Adequate iodine intake is essential during pregnancy. A previous study of pregnant women from the Pamplona healthcare region showed mild iodine deficiency (mean urinary iodine level, 125mcg/L). This study was intended to ascertain the iodine intake of pregnant women in our region and to analyze the change over time in their iodine nutritional status.
MethodsAn observational study of 400 women in their first trimester of pregnancy. An iodine intake questionnaire was administered. To assess iodine status, urinary iodine concentration (UIC) was measured in a simple urine sample, and serum thyroglobulin levels were determined. In addition, thyroid volume was measured by cervical ultrasound examination.
ResultsIodized salt was used by 70.5% of all participants (55.3% since the pre-gestational period) and 98.5% of them received iodine-containing supplements (mean dose, 202.6±30.1mcg/day). Mean urinary iodine concentration was 242mcg/L (138.5–415.5mcg/L) and the mean serum thyroglobulin level was 12.3mcg/L (8.3–9mcg/L). Iodized salt intake was associated with higher UICs and lower thyroid volume. No differences were found in any of the tested parameters regarding the intake of dairy products, fish, or eggs.
ConclusionsIodine intake by pregnant women in Pamplona has increased due to a greater use of iodized salt and to higher doses of iodine supplements. As a result of this, an adequate iodine status has been achieved in the last decade.
La ingesta adecuada de yodo es esencial durante el embarazo. Sin embargo, una parte de la población gestante de nuestro país persiste en una situación de yododeficiencia. Un estudio previo realizado en embarazadas del área sanitaria de Pamplona mostró una yoduria insuficiente (125mcg/l) y un bajo consumo de sal yodada. El objetivo del presente trabajo es conocer la ingesta de yodo y analizar la evolución del estado de yodación en gestantes de nuestro medio en los últimos años.
MétodosEstudio observacional de 400 gestantes de primer trimestre sin antecedentes conocidos de enfermedad tiroidea. Se cumplimentó un cuestionario de consumo de yodo. Como marcadores del estado de yodación se analizaron la yoduria en una muestra simple de orina y la tiroglobulina sérica, y se calculó el volumen tiroideo mediante ecografía cervical.
ResultadosEl 70,5% de las participantes consumía sal yodada (55,3% pregestacional) y el 98,5% suplementos farmacológicos con yodo (dosis 202,6±30,1mcg/día). La mediana de la yoduria fue 242mcg/l (138,5-415,5mcg/l) y de la tiroglobulina 12,3mcg/l (8,39mcg/l). El consumo de sal yodada se asoció a mayor yoduria y a un menor volumen tiroideo. No se encontraron diferencias en los parámetros estudiados en función del consumo de lácteos, pescado o huevos.
ConclusionesLa ingesta de yodo en gestantes de Pamplona ha aumentado, tanto a expensas del empleo de sal yodada como de la dosis de la suplementación farmacológica. Esto ha permitido alcanzar un estado de yodación adecuado.
