmol/l in the first 48h. Close monitoring of electrolytes is recommended in the first 24–48h and, if the correction rate exceeds that recommended, do not hesitate to increase the glucose saline perfusion rate and prescribe desmopressin intravenously or subcutaneously in a timely manner (1–2mg).
Mineralocorticoid replacement is not required on an acute basis, since high GC stress doses have an MC effect.
After starting GC treatment and hydration, an attempt should be made to identify and treat the triggering factor or disease.
With appropriate treatment, hemodynamic recovery occurs in the first 6–12h, and clinical recovery in the first 24h. Recovery may be slower in cases of prolonged corticosteroid deficiency with impaired consciousness.
Subacute treatment (24–48h)Once hemodynamic stabilization of the patient has been achieved, intravenous hydration should be continued for the next 24–48h (total 72h), with the administration rate being reduced and the dose of intravenous GC being gradually lowered. Finally, this should lead to a return to the usual dose via the oral route when so allowed by the underlying condition or factor causing AAI.
In the case of the onset of adrenal insufficiency, in addition to identifying and treating the triggering factor or disease, an etiological diagnosis should be established (see Table 1).
It is advisable to consult the Endocrinology Department to ensure due assessment in the event of the onset of adrenal insufficiency, and to reinforce educational measures and adjust the basal regimen in special situations.
In cases of primary adrenal insufficiency, MC replacement should be restarted with fludrocortisone (0.1mg daily via the oral route) when saline infusion is suspended and the hydrocortisone dose is less than 50mg/day.
It should be noted that the diagnostic algorithm and the management of chronic replacement therapy in adrenal insufficiency are not the objective of this guide, but can be found in the documents cited in the references.
Prevention of acute adrenal insufficiencyIn the management of patients with adrenal insufficiency, it is essential for both the patients and their relatives to have the basic tools to prevent, identify, and treat an adrenal crisis. This involves the following:
- •
An awareness of those situations that can trigger hormone deficiency and of the reasons why early treatment is so important. Likewise, the administration of high GC doses is not intended to mimic the mean values of normal subjects during stress, but to mimic the maximum cortisol increase that may be required in order to cover additional unexpected needs. The damage caused by these doses has not been demonstrated, and there are no direct studies indicating which lowest doses are safe.
- •
Education and regular follow-up (annually or more often in patients with a recent history of AAI), reviewing how to increase the GC dose according to the severity of intercurrent illness, and when and how to administer hydrocortisone via the intramuscular, subcutaneous or rectal route at home.
- •
Table 6 summarizes the main recommendations that healthcare professionals should be familiar with so that they can adjust the GC dose in relation to stress and medical or therapeutic procedures.
Table 6.Recommendations for corticosteroid dose adjustment in stress situations and medical/therapeutic procedures.
Condition Treatment Disease with fever, requiring home rest (e.g., respiratory tract infection) or antibiotics, after vaccination (influenza) Hydrocortisone: double (>38°C) or triple (>39°C) the oral dose while fever persists, and then reduce it to the usual dose in 1–2 and 2 days, respectively. Increase the intake of fluids with electrolytes (isotonic drinks)a Gastroenteritis with persistent vomiting or diarrhea, oral intolerance Hydrocortisone 50mg/12h i.m. or i.v.aHydration: saline solution 0.9% 1000ml 1st hour; 500ml 2nd hour, subsequent continuous infusion. Consider hospital admissionChildren: hydrocortisone 50mg/m2 i.m. (or 25mg in preschool children, 50mg in schoolchildren, 100mg in adolescents)a Wound with considerable bleeding, severe trauma, fracture, or severe disease (e.g., pneumonia, altered level of consciousness, severe sepsis, myocardial infarction, pancreatitis, etc.) Hydrocortisone i.v. 50–100mg/8h or continuous i.v. perfusion of 150–300mg/24h, decreasing to half daily when improvement is seenaChildren: hydrocortisone 50mg/m2 i.v., followed by hydrocortisone 50–100mg/m2 daily or divided and given every 6ha Pregnancy Increase the dose by 20–50% of the initial dose (an increase in hydrocortisone of 2.5–10mg/day) in the