To determine the behavior of the triglycerides/HDL-cholesterol ratio (TG/HDL) as a cardiometabolic risk marker in children and adolescents from Mérida, Venezuela.
MethodsA total of 1292 children and adolescents aged 7–18 years who attended educational institutions in the Libertador Municipality were enrolled into this study. Anthropometric measurements and blood pressure values were recorded. Fasting blood glucose, insulin and lipid levels were measured. The TG/HDL ratio, HOMA-IR, and QUICKI indexes were calculated. Subjects were categorized as with and without cardiometabolic risk based on the presence or absence of 2or more risk factors. Cut-off points for the TG/HDL ratio were determined by constructing ROC curves.
ResultsSignificantly higher mean TG/HDL ratios were found in pubertal (2.2±1.7) as compared to prepubertal subjects (1.8±1.5; p=.001), with no sex differences. Two or more risk factors were found in 14.7% (n=192) of the participants, in whom TG/HDL ratios were significantly higher as compared to those with no risk (3.5±2.9 versus 1.6±0.8 in prepubertal and 4.1±3.5 versus 1.8±0.9 in pubertal subjects; p=.0001). According to cardiometabolic risk, cut-off points for the TG/HDL ratio of 1.8 and 2.5 were found for prepubertal and pubertal children respectively. These cut-off points showed risks (odds ratio) higher than 2.5 for conditions such as metabolic syndrome, elevated non-HDL-C, abdominal obesity, and elevated HOMA-IR.
ConclusionIn this sample of children and adolescents, an elevated TG/HDLc ratio was found to be a good marker for predicting cardiometabolic risk.
Determinar el comportamiento de la relación triglicéridos/colesterol HDL (TG/cHDL) como indicador de riesgo cardiometabólico en niños y adolescentes escolarizados de la ciudad de Mérida.
MétodosSe estudió a 1.292 niños y adolescentes entre 7 y 18 años de edad, de instituciones educativas del Municipio Libertador. Se registraron medidas antropométricas y la presión arterial. Se determinaron glucemia, insulina y lípidos en ayunas. Se calcularon la relación TG/cHDL y los índices HOMA-IR y QUICKI. Se realizó la clasificación de individuos con riesgo y sin riesgo cardiometabólico a partir de la presencia o no de 2 o más factores de riesgo. Se determinaron puntos de corte de la relación TG/cHDL a través de la construcción de curvas operador receptor (COR).
ResultadosLa relación TG/cHDL presentó medias significativamente superiores en individuos púberes (2,2 ± 1,7) en comparación con prepúberes (1,8 ± 1,5; p = 0,001), sin diferencias según el género. El 14,7% (n = 192) de los participantes presentaba 2 o más factores de riesgo y los valores de la relación TG/cHDL fueron significativamente mayores en comparación con aquellos sin riesgo (3,5 ± 2,9 frente a 1,6 ± 0,8 en prepúberes y 4,1 ± 3,5 frente a 1,8 ± 0,9 en púberes; p = 0,0001). De acuerdo con el riesgo cardiometabólico, se obtuvieron puntos de corte para la relación TG/cHDL de 1,8 y 2,5 en prepúberes y púberes, respectivamente. Estos puntos de corte muestran riesgos (odds ratios) superiores a 2,5 para alteraciones como síndrome metabólico, colesterol no HDL elevado, obesidad abdominal y HOMA-IR elevado.
ConclusiónEn esta muestra de niños y adolescentes, la relación TG/cHDL elevada demostró ser un buen marcador para predecir riesgo cardiometabólico.
Alterations in lipid metabolism are a key element in atheroma plaque formation, representing up to 50% of cardiovascular risk.1 Low density lipoprotein cholesterol (LDLc) currently constitutes the main management objective in the primary and secondary prevention of cardiovascular disease (CVD).2 However, it is acknowledged that the measurement of coronary risk based only on LDLc is insufficient,3,4 since approximately 40% of all patients who have suffered a coronary event exhibit normal LDLc levels.5,6
A small and dense LDL particle phenotype (pattern B) has been related to a three-fold greater cardiovascular risk than a large LDL particle phenotype,7,8 and has been significantly correlated to so-called atherogenic dyslipidemia, characterized by elevated triglyceride (TG) concentrations and low high density lipoprotein cholesterol (HDLc) levels, which could explain 50–67% of the variance in LDL particle size.9,10 The prevalence in adults of so-called LDL pattern B is 31–44% of the general population in the United States,11,12 versus up to 34% in Japan.13 In 2004, Shimabukuro et al.,14 in 586 Japanese children between 7 and 12 years of age, found 10.8% of the boys and 4.4% of the girls to have small LDL particles, and the main determinants of this lipoprotein pattern were higher plasma concentrations of glucose, insulin and TGs, and lower HDLc levels.
The TG/HDLc ratio may reflect the balance between atherogenic and protective lipoproteins, and shows a positive correlation to the HDL esterification rate and an inverse correlation to LDL particle size.15 In a classic study, McLaughlin et al.16 found the TG/HDLc ratio to be the best predictor of insulin resistance (IR) and of LDL particle diameter. In this respect, a cut-off point of 3.5 showed high sensitivity and specificity in identifying individuals with LDL phenotype B and subjects with IR.