Iodine is an essential micronutrient needed for thyroid hormone synthesis. These hormones play a key role in the metabolism of most cells and in the growth and development of all organs, especially the brain.1 Human brain development takes place during prenatal life and early childhood. Consequently, an adequate iodine intake during pregnancy is essential for the maternal production of thyroxine and for a normal thyroid state in the fetus. Iodine insufficiency in pregnancy may result in inadequate thyroid hormone production, which has been associated to different degrees of altered fetal brain development.2,3
The iodine requirements increase by 50% during pregnancy; pregnant women are therefore at an increased risk of iodine deficiency, and moreover constitute a population group in which iodine deficiency has a greater impact.3 The World Health Organization (WHO) recommends a daily intake of 250μg of iodine during pregnancy.4 The main sources of iodine in the diet are iodized salt, dairy products and seafood. As the daily iodine intake from the diet may be insufficient, and since the use of iodized salt is not a universal practice, pharmacological supplementation with potassium iodide is recommended at the start of pregnancy or, ideally, before conception.5–7
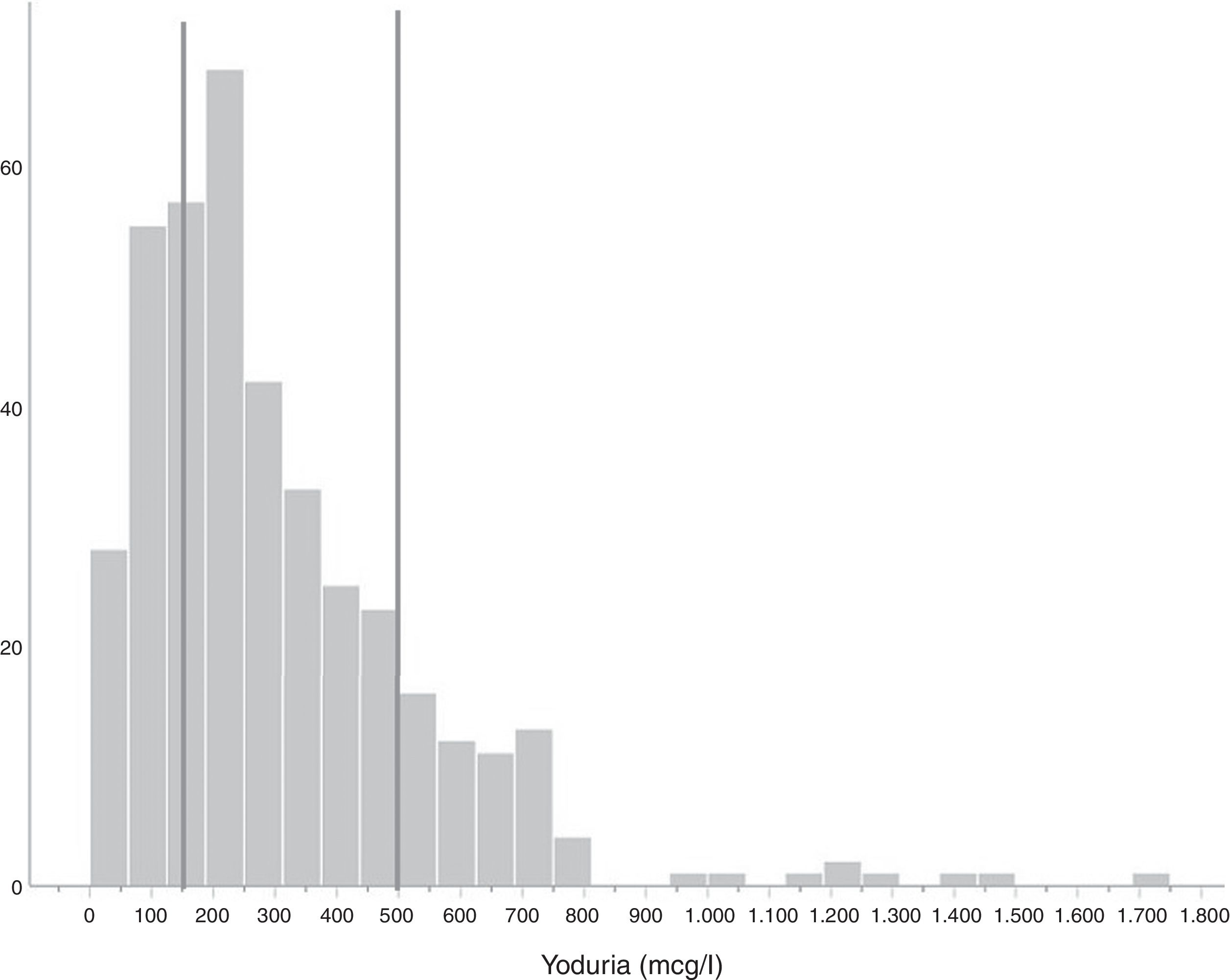
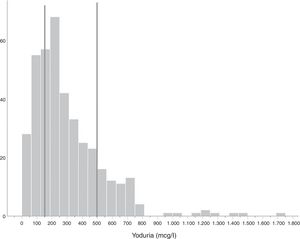
In order to determine the iodine status of the pregnant population, the WHO recommends determining urinary iodine concentration (UIC) in simple urine samples and comparing median urinary iodine levels to the established cut-off values. A median value of less than 150μg/l is considered insufficient, 150–249μg/l is regarded as adequate, 250–499μg/l is higher than required, and over 500μg/l is considered to be excessive.8 The determination of UIC is not useful for classifying individual iodine status, though it is the measure that best reflects recent iodine nutritional status in the studied population, since 90% of ingested iodine is excreted in the following 24–48h.1 Thyroglobulin (Tg) is considered a good marker of longer-term iodine status (weeks-months), but the data in the pregnant population are still limited.9 Thyroid volume also inversely reflects iodine intake, though over a much longer term (months-years).1
Even in countries traditionally considered to be iodine-sufficient, a large proportion of pregnant women have an inadequate iodine intake.10,11 Since the year 2004, the WHO has included Spain among the countries with optimum iodine nutrition.12 However, different studies in Spain indicate that an important proportion of pregnant women suffer iodine deficiency.13–16 In Navarra, a cross-sectional study conducted in 2005 on 332 full-term pregnant women showed a median ioduria level of 125μg/l, which is consistent with inadequate iodization.17 In that study, 32% of the women had used iodized salt continuously during pregnancy, and only 2% of the total consumed such salt since before pregnancy. Ninety-six percent of the pregnant women received iodine-containing drug supplements, which in most cases provided 100μg of iodine a day. Following these results, and in addition to specifically recommending the consumption of iodized salt, pharmacological supplementation was increased to 200μg of iodine a day. These measures should guarantee adequate iodization in pregnant women in our population.