third trimester (from week 24) Anticonvulsants and barbiturates, tuberculostatic agents, etomidate, topiramate, growth hormone, tamoxifen, estrogens, thyroxine Assess the need for increased fludrocortisone after increased hydrocortisoneIncrease the dose (start with 10–20%) Liquorice, grapefruit juice and colestipol Reduce the dose (start by 10–20%) Antifungal drugs Dose adjustment may be required Prolonged or severe emotional or mental stress (university or workplace exams, death of a family member, grief, acute depression)Exercise of unusual intensity or duration (marathon, match) An additional hydrocortisone dose of 5–20mg 30–60min before. In the case of physical exercise: additional fluid and salt intakeb Working in shifts or travel with changes in time zone Adapt the hydrocortisone dose to the sleep-wake cycle Ramadan Instead of hydrocortisone, longer-acting glucocorticoids, such as prednisolone or dexamethasone, should be considered for fasting compliance (approximately 15h)A combination of prednisolone in the morning and hydrocortisone in the afternoon-evening may also be considered. If possible, it should be started a few weeks earlier for appropriate dose adjustment. During fasting hours, strenuous work and excessive heat should be avoided, and attempts should be made to rest during this period to avoid stress Lithium, progesterone (synthetic non-progestins), drospirenone, phenytoin, beta-blockersExposure to excessive humidity and heat (tropical climate) Increase the dose of fludrocortisone (start with 20%)Increase the intake of fluids with electrolytes (isotonic drinks); free access to saltIncrease the dose of fludrocortisone (start with 20–50%) Procedure Pre-procedure Post-procedure Major surgery with a long recovery time (e.g., intraabdominal surgery, cardiac surgery), general anesthesia, intensive care 100mg of hydrocortisone i.v. or i.m. just before anesthesia and continue with 200mg/day as a continuous infusion or bolus of 100mg every 12h or 50mg every 6haChildren: hydrocortisone 50mg/m2 i.v. followed by hydrocortisone 50–100mg/m2 daily or divided into every 6ha On the first day, 100mg of hydrocortisone every 8–12h or continuous i.v. infusion of 200–300mg/24h. After the uncomplicated procedure, gradually reduce the dose (30%) every day until the patient is able to drink and eataDouble the oral dose for 48h, then reduce to the normal doseChildren: hydrocortisone 50mg/m2 i.v. followed by hydrocortisone 50–100mg/m2 daily or divided into every 6ha Major surgery with rapid recovery (joint replacement surgery, cesarean section) 100mg of hydrocortisone i.v. or i.m. just before anesthesiaaChildren: hydrocortisone 50mg/m2 i.v.a Hydrocortisone 50mg/8h i.v. on the day of surgery, halve in the next 24h and return to the usual replacement doses in the following daysaChildren: hydrocortisone 50mg/m2 followed by hydrocortisone 25–50mg/m2 daily or divided and given every 6ha Prepartum and delivery maneuvering Start of hydrocortisone with labor maneuvers: infusion of 100mg in 12h until deliveryDuring labor: hydrocortisone i.v.a25mg every 6hIf labor is prolonged, 100mg/8h or continuous infusion (200–300mg/24h) until delivery Double the oral dose for 24–48h after delivery, then reduce to the normal dose Minor surgery (cataracts, hernia) and major dental surgery (tooth extraction under general anesthesia, molars) 100mg of hydrocortisone i.m. (or s.c.) just before anesthesia or i.v. infusion during surgeryaChildren: hydrocortisone 50mg/m2 i.m.a Double the oral dose for 24h, then return to the normal doseb Invasive procedures requiring laxatives: colonoscopy, barium enema, etc. Consider hospital admission the night before to administer 100mg of hydrocortisone i.v. or i.m. (or s.c.) and fluids (isotonic saline solution), repeat the dose just before the procedureaChildren: hydrocortisone 50mg/m2 i.m. before the procedurea Double the oral dose for 24h, then return to the normal doseb Other invasive procedures: arteriography, endoscopy, etc. 100mg hydrocortisone i.v. or i.m. just before the procedure Double the oral dose for 24h, then return to the normal doseb Minor dental procedure (filling, skin lesion) Not normally required. An extra dose can be administered 1h before the procedure (10–20mg of hydrocortisone in adults) Extra dose in the event of symptoms of hypocortisolismb Minor dental procedure (endodontic treatment) An extra dose can be administered 1h before the procedure (10–20mg of hydrocortisone in adults) Double the oral dose for 24h, then return to the normal doseb i.m.: intramuscular; i.v.: intravenous; s.c.: subcutaneous: p.o.: oral.