In a pediatric population involving 35 overweight adolescents, Hannon et al.17 found those subjects with a TG/HDLc ratio ≥3 to have less insulin sensitivity as determined by the euglycemic-hyperinsulinemic clamp technique, and greater visceral fat, than those with values below this cut-off point. In Mérida (Venezuela), Quijada et al., 18 in a group of 67 prepubertal children, found that 69% of the obese children, 83% of those with hypertension, and 95% of those with metabolic syndrome (MS) presented a TG/HDLc ratio ≥3.5. In a group of 884 predominantly (61%) obese individuals between 6 and 16 years of age, interventricular septal thickness, relative wall thickness and left ventricular mass increased with the TG/HDLc ratio, and those who exceeded a value of 2 presented a 2- to 3-fold higher risk of alanine aminotransferase (ALT) elevation and concentric left ventricular hypertrophy.19
Since molecular changes precede the clinical manifestations of disease, and bearing in mind that many of the biochemical variables implicated in disorders such as MS, type 2 diabetes mellitus or CVD exhibit variations according to ethnicity or age group, it is important to establish clear criteria in the pediatric population in order to ensure early detection and the adoption of adequate preventive measures in children and adolescents at risk. Thus, the purpose of this study was to determine the behavior of the TG/HDLc ratio as an indicator of cardiometabolic risk, and to define the cut-off points of this relationship based on the plotting of receiver operating curves (ROCs) in schoolchildren and adolescents in the city of Mérida (Venezuela).
Materials and methodsSubjectsA cross-sectional, analytical observational study was carried out. The sample of subjects came from two databases: (a) that of the study entitled “Obesity in schoolchildren from Mérida, Venezuela: association with cardiovascular risk factors”,20 by Paoli et al., involving subjects between 7 and 9 years of age; and (b) that of the project called “Evaluation of growth, development and cardiometabolic risk factors in schoolchildren and adolescents from Mérida, Venezuela (CREDEFAR)”,21,22 which was carried out at the Instituto Autónomo Hospital Universitario de Los Andes (IAHULA) between March 2010 and June 2011, with the participation of subjects between 9 and 18 years of age. Both studies involved stratification, proportional, randomized and multistage sampling, guaranteeing adequate representation in terms of gender, public or private institutions (socioeconomic level) and geographical setting. A representative sample was thus obtained, comprising 1292 children and adolescents between 7 and 18 years of age from the second basic grade to the fifth year of the different public and private Educational Units of Libertador Municipality in the city of Mérida.20–22 This city is the state capital, and Libertador Municipality is the most important municipality in terms of economic development and population density, forming part of the Metropolitan Area of Mérida. Schoolchildren and adolescents with chronic diseases such as heart disease, kidney disease, endocrine disorders, immune alterations and infections were excluded, as were those receiving medications capable of affecting the study variables, and pregnant adolescents. The ethical standards set down in the Declaration of Helsinki were met.
ProcedureAll the parents received an information sheet explaining the characteristics and objectives of the study, and an informed consent form, through the management boards of the participating Educational Units. The participants in the CREDEFAR study21,22 were instructed to report to the Hormones Laboratory of the IAHULA on a specific day of the week at 7:00a.m. under fasting conditions, accompanied by their representative, while the subjects under 9 years of age in the study of Paoli et al.20 were seen at school. A data sheet designed for the study was completed. We recorded the personal and family identification data, the personal history of disease, current medication, and the family history, with special reference to cardiometabolic disorders.
Anthropometric variables and arterial pressureThe anthropometric variables were recorded with the patient in underwear and barefoot, following the standards and techniques described by the National Health and Nutrition Examination Survey 2000.23 Body weight (in kg) was recorded using a calibrated standard scale, while height was measured (in cm) as the average of three recordings using a Harpenden stadiometer, with the subject erect and with the head placed in the Frankfurt horizontal plane. The body mass index (BMI) was calculated as body weight (kg)/height2 (m). The waist circumference (WC) was measured with a non-elastic metric tape at the midpoint between the margin of the ribcage and the iliac crest, in expiration. Arterial pressure was recorded with the patient seated and the arm at heart level, using a mercury sphygmomanometer. An appropriate size cuff was fitted. The auscultation method was used, and the reading corresponding to the first Korotkoff sound was recorded as systolic blood pressure (SBP), while diastolic blood pressure (DBP) was defined as the point where the sounds disappeared or decreased in intensity.
Biochemical variablesThe biochemical parameters in the CREDEFAR study22 comprised basal insulin and blood glucose, and a lipid profile under fasting conditions. The recordings of blood glucose, total cholesterol (TC), HDLc and TG were obtained employing enzyme methods with reagents from the company CIENVAR, using a HITACHI 911 autoanalyzer (Roche Diagnostics, USA). The LDLc values were calculated using the Friedewald formula: LDLc=TC−(TG/5+HDLc). Insulin concentration was recorded in 917 participants from serum samples stored at −20°C, using Immulite/Immulite 1000 analyzers, (chemiluminescent immunometric assay from SIEMENS, Diagnostic Products Corporation-DPC [Los Angeles, CA, USA]), with between- and within-assay coefficients of variation of 6.5% and 5.4%, respectively.24 The analyses were made in the Hormones Laboratory of the IAHULA, and where possible were processed in duplicate. In the study of Paoli et al.,20 the blood glucose and lipid profile determinations were made from a capillary blood sample that was immediately processed using the LDX System Plus (Cholestech) and the corresponding reagents cassette. Validity was previously determined, and correlation was found to be strong, exhibiting adequate sensitivity and specificity with respect to the standard laboratory determinations.25 These data were used to obtain the TG/HDLc ratio, while insulin resistance was calculated from the Homeostasis Model Assessment (HOMA-IR)26 formula: HOMA-IR=fasting insulin (μIU/ml)×fasting glucose (mmol/l)/22.5, and insulin sensitivity was determined based on the Quantitative Insulin-Sensitivity Index (QUICKI), using the formula: 1/([log insulin 0min]+[log blood glucose 0min]).27
Categorization of variablesIn relation to nutritional status, obesity was defined as a BMI above percentile (pc) 97, while overweight was defined as a BMI between pc 90 and 97. Normal body weight was regarded as a BMI between pc 10 and 90, and low weight was defined as a BMI below pc 10, based on the age and gender curves for Venezuelan children and adolescents generated by FUNDACREDESA.28 Prepubertal subjects were those in Tanner stage 1, while pubertal individuals were those in Tanner stage 2 or higher. The determination of dyslipidemia was based on the CREDEFAR local references obtained from the same population and which had already been published.22 Alterations in TG, TC, LDLc and non-HDLc were defined by values above pc 90 adjusted for age and gender, while alterations in HDLc were defined by values below pc 10. Hyperglycemia was defined by a fasting blood glucose concentration of >100mg/dl. For diagnosing high HOMA-IR and insulin values, use was made of the CREDEFAR references above pc 95,24 i.e., high insulin ≥9μIU/ml in prepubertal subjects and ≥12μIU/ml in pubertal subjects; high HOMA-IR ≥2 in prepubertal subjects and ≥2.5 in pubertal subjects. Metabolic syndrome was diagnosed based on the ATP III classification modified by Cook et al.,29 and using the local references as cut-off points.28,29 Metabolic syndrome was defined by the compliance of at least three of the following diagnostic criteria: WC (cm)>pc 90 (abdominal obesity), SBP and DBP (mmHg)>pc 90, TG (mg/dl)>pc 90, HDLc (mg/dl)<pc 10 according to age and gender, and fasting blood glucose ≥100mg/dl.