The purpose of the present study was to determine iodine consumption and iodine status among pregnant women in the healthcare area of Pamplona (Navarra, Spain) and to compare the results obtained with those of the aforementioned 2005 study. An analysis was also made of the influence of iodine consumption upon the different parameters that assess iodine status.
MethodsStudy populationAn observational study was made of 400 women in the first trimester of pregnancy with no history of thyroid disease and receiving no treatment with drugs exerting an influence upon thyroid function (amiodarone, antithyroid drugs, levothyroxine or lithium). The sample size was calculated for the primary study objective, which was to establish the reference thyroid function values in the three trimesters of pregnancy in our population.18 The participants were recruited from two women's care centers in the healthcare area of Pamplona (Andraize and Buztintxuri) on occasion of the first prenatal visit, and were evaluated in person at the Department of Endocrinology of Complejo Hospitalario de Navarra (CHN) between 2014–2016. At this visit, a case history was compiled, with physical examination, thyroid ultrasound and the collection of a simple urine sample for measuring UIC. In a subgroup of pregnant women, serum Tg levels were also measured in the first trimester.
The study was approved by the Clinical Research Ethics Committee of Navarra, and all the participants signed the corresponding informed consent form.
Iodine consumption questionnaireThe iodine consumption questionnaire was completed through personal interview on occasion of the visit to the endocrinology clinic. The following points were addressed:
- a.
Iodized salt intake: yes/no/unknown.
- b.
Start of iodized salt intake: regular use from before pregnancy (pregestational) or starting in current pregnancy.
- c.
Regular intake of cow's milk and other dairy products (daily servings). A serving was regarded as a glass of milk, two yoghurts, or 40–60g of cheese.
- d.
Regular intake of fish or shellfish (weekly frequency).
- e.
Regular intake of eggs (weekly number).
- f.
Seaweed consumption: yes/no.
- g.
Use of iodine-containing drug supplements: yes/no.
- h.
Start of iodine drug supplementation: preconception (at least one month before conception) or in the first trimester of pregnancy.
- i.
Iodine dose of the drug supplement used (μg/day).
The determination of ioduria was carried out with a simple urine sample at the Public Health Standard Laboratory of the Basque Government in Derio (Vizcaya) using ion-pair reversed-phase liquid chromatography with electrochemical detection and a silver electrode (Waters Chromatography, Milford, MA, USA). Detailed information on the procedure and validation of the method and its within- and between-serial precision has been published elsewhere.19 The urine samples were stored in the Biobank, frozen at −20°C, duly identified, until submission for analysis. Thyroglobulin was analyzed by the NHC core laboratory using the Immulite 2000 analytical method (Siemens).
For thyroid ultrasound, the pregnant woman was placed in supine decubitus, and the MicroMaxx® portable ultrasound device was used (Sonosite, Bothell, WA, USA) with a 5–12MHz linear probe. Thyroid volume (in ml) was calculated by summing the volume of both thyroid lobes (volume of each lobe=longitudinal axis [cm]×transverse axis [cm]×anteroposterior axis [cm]×0.479). Goiter was defined as a thyroid volume>18ml.20
Statistical analysisQualitative variables were reported as frequencies and percentages, while quantitative variables were expressed as measures of central tendency (mean, median) and dispersion (standard deviation [SD] and interquartile range [IQR]). Normal distribution of the variables was assessed using the Kolmogorov–Smirnov test. The Student t-test for independent samples or the Mann–Whitney U-test was used for the comparison of quantitative variables, as applicable. Comparisons between more than two groups were based on analysis of variance (ANOVA) or the Kruskal–Wallis test, as applicable. Associations between categorical variables were assessed using the χ2 test. The correlations among serum Tg, thyroid volume and ioduria were assessed using the Spearman test. The SPSS version 25 statistical package was used throughout. Statistical significance was considered for p<0.05.
