Diabetes is one of the leading causes of morbidity-mortality in the Western world, and is independently correlated to chronic kidney disease (CKD) and cardiovascular disease (CVD).1 Diabetes and CVD have an individualized impact upon CKD.1 Banerjee and Panas showed patients with CVD and diabetes to be twice as likely to develop CKD versus those without CVD.1
Today, a large proportion of patients admitted to hospital suffer different degrees of heart failure (HF) and kidney failure. The dysfunction of one of these organs leads to the dysfunction of the other, in a complex interaction clinically referred to as cardiorenal syndrome (CRS).2 This syndrome can be defined as a pathological renal and cardiac deterioration secondary to acute or chronic dysfunction of either organ (kidney or heart), and which induces the dysfunction of the other.1,2
Several studies have shown the existence of bidirectional regulation between the heart and kidneys, so that changes in the function of one organ lead to the failure of both, with resultant changes in the levels of nitric oxide (NO) and reactive oxygen species (ROS), thus promoting systemic inflammation and activation of the sympathetic nervous system and the renin–angiotensin system (RAS).2,3 Furthermore, it has been seen that patients with cardiovascular risk factors (smoking, hypertension, dyslipidemia, age and diabetes) are also at an increased risk of suffering kidney disease.2
Diabetic nephropathy (DN) is the main cause of end-stage chronic kidney failure in developed countries, and one of the leading causes of cardiovascular mortality. The early stages of DN are characterized by increased blood pressure (BP) and an increase in urinary albumin excretion, so-called microalbuminuria. In the more advanced stages of the disease, hypertension and proteinuria appear.4 In this regard, the proteinuria levels are a CKD progression marker.5 It should be mentioned that microalbuminuria is not only an indicator of the risk of kidney disease but is also associated with an increased risk of cardiovascular events and mortality.6
Patients with type 1 diabetes (DM1) and with microalbuminuria or proteinuria show a 2- to 10-fold greater progression of cardiovascular complications compared with patients presenting normal albumin excretion.7 In the case of type 2 diabetes (DM2), microalbuminuria is a risk factor for the development of atherosclerosis, coronary disease and other vascular disorders.8 In this regard, it is well known that patients with DM2 have higher cardiovascular event rates than patients without diabetes who have suffered a previous cardiovascular event.9