All other specifications referring to the population, sample and methodology can be found in the previously published articles.20–22,24,30 In the present study, the following risk factors were taken into consideration for the categorization of cardiometabolic risk: obesity, TG elevation, LDLc elevation, HDLc reduction, arterial hypertension or pre-hypertension, and hyperglycemia. The participants were classified as presenting no risk (the absence or presence of only one risk factor) or at risk (the presence of two or more risk factors).
Statistical analysisAll data were processed using the SPSS version 20 statistical package for MS Windows. Qualitative variables were expressed as absolute frequencies and percentages, while quantitative variables were reported as the arithmetic mean±standard deviation. Associations between qualitative variables were evaluated by means of the chi-squared test. Statistically significant differences between quantitative variables were explored using the Student t-test for unpaired samples. Receiver operating characteristic (ROC) curves were plotted in search of the cut-off points for the TG/HDLc ratio. To this effect, the study sample was divided into prepubertal and pubertal subjects, and these in turn were classified into individuals with or without cardiometabolic risk. An optimum area under the curve (AUC) of 1.000 was established, while an AUC of under 0.500 was regarded as an invalid test. To estimate the optimum cut-off point for the pubertal group from the ROC, the Youden index was calculated using the formula: (J=sensitivity+specificity−1=S–[1−Sp]).31 The probability (odds ratio [OR]) of presenting cardiometabolic risk factors was calculated using the cut-off points obtained for the TG/HDLc ratio. Statistical significance was considered for p<0.05.
ResultsThe total study sample consisted of 1292 children and adolescents from the city of Mérida: 639 males (49.5%) and 653 females (50.5%). The mean age of the population was 11.70±3.30 years (range 7–18.9 years); 558 were prepubertal (43.2%) and 734 pubertal subjects (56.8%). A total of 70.1% of the study population presented normal weight while 10.8% were overweight and 8.4% obese.
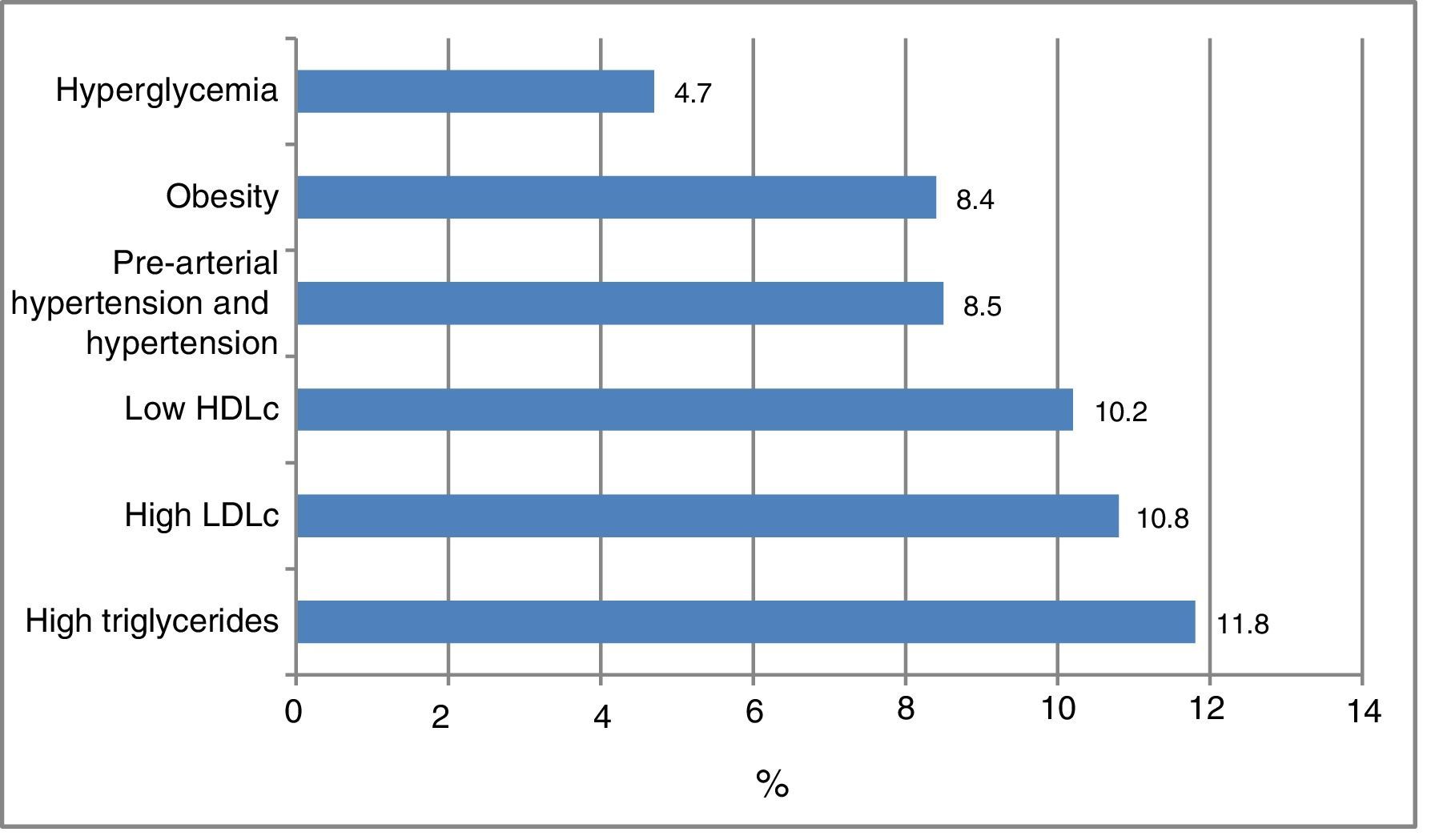
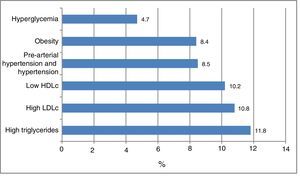
Fig. 1 shows the frequency of the cardiometabolic risk factors that were used to categorize the subjects according to risk; 28.1% of the participants had dyslipidemia, 11.8% elevated TG levels, 10.8% high LDLc concentrations, and 10.2% low HDLc levels. A total of 8.5% presented arterial hypertension or pre-hypertension, 8.4% were obese, and 4.7% presented hyperglycemia. There were no differences according to gender. Other cardiometabolic risk factors not used for the risk classification of the patients were abdominal obesity (in 10.2% of the cases), elevated non-HDLc (5.3%), elevated insulin levels (2.6%), elevated HOMA-IR (2.8%) and MS (2.6%). The insulin values were available for 917 individuals.