ResultsCharacteristics of the study populationThe study comprised a population of 400 mostly (93.5%) Caucasian pregnant women aged 33.4±4.1 years, of which 51.5% were nulliparous. The iodine consumption questionnaire, cervical ultrasound and urine sample collection were carried out on occasion of the physical presence visit to Endocrinology, which took place in gestational week 10 (range 9–12). Urine samples were collected for the determination of ioduria in 397 participants (99.3%). Serum Tg levels were analyzed in 77 pregnant women (19.3%) in the first trimester.
Iodine intake from the dietA total of 70.5% (n=282) of the pregnant women consumed iodized salt. Sixty-one women had started to use it in pregnancy, while 221 consumed iodized salt from before pregnancy (55.3% of the total). A total of 9.7% of the participants did not know the type of salt they used. Pregestational iodized salt consumption was higher in women with children than in nulliparous women (63.9 vs. 47.1%; p=0.003).
Mean dairy product consumption was 1.7±1.0 servings a day (1.2±0.9 servings/day of milk and 0.5±0.4 servings/day of other dairy products). The mean frequency of fish or shellfish consumption was 2.0±1.0 times per week, while mean egg consumption was 2.8±1.2 units a week. A total of 6.3% occasionally consumed seaweed.
Only 30.2% of the participants consumed iodized salt and more than two daily servings of dairy products.
Iodine-containing drug supplementsAt the time of the physical presence visit, 98.5% of the pregnant women (n=394) were taking iodine drug supplements – the latter having started before pregnancy in 42.5% of the total sample (n=170). The mean iodine dose in the supplement was 202.6±30.1μg/day. Only 6 women (1.5%) were not taking iodine-containing supplements at the time of the evaluation (one due to personal choice, while the other 5 were pending the start of supplementation).
