Ga-68-DOTATOC PET/CT is a recently introduced imaging technique for the diagnosis and follow-up of neuroendocrine tumors.
A prospective observational study was conducted in seven patients who underwent a Ga-68-DOTATOC PET/CT study. They were suspected of active neuroendocrine tumor lesions, either on initial diagnosis or as a possible recurrence and/or progression of already known tumors. The results of prior imaging studies (MRI, thoracoabdominal CT scan, octreotide…), had been negative or inconclusive.
All positive Ga-68-DOTATOC PETs were true positives, confirmed by pathological examination. There were no false positive results. Only one false negative result was found.
Ga-68-DOTATOC PET/CC is more sensitive and specific for the detection of primary neuroendocrine tumor lesions, allows for a more complete extension study, and detects recurrences in earlier stages, conditioning changes in staging and surgical treatment. It provides additional information on somatostatin receptor overexpression, which is essential for the indication of PRRT (peptide receptor radionuclide therapy) with Lu177 dotatate.
El PET/TC Ga68-DOTATOC es una técnica de diagnóstico por la imagen, de reciente introducción en nuestro medio, para el diagnóstico y seguimiento de tumores neuroendocrinos.
Estudio observacional prospectivo de 7 pacientes a quienes se les realizó un estudio PET/TC Ga68-DOTATOC. Los pacientes tenían sospecha de lesiones tumorales neuroendocrinas activas, en diagnóstico inicial o con posible recidiva y/o progresión de tumores ya conocidos. Los resultados de los estudios de imagen realizados previamente (RM, TC toracoabdominal, octreotida…) habían sido negativos o no concluyentes.
Todos los PET Ga68-DOTATOC positivos fueron verdaderos positivos, confirmándose por anatomía patológica. No se obtuvieron resultados falsos positivos. Solo se obtuvo un falso negativo.
PET/TC Ga68-DOTATOC es más sensible y específico en la detección de lesiones tumorales primarias neuroendocrinas, permite un estudio de extensión más completo y detecta recidivas en estadios más precoces, condicionando cambios en la estadificación y el tratamiento quirúrgico. Aporta información adicional sobre la sobreexpresión de receptores de somatostatina, esencial para la indicación de PRRT (terapia con péptidos marcados con radionúclidos) con Lu177-Dotatate.
Neuroendocrine tumors comprise a heterogeneous group of lesions derived from the neural crest and endoderm, with neuroendocrine differentiation. The behavior and course of these tumors vary according to their histological differentiation and degree of proliferation.
Despite their heterogeneity, most of these neoplasms (80%) are characterized by the overexpression of somatostatin receptors, particularly subtype 2 receptors. The expression of somatostatin receptors has relevant clinical implications: on one hand, the intrinsic regulatory function they exert upon the tumor (hormone secretion, tumor growth and angiogenesis), with the corresponding therapeutic implications, and on the other hand, they play an important role in the identification and typing of these tumors1. The overexpression of somatostatin receptors constitutes the physiopathological basis for the diagnosis of these tumors using nuclear medicine techniques (scintigraphy and PET), as well as for patient treatment with cold somatostatin analogs (lanreotide, octreotide, etc.) or radioisotope markers such as lutetium177.
In 2017, the World Health Organization (WHO), in an attempt to standardize the nomenclature referred to this varied group of tumors, published a new classification based on histopathological criteria: degree of cell differentiation, degree of proliferation (Ki67) and mitotic index2. Neuroendocrine tumors were classified according to their Ki67 or mitotic index into: G1, G2, and G3. On the other hand, neuroendocrine carcinomas refer to tumors that are poorly differentiated and with a high mitotic index.
Neuroendocrine tumors are considered to be rare lesions, with an incidence in Spain of 5-6 cases per 100,000 inhabitants. Sixty percent are located in the gastrointestinal tract (where they are the most prevalent tumors after colon cancer) and 27% in the tracheobronchial tree3. Most of these lesions are non-secretory, and 50% present metastatic spread at the time of diagnosis.
An increase in the diagnosis of these tumors has been observed in recent years, though it is difficult to determine whether this is due to a genuine increase in the incidence of such lesions or to improved knowledge and greater sensitivity and availability of diagnostic techniques.
In 2018, the Spanish Agency for Medicinal Products and Medical Devices (Agencia Española del Medicamento [AEMPS])4,5 approved the use of PET/CT with somatostatin analogs: 68Ga-edotreotide (SomaKit TOC®)5 in Spain.