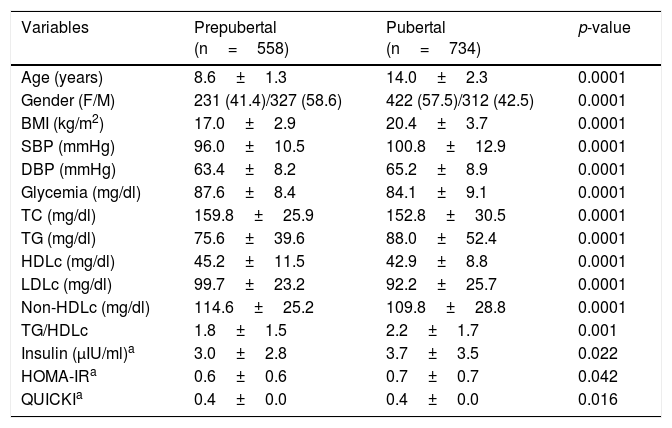
On comparing the anthropometric and biochemical values of the children and adolescents according to gender, no differences were noted in relation to age, the BMI, TG and HDLc values, the TG/HDLc ratio, basal insulin or the HOMA-IR index. Analysis of the population according to the presence or absence of puberty (Table 1) revealed significant differences for all the parameters studied, the values corresponding to the BMI, SBP and DSB, TG, the TG/HDLc ratio, insulin and HOMA-IR being higher in the pubertal individuals, while non-HDLc and the QUICKI score were higher in the prepubertal subjects. The TG/HDLc ratio was 1.89 on average among the prepubertal children and 2.20 in the pubertal group (p=0.001). There were no statistically significant gender differences in the TG/HDLc ratio in each pubertal group. In view of the differences according to pubertal stage, the following results are presented and analyzed with this variable being taken into consideration.
Anthropometric, clinical and biochemical parameters of the study sample according to pubertal stage.
| Variables | Prepubertal (n=558) | Pubertal (n=734) | p-value |
|---|---|---|---|
| Age (years) | 8.6±1.3 | 14.0±2.3 | 0.0001 |
| Gender (F/M) | 231 (41.4)/327 (58.6) | 422 (57.5)/312 (42.5) | 0.0001 |
| BMI (kg/m2) | 17.0±2.9 | 20.4±3.7 | 0.0001 |
| SBP (mmHg) | 96.0±10.5 | 100.8±12.9 | 0.0001 |
| DBP (mmHg) | 63.4±8.2 | 65.2±8.9 | 0.0001 |
| Glycemia (mg/dl) | 87.6±8.4 | 84.1±9.1 | 0.0001 |
| TC (mg/dl) | 159.8±25.9 | 152.8±30.5 | 0.0001 |
| TG (mg/dl) | 75.6±39.6 | 88.0±52.4 | 0.0001 |
| HDLc (mg/dl) | 45.2±11.5 | 42.9±8.8 | 0.0001 |
| LDLc (mg/dl) | 99.7±23.2 | 92.2±25.7 | 0.0001 |
| Non-HDLc (mg/dl) | 114.6±25.2 | 109.8±28.8 | 0.0001 |
| TG/HDLc | 1.8±1.5 | 2.2±1.7 | 0.001 |
| Insulin (μIU/ml)a | 3.0±2.8 | 3.7±3.5 | 0.022 |
| HOMA-IRa | 0.6±0.6 | 0.7±0.7 | 0.042 |
| QUICKIa | 0.4±0.0 | 0.4±0.0 | 0.016 |
Data expressed as the mean±SD and n (%).
HDLc: high density lipoprotein cholesterol; LDLc: low density lipoprotein cholesterol; Non-HDLc: non-HDL cholesterol; TC: total cholesterol; F: female; HOMA-IR: Homeostasis Model Assessment for Insulin Resistance; BMI: body mass index; M: male; DBP: diastolic blood pressure; SBP: systolic blood pressure; QUICKI: Quantitative Insulin Sensitivity Check Index; TG: triglycerides.