The pregestational start of iodine-containing supplements was more frequent among women receiving fertility treatment than in those without such treatment (85.7% vs. 38.4%; p<0.001), and in pregnant women over 35 years of age (50.0% vs. 38.9%; p=0.035). There were no significant differences in the pregestational start of iodine-containing supplements between women with children and nulliparous women (39.7% vs. 45.1%; p=0.270).
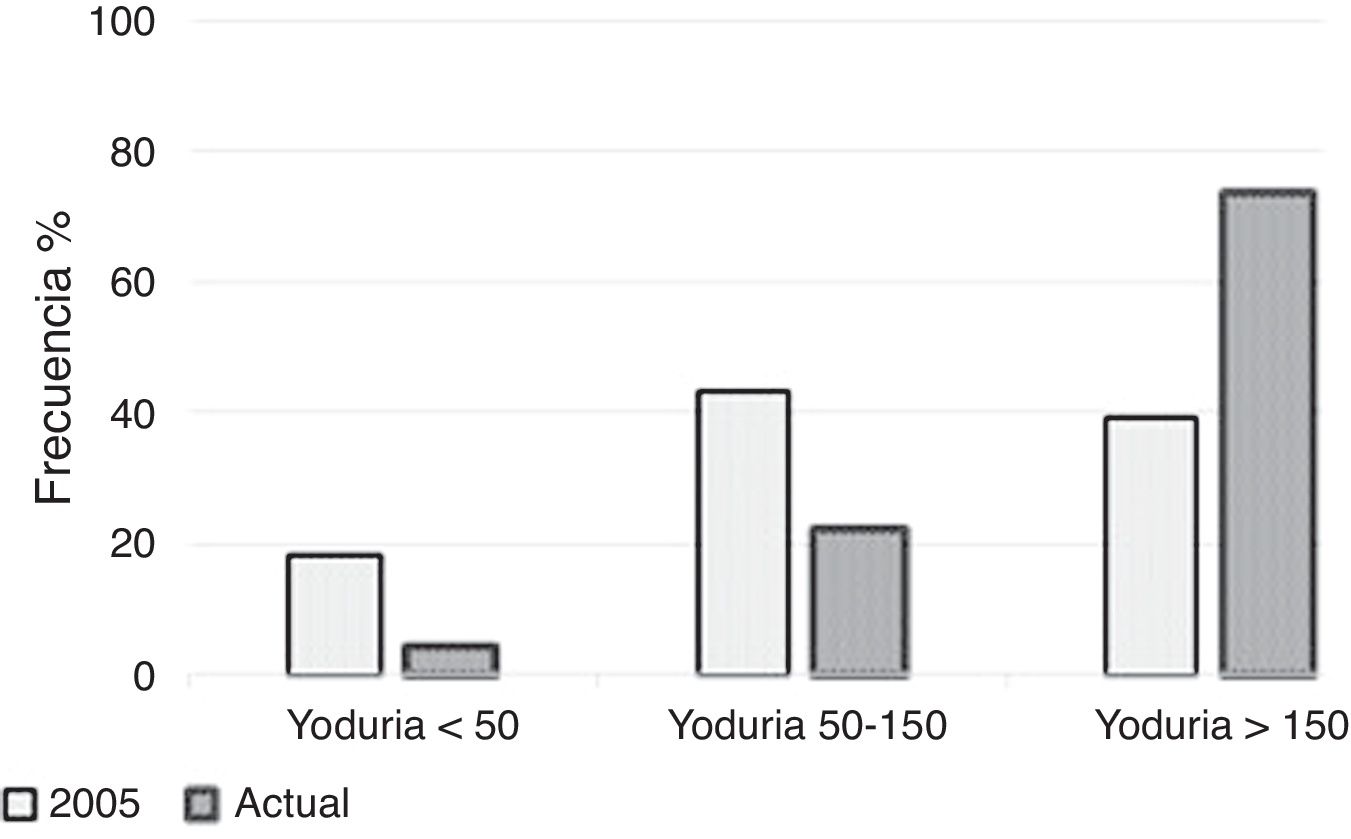
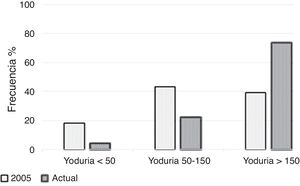
Iodine statusMedian ioduria in the study population was 242μg/l (138.5–415.5μg/l) (Fig. 1). In the 6 women not taking iodine-containing supplements, median ioduria was 156.5μg/l (20.0–272.8μg/l); in three of them, ioduria was ≤100μg/l (none used iodized salt). Fig. 2 compares the distribution of ioduria in the 2005 study with that reported in the present study.
The median serum Tg level after excluding pregnant women with positive anti-Tg antibodies (n=9) was 12.3μg/l (8.3–9.0μg/l). No pregnant woman presented Tg>40μg/l. The thyroid gland volume calculated from the ultrasound exploration was 8.2±3.6ml. Only four pregnant women had a thyroid volume>18ml, defined as goiter. There was no correlation between Tg and UIC (r: −0.096; p=0.438) or thyroid volume (r: −0.055; p=0.657).
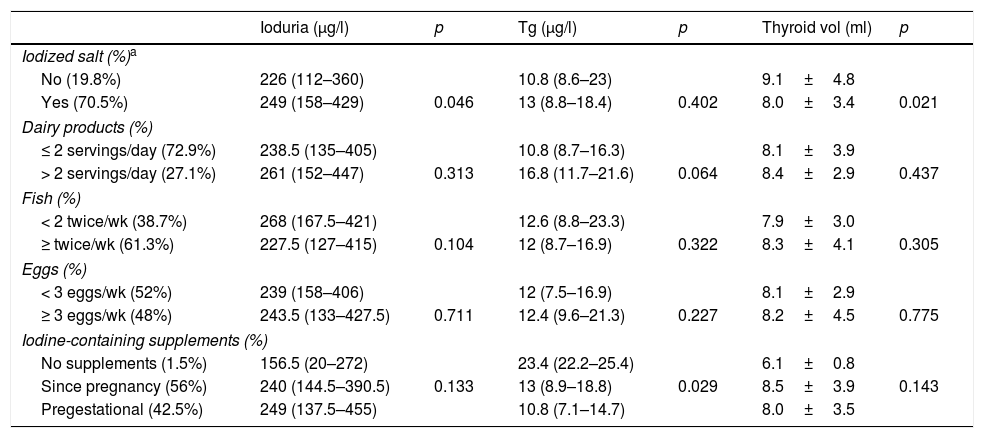
Influence of iodine consumption upon iodine statusTable 1 shows the ioduria, Tg and thyroid volume values according to iodine intake of the study participants.