Edotreotide (DOTATOC) is a somatostatin analog which, when bound to 68Ga in PET imaging, allows the identification of cells that over-express somatostatin receptors, particularly subtype 2 and 5 receptors. Such receptors are identified in 80-85% of all neuroendocrine tumors. This technique is therefore very sensitive in diagnosing these neoplasms.6,7 Although only recently introduced in Spain, PET-CT with somatostatin analogs is supported by over 10 years of experience in other European countries. The published literature is extensive and indicates sensitivity and specificity values higher than those of other explorations (90% and 90-92%, respectively) in the diagnosis of well differentiated neuroendocrine tumors (NETs) G1, G2 and G3. These figures are greater than those of an Octreoscan (60-80%)6,7, and other radiological techniques.
The very recent approval by the AEMPS of this radiopharmaceutical implies that the experience gained in Spain is still very incipient8,9.
We believe it to be important to validate the promising results reported in international literature in our own setting.
The present study describes the first 7 patients subjected PET with somatostatin analogs in our healthcare area. Emphasis will be placed on the indications and rationale for conducting the study, establishing comparisons with the previously conducted imaging studies, and exploring correlations with the histopathological findings (if any). Lastly, the clinical impact of PET will be assessed in terms of changes in the diagnosis and/or extent of the disease, as well as in the initially planned treatment strategy.
Material and methodsA prospective descriptive study was made of 7 patients with suspected neuroendocrine tumors subjected to Ga68-DOTATOC PET/CT study. The patients came from two centers, Hospital del Mar and Hospital Quironsalud (Barcelona, Spain), and the studies were conducted in the period between March 2018 and November 2018.
We selected patients with suspected primary tumors of probable neuroendocrine origin and/or patients with suspected relapse or progression of confirmed neuroendocrine tumors. All of the patients had previously undergone the imaging tests routinely used in the diagnostic protocol for these tumors (ultrasound, MRI, thoracoabdominal CT, octreotide scan and/or F18-FDG PET/CT), with negative or inconclusive results. The results were defined as inconclusive when the findings obtained were unable to establish a diagnosis allowing the adoption of a treatment strategy.
The reasons for the study were: confirmation of the neuroendocrine origin of the lesion, staging and the assessment of relapse in patients diagnosed with neuroendocrine tumors. On the other hand, the test allowed us to select candidates for both pharmacological and radiolabeled somatostatin analog (peptide receptor radionuclide therapy [PRRT]) treatments.
The final decision to perform the PET study with somatostatin analogs was made on a multidisciplinary basis by the committees where these patients were studied. The indications of PET with analogs were based on the American and European guidelines of the respective neuroendocrine tumor societies10–12.
The PET/CT study was performed after the intravenous administration of 1.5 MBq/kg (100-185 MBq) of Ga68-edotreotide (Somakit®, Advanced Accelerator Applications). Images were acquired after 60 minutes under physical and sensory resting and postmicturition conditions, using the Siemens Biograph 6 tomograph of the Nuclear Medicine Department of Hospital Quirón Barcelona.
Images were systematically obtained from the skull to the proximal third of the thigh in all patients, and the upper and/or lower extremities were added if these zones were susceptible to present tumor involvement.
Computed tomography from 80-150 mA (care dose) with intravenous iodinated contrast was performed in the absence of contraindications. Three-dimensional (3D) emission PET scanning was performed, 3 min × bed. Iterative reconstruction. Matrix 128 × 128, pixel size 2-4 mm. Attenuation correction using the CT transmission images.
The study was reconstructed, obtaining images in the transverse, coronal and sagittal axes of the corrected PET, uncorrected PET and PET/CT fusion.
Analysis of the PET/CT imagesThe PET/CT images were visually evaluated by two experienced specialists in nuclear medicine on an independent basis - one from each participating center. The final report was prepared based on consensus between the two specialists.
A positive PET study was defined by an area of enhanced uptake of the radiopharmaceutical that was not identifiable as physiological (hypophysis, thyroid gland, spleen, liver, adrenal glands, pancreatic uncinate process and urinary tract), with an intensity greater than the background signal of the organ or region where it was located, and greater than the uptake to be expected physiologically.