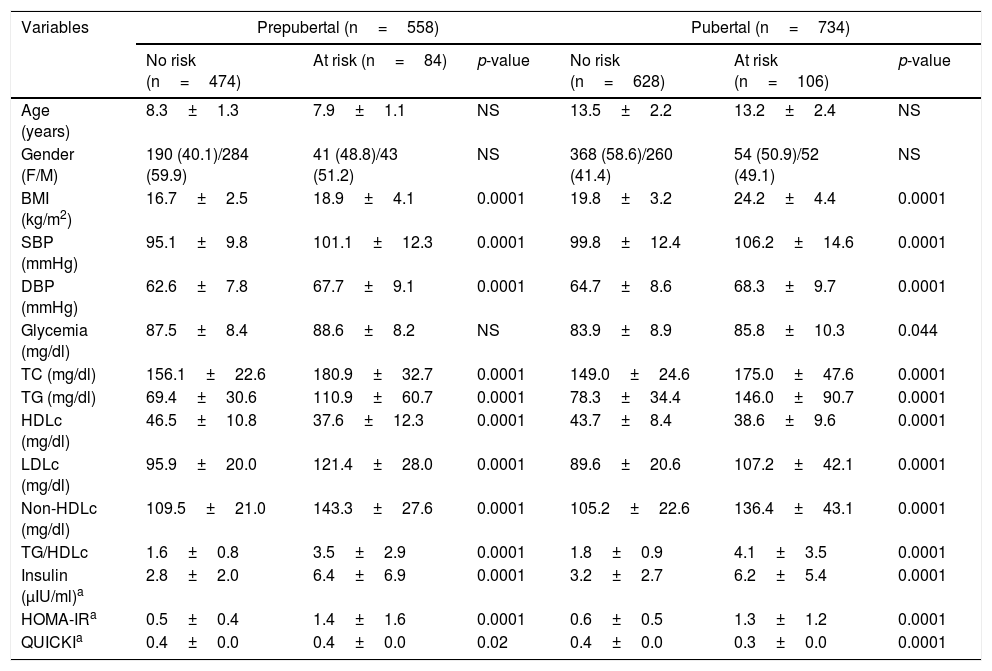
The sample was classified into individuals with and without cardiometabolic risk, based on the presence of two or more risk factors (Table 2). Of the 1292 participating children and adolescents, 192 presented two or more risk factors (14.7%). In the prepubertal group, 85% presented no risk while 15% presented risk. In the pubertal group these figures were 86% and 14%, respectively. There were no differences in age or gender distribution. The values corresponding to the BMI, SBP and DBP and the lipid parameters TC, TG, LDLc and non-HDLc were significantly higher (p<0.0001) among the individuals at risk, including the TG/HDLc ratio (p<0.001), while HDLc showed lower values in both the prepubertal and pubertal subjects (p<0.001). No differences were observed in terms of the blood glucose concentrations in the prepubertal group, though higher values were recorded in the pubertal group at risk (p=0.04). The variables indicative of insulin resistance, such as basal insulin (p<0.001) and HOMA-IR (p<0.001) were higher and the QUICKI score (p<0.02) lower, in both the prepubertal and pubertal individuals at risk.
Anthropometric, clinical and biochemical parameters according to pubertal stage and the presence of cardiometabolic risk factors in the study sample.
| Variables | Prepubertal (n=558) | Pubertal (n=734) | ||||
|---|---|---|---|---|---|---|
| No risk (n=474) | At risk (n=84) | p-value | No risk (n=628) | At risk (n=106) | p-value | |
| Age (years) | 8.3±1.3 | 7.9±1.1 | NS | 13.5±2.2 | 13.2±2.4 | NS |
| Gender (F/M) | 190 (40.1)/284 (59.9) | 41 (48.8)/43 (51.2) | NS | 368 (58.6)/260 (41.4) | 54 (50.9)/52 (49.1) | NS |
| BMI (kg/m2) | 16.7±2.5 | 18.9±4.1 | 0.0001 | 19.8±3.2 | 24.2±4.4 | 0.0001 |
| SBP (mmHg) | 95.1±9.8 | 101.1±12.3 | 0.0001 | 99.8±12.4 | 106.2±14.6 | 0.0001 |
| DBP (mmHg) | 62.6±7.8 | 67.7±9.1 | 0.0001 | 64.7±8.6 | 68.3±9.7 | 0.0001 |
| Glycemia (mg/dl) | 87.5±8.4 | 88.6±8.2 | NS | 83.9±8.9 | 85.8±10.3 | 0.044 |
| TC (mg/dl) | 156.1±22.6 | 180.9±32.7 | 0.0001 | 149.0±24.6 | 175.0±47.6 | 0.0001 |
| TG (mg/dl) | 69.4±30.6 | 110.9±60.7 | 0.0001 | 78.3±34.4 | 146.0±90.7 | 0.0001 |
| HDLc (mg/dl) | 46.5±10.8 | 37.6±12.3 | 0.0001 | 43.7±8.4 | 38.6±9.6 | 0.0001 |
| LDLc (mg/dl) | 95.9±20.0 | 121.4±28.0 | 0.0001 | 89.6±20.6 | 107.2±42.1 | 0.0001 |
| Non-HDLc (mg/dl) | 109.5±21.0 | 143.3±27.6 | 0.0001 | 105.2±22.6 | 136.4±43.1 | 0.0001 |
| TG/HDLc | 1.6±0.8 | 3.5±2.9 | 0.0001 | 1.8±0.9 | 4.1±3.5 | 0.0001 |
| Insulin (μIU/ml)a | 2.8±2.0 | 6.4±6.9 | 0.0001 | 3.2±2.7 | 6.2±5.4 | 0.0001 |
| HOMA-IRa | 0.5±0.4 | 1.4±1.6 | 0.0001 | 0.6±0.5 | 1.3±1.2 | 0.0001 |
| QUICKIa | 0.4±0.0 | 0.4±0.0 | 0.02 | 0.4±0.0 | 0.3±0.0 | 0.0001 |
Data expressed as the mean±SD and n (%).