Ioduria, serum thyroglobulin and thyroid volume according to iodine intake of the pregnant women included in the study.
| Ioduria (μg/l) | p | Tg (μg/l) | p | Thyroid vol (ml) | p | |
|---|---|---|---|---|---|---|
| Iodized salt (%)a | ||||||
| No (19.8%) | 226 (112–360) | 10.8 (8.6–23) | 9.1±4.8 | |||
| Yes (70.5%) | 249 (158–429) | 0.046 | 13 (8.8–18.4) | 0.402 | 8.0±3.4 | 0.021 |
| Dairy products (%) | ||||||
| ≤ 2 servings/day (72.9%) | 238.5 (135–405) | 10.8 (8.7–16.3) | 8.1±3.9 | |||
| > 2 servings/day (27.1%) | 261 (152–447) | 0.313 | 16.8 (11.7–21.6) | 0.064 | 8.4±2.9 | 0.437 |
| Fish (%) | ||||||
| < 2 twice/wk (38.7%) | 268 (167.5–421) | 12.6 (8.8–23.3) | 7.9±3.0 | |||
| ≥ twice/wk (61.3%) | 227.5 (127–415) | 0.104 | 12 (8.7–16.9) | 0.322 | 8.3±4.1 | 0.305 |
| Eggs (%) | ||||||
| < 3 eggs/wk (52%) | 239 (158–406) | 12 (7.5–16.9) | 8.1±2.9 | |||
| ≥ 3 eggs/wk (48%) | 243.5 (133–427.5) | 0.711 | 12.4 (9.6–21.3) | 0.227 | 8.2±4.5 | 0.775 |
| Iodine-containing supplements (%) | ||||||
| No supplements (1.5%) | 156.5 (20–272) | 23.4 (22.2–25.4) | 6.1±0.8 | |||
| Since pregnancy (56%) | 240 (144.5–390.5) | 0.133 | 13 (8.9–18.8) | 0.029 | 8.5±3.9 | 0.143 |
| Pregestational (42.5%) | 249 (137.5–455) | 10.8 (7.1–14.7) | 8.0±3.5 | |||
Tg: thyroglobulin; Thyroid vol: thyroid volume.
Pregnant women using iodized salt had higher ioduria and lesser thyroid volume than those who did not use iodized salt. No significant differences were found in UIC, Tg or thyroid volume according to the consumption of dairy products, fish, seaweed or eggs.
The Tg levels in pregnant women taking iodine drug supplements were lower than those who did not take such supplements.
DiscussionThe results of this study show that iodine intake among pregnant women in the healthcare area of Pamplona (Navarra, Spain) has increased in recent years. This is evidenced by an increase in median urinary iodine levels from 125μg/l to 242μg/l, which implies adequate iodine status.
The most effective measure for ensuring adequate iodine intake in the population is the use of iodized salt. The WHO and the International Council for the Control of Iodine Deficiency Disorders advocate the goal of ensuring regular iodized salt consumption in 90% of all homes.8 Its continued use has been associated to improved iodization in pregnant women, but the iodized salt consumption rate is less than 50% in most studies involving the Spanish population.14,15,21–23
In the present study, 70.2% of the participants used iodized salt at the time of the survey, as compared to 32% in the 2005 study – this representing an important increase in the consumption of iodized salt over the last decade. As reported in other populations, the use of iodized salt was associated with higher UIC levels. Iodized salt was consumed by 55.3% of the women from before pregnancy, as compared to only 2% in the previous study. Pregestational iodized salt intake was higher in women who already had children than in nulliparous women. This underscores the importance of actively recommending the use of iodized salt in pregnancy, since the habit of doing so appears to persist over time. In this way, adequate iodine provision would be favored in those populations most vulnerable to iodine deficiency (i.e., children and women of childbearing age).
Dairy products are currently another important source of iodine, and their consumption has been associated to improved iodine nutritional status and greater UIC in both schoolchildren and pregnant populations.21,22,24,25 The consumption of fish or eggs has also been associated in some studies to improved iodization in pregnancy.26 However, we observed no differences in the evaluated iodization parameters according to the consumption of these foods. This possibly could be explained by the fact that the systematic use of iodine-containing supplements would minimize the impact of iodine supplied through the diet. No data are available on the intake of dairy products, fish or eggs in the 2005 study. It is therefore not clear whether the consumption patterns of these foods have changed in recent years.