The RAS plays an important role in BP regulation and in the kidneys and the heart. Blockade of the RAS has been related to an improvement of proteinuria and a reduction in the progression of CKD.10 The formation of angiotensin-II (Ang-II) mediated by angiotensin converting enzyme (ACE) favors smooth muscle contraction in the arterioles, sodium reabsorption in the renal tubules, the stimulation of aldosterone secretion, and an increased sympathetic response. Over the long term, it stimulates fibrosis, cell proliferation and differentiation, inflammation, apoptosis, the generation of ROS and ultimately also the inhibition of renin release into the bloodstream.11
In diabetic patients, poorly controlled hyperglycemia and hypertension favor RAS activation, facilitating the accumulation of Ang-II through interaction with Ang-II receptor I. The excess Ang-II increases the TGF-β levels, which stimulate oxidative stress, favor the appearance of endothelial damage, raise BP, cause vasoconstriction and increase cardiovascular risk. In the kidneys, the accumulation of Ang-II exerts a negative effect upon podocyte structure, favoring apoptosis and epithelial-mesenchymal transition, causing damage and reducing the number of podocytes. This in turn leads to the appearance of proteinuria and glomerulosclerosis, with the consequent loss of renal function.11
Angiotensin-converting enzyme 2 (ACE2), a homolog of ACE, was discovered in 2000. While the main function of ACE is to generate Ang-II from Ang-I, ACE2 avoids the accumulation of Ang-II by degrading it to Ang-(1-7). In turn, it converts Ang-(1-7) to angiotensin 1-9, though its affinity for Ang-I is less than that for Ang-II.12 Accordingly, in the kidneys and the heart Ang-(1-7) counters the negative effects of the accumulation of Ang-II. It has vasodilator properties, inhibits tubular epithelial cell proliferation signals, and avoids kidney damage.11,13
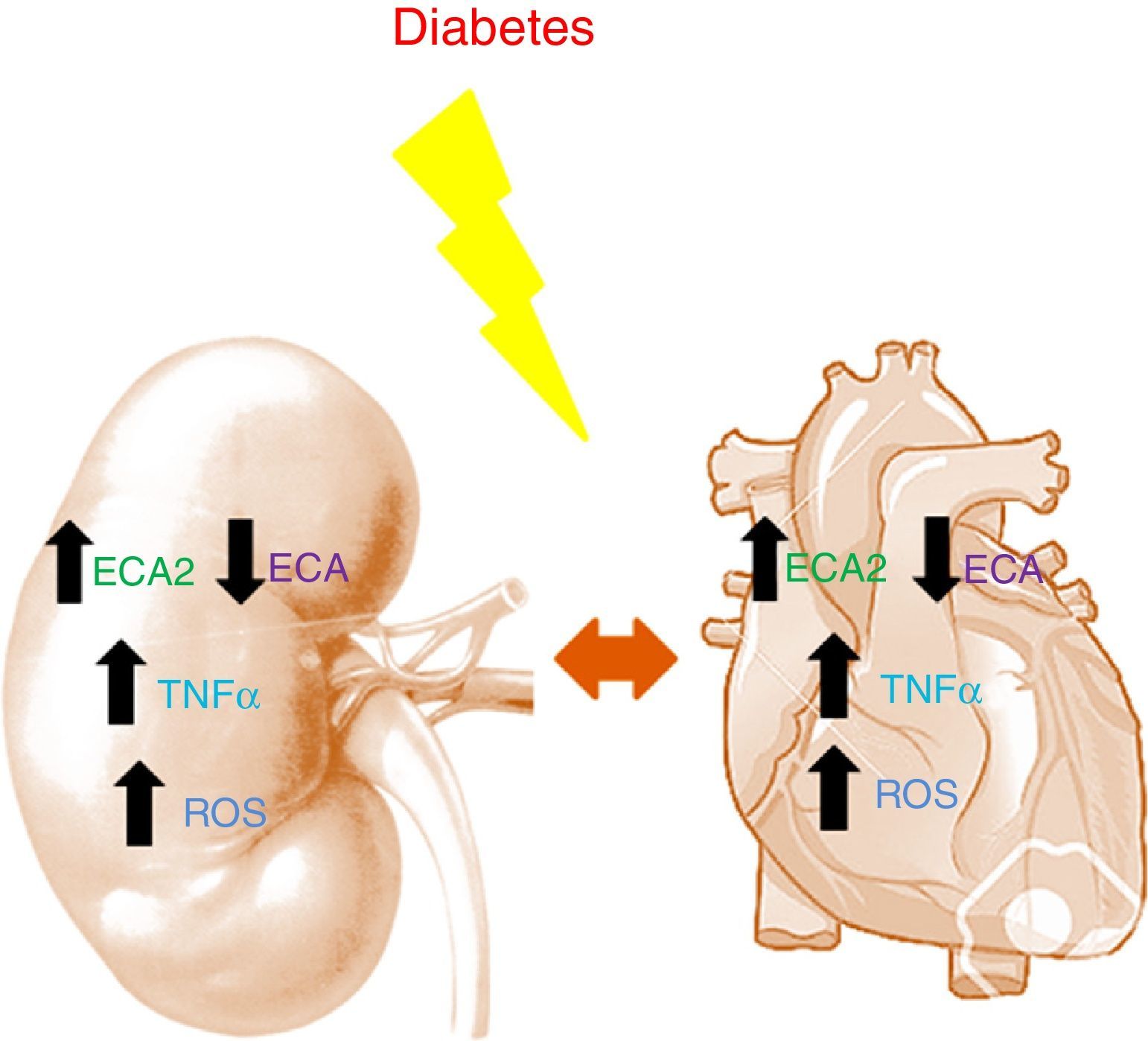
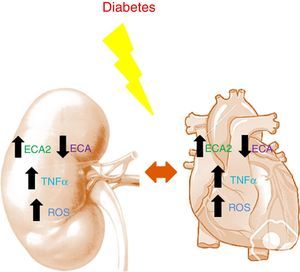
Studies in murine models have shown diabetes to be characterized by an increase in ACE2 and a decrease in ACE in both the kidneys and the heart.14,15 The decrease in ACE, combined with the increase in ACE2, appears to have a renal and cardiac protective effect during the early stages of diabetes, helping to slow the accumulation of Ang-II to promote the formation of Ang-(1-7). Angiotensin converting enzyme 2 deficiency in murine models of type 1 diabetes is histologically characterized by glomerular hypertrophy and mesangial matrix expansion, worsening the renal damage due to the accumulation of Ang-II.16 In the heart, ACE2 deficiency results in left ventricular dilatation and systolic dysfunction that are reversed with ACE gene deletion.17 In patients with acute myocardial infarction, the activity of circulating ACE2 is related to infarct size, ventricular ejection fraction and mortality.12 In turn, in CKD the activity of circulating ACE2 is found to be increased in patients with diabetes.18 It should be mentioned that in such patients the increase in ACE2 activity is associated with an increased risk of silent atherosclerosis after two years of follow-up19 (Fig. 1).