HDLc: high density lipoprotein cholesterol; LDLc: low density lipoprotein cholesterol; Non-HDLc: non-HDL cholesterol; TC: total cholesterol; HOMA-IR: Homeostasis Model Assessment for Insulin Resistance; BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure; QUICKI: Quantitative Insulin Sensitivity Check Index; TG: triglycerides.
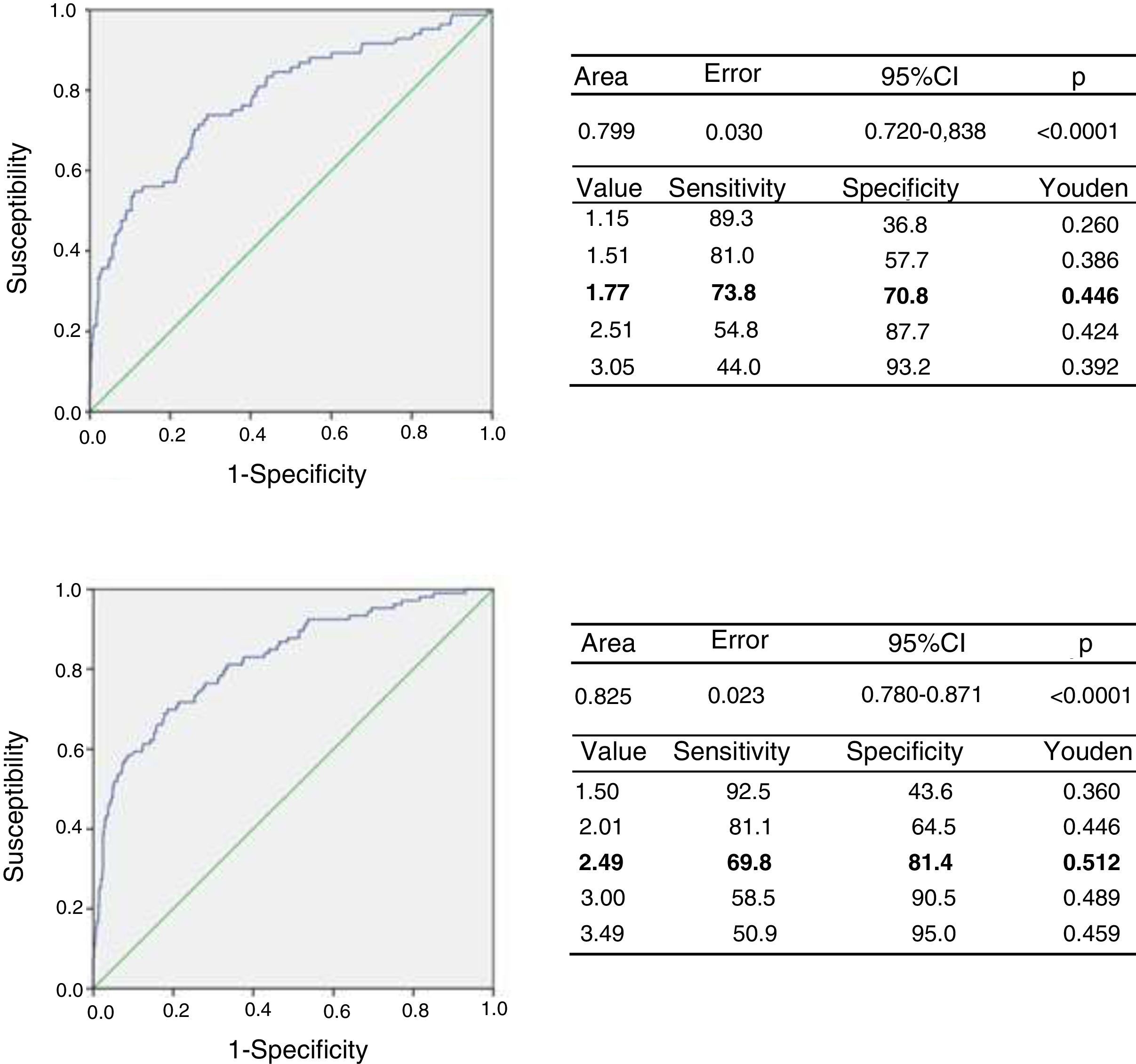
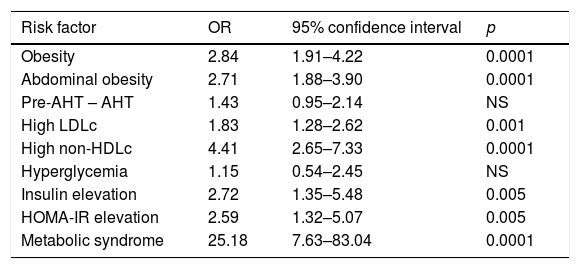
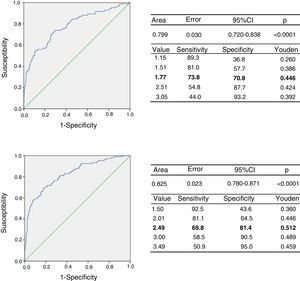
Based on the classification of the subjects according to cardiometabolic risk, we determined the cut-off point for the TG/HDLc ratio using the ROC curves and the Youden index. Fig. 2 (upper panel) shows the ROC curve for the prepubertal children, with an AUC of 0.779 (95% confidence interval [CI]: 0.720–0.838), a sensitivity of 73.8% and a specificity of 70.8% for the TG/HDLc ratio cut-off point of 1.77 (J=0.446), which could be rounded to 1.8. Fig. 2 (lower panel) also shows the ROC curve for the pubertal group, with an AUC of 0.826, a sensitivity of 69.8% and a specificity of 81.4% for the cut-off point of 2.49 (J=0.512), which could be rounded to 2.5. The statistical power of this test for an alpha error of 0.05 and a 95% confidence interval in both the prepubertal and pubertal subjects was 100%. We found that 196 of the prepubertal participants (35.1%) had a TG/HDLc ratio of ≥1.8, while 190 of the pubertal subjects (25.9%) had a ratio of ≥2.5.