In order to meet the iodine requirements during pregnancy, iodized salt and at least 2–3 servings of dairy products a day should be advised. However, only a minority of women consume these foods in the recommended amounts.13,21,22 In populations where adequate iodine intake from the diet is not guaranteed, iodine drug supplementation is recommended,5–7 preferably starting before conception. Different studies have consistently associated the use of iodine drug supplements to adequate ioduria levels.21,22,27 In Navarra, iodine supplementation has been systematically indicated for several years, but the usual dose is currently 200μg/day as compared to a dose of 100μg/day predominantly used in the 2005 study. Supplementation started in the pregestational period in 42.5% of the women, though the percentage reached 85.7% in those receiving fertility treatments. The few women not taking iodine-containing supplements in our study complicates the drawing of firm conclusions, though it should be noted that half of them had ioduria <100μg/l, and the serum Tg levels were significantly higher.
The median ioduria recorded in the study confirms adequate iodine status according to the WHO classification. A total of 26.3% of the pregnant women had UIC <150μg/l. This percentage is lower than that reported in other iodine-sufficient pregnant populations in Spain,21,22 and lower than the 61% recorded in the previous study in Pamplona.17
On the other hand, serum Tg has been shown to be useful as an iodization marker, fundamentally in children, where median Tg <13μg/l or <3% of all Tg values >40μg/l would indicate an adequate status.9 These cut-off points could also be valid in pregnant women,28 but the data available are limited and are mainly limited to iodine-deficient populations. In most of these studies, the median Tg levels were ≥13μg/l.9 It must be taken into account that Tg could increase physiologically in pregnancy due to the associated increase in thyroid activity, and it is not clear whether the values vary in the course of pregnancy. In addition, there is wide variation in relation to the analytical method used for measuring Tg.29 The median serum Tg concentration in our study was 12.5μg/l, though it was only measured in 77 pregnant women. There were no differences in Tg values according to iodine intake from the diet. As in other studies, no correlation was found between Tg and UIC.30 This could be due to recent changes in iodine intake, particularly at the start of drug supplementing in the first weeks of pregnancy. On the other hand, thyroid volume, which reflects much longer-term iodine status, was only inversely associated to the consumption of iodized salt.
The main limitation of this study is that both iodized salt intake and the consumption of other sources of iodine were reported by the participants, and were not confirmed. In all cases, the pregestational start of iodine supplements was contrasted against the data recorded in the electronic case history. However, no specific question was asked about the degree of compliance with drug supplementation, which in some cases could justify notoriously low UIC values. It also should be noted that the 2005 study was conducted in full-term pregnant women, while the women in our study were in the first trimester of pregnancy. Lastly, the participants were recruited from two women's care centers – a fact that could limit extrapolation of the data, fundamentally as regards iodine intake from the diet. The prescription of iodine-containing drug supplements is a widespread practice in Navarra, so few differences can be expected in this regard.
In conclusion, iodine consumption among pregnant women in our setting has increased in recent years, resulting in a clear improvement in iodine status. Public health measures are needed to promote the use of iodized salt – this being particularly important in children and women of childbearing age. Reaching and maintaining iodine sufficiency in a specific population requires regular monitoring of the determinants of iodine status, with a view to adopting appropriate measures if necessary.
Financial supportThis study was funded in part by the Endocrinology, Nutrition and Diabetes Foundation of Navarra (Fundación de Endocrinología, Nutrición y Diabetes de Navarra).
Authorship/collaboratorsM. Dolores Ollero, Juan Pablo Martínez, Javier Pineda, Marta Toni and Emma Anda made substantial contributions to the conception and design of the study, and to data acquisition, analysis and interpretation. They also contributed to critical review of the manuscript and to final approval of the submitted version. Mercedes Espada contributed to data analysis, as well as to critical review and final approval of the submitted version of the manuscript.
Conflicts of interestNone declared.
We thank the pregnant women for their participation in the study and the Gynecology and Obstetrics Department of CHN for the collaboration provided, especially the midwives and gynecologists of the Andraize and Buztintxuri women's care centers.
Please cite this article as: Ollero MD, Martínez JP, Pineda J, Toni M, Espada M, Anda E. Evolución del estado de nutrición de yodo en gestantes del área sanitaria de Pamplona. Endocrinol Diabetes Nutr. 2020;67:643–649.