Recommendations for glucocorticoid dose adjustment in medical and therapeutic procedures.
aIf hydrocortisone administered via the intramuscular or intravenous route is not available, it is advisable to replace it with the equivalent dose of methylprednisolone or dexamethasone (100mg hydrocortisone is equivalent to 20mg of methylprednisolone or 4mg of dexamethasone), adjusting the hourly regimen according to the specific pharmacokinetics of each GC. It is important to take into account that MC replacement is not required if the hydrocortisone dose exceeds 50mg every 24h.
bIn the case of treatment with sustained-release and dual-release GC preparations (such as Plenadren®), in order to double or triple the total daily dose, the maintenance dose should be administered every 12 or 8h, respectively (e.g., if the dose is 20mg at breakfast and we want to triple it, raise it to 20mg at breakfast, 20mg at lunch and 20mg at dinner time). In the case of treatment with Plenadren®, and if an extra dose is to be administered, an extra immediate-release dose of hydrocortisone is required (5–10mg), particularly in the afternoon or evening (e.g.: Plenadren® 20mg at breakfast and hydrocortisone 10mg at the time of the afternoon snack).
- •
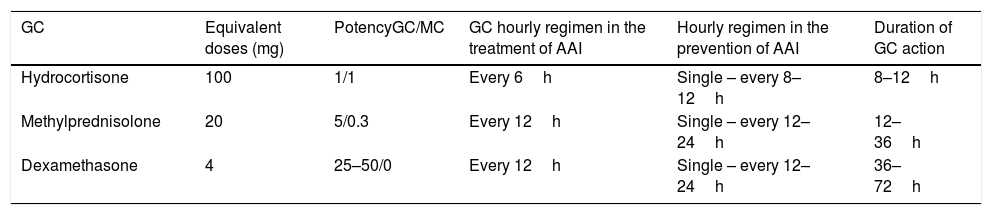
If hydrocortisone is not available for intramuscular or intravenous administration, replacement with the equivalent dose of methylprednisolone or dexamethasone is advisable. Table 7 summarizes the characteristics of the GCs recommended for administration via these routes for the treatment and prevention of AAI.
Table 7.Glucocorticoids used in the prevention and treatment of acute adrenal insufficiency.
GC Equivalent doses (mg) PotencyGC/MC GC hourly regimen in the treatment of AAI Hourly regimen in the prevention of AAI Duration of GC action Hydrocortisone 100 1/1 Every 6h Single – every 8–12h 8–12h Methylprednisolone 20 5/0.3 Every 12h Single – every 12–24h 12–36h Dexamethasone 4 25–50/0 Every 12h Single – every 12–24h 36–72h GC: glucocorticoid; h: hour; AAI: acute adrenal insufficiency; MC: mineralocorticoid.
- •
Education to recognize the warning signs and symptoms of a possible adrenal crisis, and to ensure awareness of the need to attend the nearest hospital and start treatment quickly.
- •
An awareness of how to use and ensure the availability of emergency material and treatment tools.
These are as follows:
- 1)
An emergency card or identification (necklace or bracelet) which the patient should carry or wear at all times, so that it can be located by the healthcare personnel in the event of an adrenal crisis in order to know the cause of the disease, regular treatment, and the treatment to be administered in the event of an emergency: “Adrenal insufficiency: I need glucocorticoids!”.
Both national and international (EU) emergency cards are available for printing in the adrenal disease section for patients of the SEEN (www.seen.es).
- 2)
The patient should report his/her disease before starting new treatments, in case dose adjustment is required.
- 3)
An emergency kit should be available for use by the patient or relatives, and should include the following: GC for injection (at least 2 vials of 100mg hydrocortisone or 20mg methylprednisolone or 4mg of dexamethasone), vials of saline solution (0.9%), and syringes. An alternative is the use of suppositories of 100mg prednisolone, or prednisolone enemas of 20mg/100ml or 10% hydrocortisone acetate (in the absence of diarrhea). Explain how the medication should be prepared and injected (intramuscular, subcutaneous or rectal). The subcutaneous and intramuscular injection of hydrocortisone presents similar pharmacokinetics, but the ease of subcutaneous administration makes this route the option of choice. It should be borne in mind that in the case of subcutaneous administration, two injections are needed (versus a single intramuscular injection), and more time is required to reach therapeutic levels (22 versus 11min).
- 4)
The availability of an information leaflet with indications for medical or therapeutic situations/events/procedures requiring dose adjustments or the intramuscular, subcutaneous or rectal administration of replacement therapy.
Supplementary informative material on AAI for patients and relatives is available in the adrenal disease section for patients of the SEEN (www.seen.es) and in the European support network (www.adrenal.eu).
- 5)
The provision of a phone number of the healthcare team familiar with the management of this disorder.
Please cite this article as: Araujo Castro M, Currás Freixes M, de Miguel Novoa P, Gracia Gimeno P, Álvarez Escolá C, Hanzu FA. Guía para el manejo y la prevención de la insuficiencia suprarrenal aguda. Endocrinol Diabetes Nutr. 2020;67:53–60.