The indirect relative risk (RRi or OR) of having risk factors in the presence of an elevated TG/HDLc ratio (taking as reference the cut-off points obtained in the ROC analysis) is reported in Table 3. In this sample of children and adolescents, the risk of obesity in an individual with a high TG/HDLc ratio was 2.76 times greater, while the risk of abdominal obesity was 2.82 times greater, high LDLc 1.8 times greater, elevated non-HDLc 4.29 times greater, elevated insulin 2.68 times greater, high HOMA-IR 2.54 times greater, and the association of three or more components of MS 24.5 times greater. No increased risk of pre-arterial hypertension or hypertension, or hyperglycemia, was observed.
Risk of presenting a cardiovascular risk factor according to the TG/HDLc ratio (cut-off point: prepubertal=1.8, pubertal=2.5).
| Risk factor | OR | 95% confidence interval | p |
|---|---|---|---|
| Obesity | 2.84 | 1.91–4.22 | 0.0001 |
| Abdominal obesity | 2.71 | 1.88–3.90 | 0.0001 |
| Pre-AHT – AHT | 1.43 | 0.95–2.14 | NS |
| High LDLc | 1.83 | 1.28–2.62 | 0.001 |
| High non-HDLc | 4.41 | 2.65–7.33 | 0.0001 |
| Hyperglycemia | 1.15 | 0.54–2.45 | NS |
| Insulin elevation | 2.72 | 1.35–5.48 | 0.005 |
| HOMA-IR elevation | 2.59 | 1.32–5.07 | 0.005 |
| Metabolic syndrome | 25.18 | 7.63–83.04 | 0.0001 |
LDLc: low density lipoprotein cholesterol; Non-HDLc: non-HDL cholesterol; HOMA-IR: Homeostasis Model Assessment for Insulin Resistance; Pre-AHT—AHT: pre-arterial hypertension—hypertension.
The presence of cardiometabolic risk factors is increasingly common among the pediatric population. Dyslipidemia was the most prevalent of the risk factors analyzed in our study, with a frequency of 28.1%. The most common lipid disorder was hypertriglyceridemia (11.8%), followed closely by hypercholesterolemia and lowered alpha-lipoprotein levels (10.8% and 10.2%, respectively).
Analyses of prospective studies both in patients with acute coronary syndrome and in individuals with stable coronary disease have shown hypertriglyceridemia and low HDLc to be intimately associated with high cardiovascular risk, even in the presence of optimum LDLc values.32,33 The TG/HDLc ratio has been proposed as a good indicator of the existence of cardiometabolic risk, since it is able to identify individuals with insulin resistance and small and dense LDL particles.16,34 Furthermore, as shown by a recent case-control study, this ratio may even predict a first coronary event.35 Urbina et al.36 recently found the TG/HDLc ratio to be an independent determinant of arterial stiffness in a group of 893 adolescents and young adults (10–26 years of age), and indicated that this ratio may help in identifying those young individuals requiring early aggressive intervention in order to prevent early atherosclerotic CVD.
In the present study we observed no significant gender differences in TG, HDLc or the TG/HDLc ratio. This was consistent with the findings of a study involving 943 adolescents between 11-14 years of age in the city of Buenos Aires.37 As a result of the above, most studies on the TG/HDLc ratio in pediatric populations analyze the subjects globally, without gender distinction. By contrast, significant differences were recorded on comparing the TG/HDLc ratio between prepubertal and pubertal subjects, with mean values of 1.89 and 2.20, respectively. Similar data were obtained by Olson et al.38 in a group of 217 subjects, with a TG/HDLc ratio of 1.84 in the prepubertal group and 2.59 in the pubertal group. Although most authors recognize the relevance of pubertal development in relation to observed changes in insulin sensitivity in the pediatric population and its repercussions upon the lipid profile, few studies in our country or elsewhere have taken into account the cardiometabolic risk factors on the basis of pubertal stage.
Defining a cut-off point for a given metabolic parameter is hampered by the generalization of variables, without due consideration of ethnic differences. It is well known that genetic factors play a key role in insulin sensitivity, anthropometric measures and lipid profile. Using universal cut-off points for metabolic parameters such as the TG/HDLc ratio therefore could over- or underestimate the risk profile in different populations.39 Moreover, the finding of low HDLc levels in our population—a situation that has already been described in the region22,30—and which appears to be a phenotypic feature of Latin Americans,40,41 underscores the need to use reference values inherent to each particular location in order to correctly detect lipid disorders as cardiometabolic risk factors.
Different methods have been proposed for establishing the cut-off point that is best able to differentiate individuals with cardiometabolic risk based on evaluation of the TG/HDLc ratio. A first approach consists of dividing the TG/HDLc ratio into tertiles and quintiles, and to show the correlation of the different risk factors to the higher strata. In this regard, Di Bonito et al.19 stratified the TG/HDLc ratio into tertiles in a sample of 884 children and adolescents between 6 and 16 years of age, and found that those in the upper tertile (TG/HDLc ratio≥2) increased their risk of greater WC, altered fasting blood glucose, high arterial pressure, insulin resistance and preclinical signs of target organ damage such as ALT elevation and concentric left ventricular hypertrophy, particularly in non-obese individuals. The authors, therefore, proposed a TG/HDLc ratio cut-off point of 2 for identifying individuals at risk. In turn, Weiss et al.42 conducted a 13-year longitudinal study among 770 Israeli adolescents between 16-17 years of age, in the context of the Jerusalem Lipid Research Clinic Study. Those patients with a basal TG/HDLc ratio of over 2.54 (upper quintile) had an OR of 7.5 for being assigned to the upper quartile of the small and dense LDL particle concentrations in adult life.