Treatment with ACE inhibitors (ACEIs) or Ang-II receptor antagonists (ARA2 drugs) is the mainstay in the management of patients with left ventricular systolic dysfunction, and also prevents progressive renal impairment in DN and in other forms of chronic kidney failure. The existing evidence suggests that the antihypertensive and antiproteinuric effects of ACEIs delay kidney function impairment in patients with type 1 diabetes. ACEIs are also useful for slowing the progression of early DN to established nephropathy in patients with T2DM. In patients with DM2 and incipient or established nephropathy, treatment with ARA2 drugs has been shown to reduce proteinuria and the progression of the disease or the need for dialysis due to end-stage CKD.20 RAS blockade with ACEIs or ARBs not only lowers BP, but also decreases proteinuria. Furthermore, RAS blockade reduces cardiovascular mortality in high risk patients, including individuals with CKD.20
Recent studies have shown the cardiac and renal protective effects of empagliflozin, a sodium-glucose cotransporter type 2 (SGLT2) inhibitor with known glucose-lowering activity. In this regard, Wanner et al. demonstrated, in patients with DM2 and high cardiovascular risk, that treatment with empagliflozin is associated with a reduced level of renal damage.21 In the context of diabetes, changes are not only seen in RAS, but also in other signaling pathways involved in cardiac and renal dysfunction. Metabolic changes as a consequence of hyperglycemia or insulin resistance contribute to an increase in oxidative stress in the kidneys and the heart.22 ROS are a basic product of cell metabolism. In non-diabetic subjects, the body is able to maintain ROS levels balanced, thus avoiding their accumulation. In the presence of diabetes, however, ROS levels exceed the antioxidant capacity of cells and cause tissue damage. The accumulation of ROS exerts toxic effects upon the genes and transcription factors in charge of controlling mitochondrial oxidative phosphorylation – thereby contributing to mitochondrial dysfunction and favoring the generation of free radicals.23
As commented above, patients with diabetes present RAS alterations, which result in increased Ang-II levels that favor the activity of the enzyme NADPH oxidase within the cytosol compartment, altering the mitochondria, increasing fatty acid metabolism, and contributing to the development of cardiomyopathy.23
Human studies have demonstrated the significant role of cytokines in diabetes in the kidneys and the heart. The effects of cytokines in CKD are associated with hemodynamic alterations, changes in renal structure and in the mesangial matrix, the stimulation of apoptosis and necrosis, and increased ROS production.24 In the kidneys, proinflammatory cytokines are seen to be increased in diabetes, favoring the development of cardiomyopathy.25 TNF-α levels are increased in both the kidneys and the heart in patients with diabetes. This cytokine causes renal cell toxicity and induces kidney damage, apoptosis, and necrosis. TNF-α promotes the loss of TGF by altering the balance between the factors that promote vasoconstriction and vasodilation. It induces the activation of NADH oxidase, promoting increased ROS levels and thus causing alterations at the glomerular level.24 In the heart, TNF-α plays a significant role in the pathogenesis of obesity and insulin resistance, altering insulin signaling pathways and myocardial function. Drug-induced inhibition of this cytokine improves cardiac function in heart failure, reducing intramyocardial inflammation and cardiac fibrosis.25 Different inflammation pathways have recently been postulated as therapeutic targets in the reno-cardiovascular management of patients with T2DM. In this regard, pentoxifylline, CCR2 inhibitors and interleukin-1 antagonists could represent future therapeutic strategies.26
It should be mentioned that other intervention strategies related to fibrosis could also prove useful in the treatment of such patients in the not too distant future, including mesenchymal stem cell therapies.27
Please cite this article as: Palau V, Riera M, Soler MJ. La conexión reno-cardiovascular en el paciente con diabetes mellitus: ¿qué hay de nuevo? Endocrinol Diabetes Nutr. 2017;64:237–240.