Receiver operating characteristic curves are used in Medicine to evaluate diagnostic tests allowing for the differentiation between healthy and ill persons.43 McLaughlin et al.16 were the first to use this method in 449 apparently healthy subjects, and established a TG/HDLc ratio cut-off point of 3.5 as a predictor of insulin resistance and high concentrations of small and dense LDL particles. Li et al.44 in turn established TG/HDLc ratio cut-off points for predicting insulin resistance in 2652 individuals belonging to different ethnic groups. They proposed a cut-off point of over 3 for non-Hispanic Caucasians and Mexican-Americans, and a cut-off point of over 2 for non-Hispanic black subjects.
In the pediatric population, Giannini et al.45 studied the TG/HDLc ratio cut-off point for predicting insulin resistance in a group of 1452 young obese individuals from different ethnic backgrounds. They found that a ratio of 2.27 in Caucasians yielded an OR indicating a 6.023-fold higher risk of insulin resistance, while in Hispanics and Afro-Americans the ROC curves were not significant. In a recent study involving 141 young overweight individuals between 9 and 18 years of age, Burns et al.46 conducted an ROC analysis in which a cut-off point of 3 in Caucasians and of 2.5 in black subjects was identified as the best predictor of the concentration of small and dense LDL particles.
In a search for simpler classification methods than the determination of the LDL particle profile, the quantification of insulin sensitivity, or the evaluation of subclinical atherosclerotic disease, we decided to classify our population from a clinical perspective into individuals with and without cardiometabolic risk, taking as reference the presence of two or more established risk factors, for posterior application of the ROC analysis. We found the mean TG/HDLc ratio to be 1.60 in individuals without risk versus 3.50 in subjects with cardiometabolic risk in the prepubertal group, and 1.87 in individuals without risk versus 4.18 in subjects with risk in the pubertal group (p<0.0001). The TG/HDLc ratio cut-off points for the prepubertal and pubertal groups were 1.77 and 2.49, respectively, though these figures were rounded to 1.8 and 2.5 for purposes of simplification. On analyzing the probability of presenting cardiometabolic risk factors based on these cut-off points, the highest OR for MS was seen to be 25.18, which can be explained by the fact that both hypertriglyceridemia and low HDLc form part of the diagnostic criteria of this disorder.47 Next in order of risk prediction was elevated non-HDLc (OR=4.41), which estimates the concentration of all the lipoproteins containing Apo B, such as VLDL, IDL, LDL and even Lp(a), in contrast to LDLc, which does not include VLDLc. According to some authors, the TG/HDLc ratio appears to be a better marker than the estimation of LDLc for defining cardiovascular risk and the monitoring of treatment, and exhibiting a good correlation to the concentration of small LDL particles.48,49
The above-mentioned cut-off points also predict a more than 2.5-fold higher risk of elevated parameters indicative of insulin resistance, such as the HOMA-IR index and basal insulin. This is explained by the influence of insulin upon the metabolism of TG-rich particles. In the presence of insulin resistance, lipoprotein lipase, cholesterol ester transfer protein, possibly liver lipase and phospholipid transfer protein exhibit a decrease in the metabolism of TG-rich particles, favoring an exchange of TG from lipoproteins containing Apo B with cholesterol esters from HDL. This results in so-called atherogenic dyslipidemia, which is characterized by elevated TG and LDL (pattern B), with TG-enriched HDL, which exhibit less activity in the reverse transport of cholesterol and greater renal clearance.50 In this same line, and assuming that visceral adipose tissue inflammation, the ectopic accumulation of fat, and adipose tissue dysfunction are the mediators of insulin resistance in humans, independently of total body fat mass,51–53 our study shows a high TG/HDLc ratio to predict a 2.71-fold increase in the risk of abdominal obesity, the latter being a surrogate index of the presence of visceral adipose tissue in the body.
To date, few studies have involved samples representative of the different populations in which the TG/HDLc ratio has been studied as a predictor of cardiometabolic risk. It is therefore difficult to compare our results with those of other authors, particularly bearing in mind the population differences and the diverse methodologies used in the different publications. Nevertheless, we propose TG/HDLc ratios of 1.8 and 2.5 for prepubertal and pubertal individuals, respectively, as cut-off points for this parameter in the children and adolescents of the city of Mérida. Future studies are required to examine these cut-off points with the introduction of subclinical atherosclerosis markers such as the determination of carotid intima/media thickness. In addition, if possible, long-term follow-up should be undertaken in order to determine the incidence of outcomes related to the above-mentioned study parameter.
Financial supportWe would like to give thanks for funding received from the Council of Scientific, Humanistic, Technological, and Art Development of Los Andes University (Consejo de Desarrollo Científico, Humanístico, Tecnológico y del Arte de la Universidad de Los Andes [CDCHTA-ULA]) under project M-1013-11-07-AA and ADG M-10, and also from the National Fund for Science, Technology and Innovation (Fondo Nacional de Ciencia, Tecnología e Innovación [FONACIT]) of the Ministry of Science, Technology and Innovation, with project no.° 2012000970.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Aguirre M, Briceño Y, Gómez-Pérez R, Zerpa Y, Camacho N, Paoli M. Relación triglicéridos/colesterol de la lipoproteína de alta densidad como indicador de riesgo cardiometabólico en niños y adolescentes de la ciudad de Mérida, Venezuela. Endocrinol Diabetes Nutr. 2018;65:74–83.