The result of the Ga68-DOTATOC PET/CT study was correlated to the histopathological findings in those cases in which a pathology study was available (6 of the 7 cases); to the therapeutic approach established after performing the PET scan; and to the clinical findings over a minimum follow-up of 9 months. The PET study results were considered true positive, true negative, false positive or false negative. These results were subjected to statistical analysis.
The clinical usefulness of Ga68-DOTATOC PET/CT was analyzed based on its clinical impact upon patient management. In this respect, we examined whether the PET findings resulted in changes in the clinical and therapeutic management of the patient: indications or variation of surgical approach, changes in drug treatment, changes in radiotherapy planning, and the selection of patients amenable to PRRT.
ResultsSeven patients (2 females and 5 males) aged 45–77 years (mean: 60.7) were included in the study.
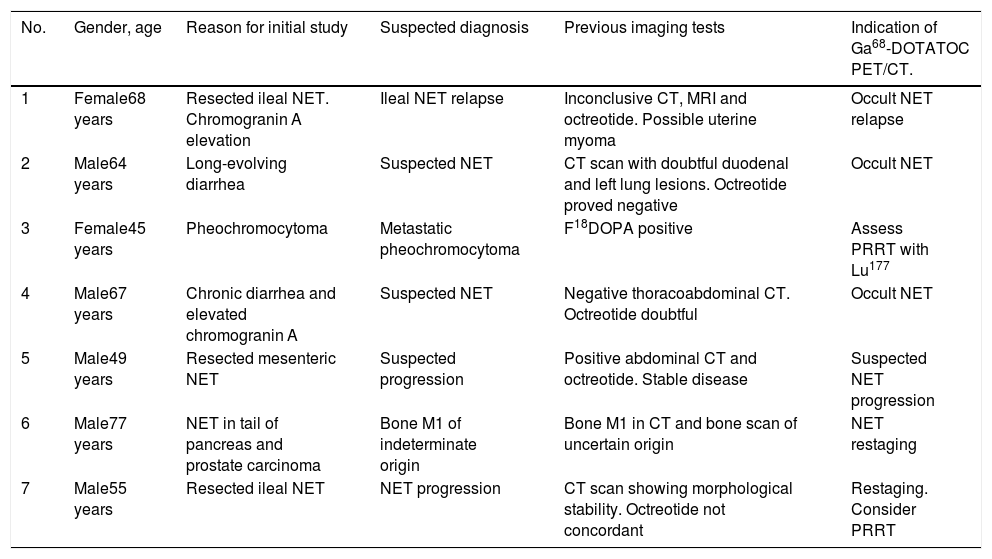
All the patients had a reasoned suspicion of neuroendocrine tumor or suspected relapse/progression of an already diagnosed neuroendocrine tumor, with inconclusive imaging findings. The individual reasons why the patients were being studied, the diagnostic suspicion of the ongoing episode, as well as the rest of the clinical and follow-up data and the complementary tests performed (mainly diagnostic imaging tests), are summarized in Table 1.
Patient details. Indication of Ga68-DOTATOC PET/CT.
| No. | Gender, age | Reason for initial study | Suspected diagnosis | Previous imaging tests | Indication of Ga68-DOTATOC PET/CT. |
|---|---|---|---|---|---|
| 1 | Female68 years | Resected ileal NET. Chromogranin A elevation | Ileal NET relapse | Inconclusive CT, MRI and octreotide. Possible uterine myoma | Occult NET relapse |
| 2 | Male64 years | Long-evolving diarrhea | Suspected NET | CT scan with doubtful duodenal and left lung lesions. Octreotide proved negative | Occult NET |
| 3 | Female45 years | Pheochromocytoma | Metastatic pheochromocytoma | F18DOPA positive | Assess PRRT with Lu177 |
| 4 | Male67 years | Chronic diarrhea and elevated chromogranin A | Suspected NET | Negative thoracoabdominal CT. Octreotide doubtful | Occult NET |
| 5 | Male49 years | Resected mesenteric NET | Suspected progression | Positive abdominal CT and octreotide. Stable disease | Suspected NET progression |
| 6 | Male77 years | NET in tail of pancreas and prostate carcinoma | Bone M1 of indeterminate origin | Bone M1 in CT and bone scan of uncertain origin | NET restaging |
| 7 | Male55 years | Resected ileal NET | NET progression | CT scan showing morphological stability. Octreotide not concordant | Restaging. Consider PRRT |
M1: metastasis; PRRT: peptide receptor radionuclide therapy; NET: neuroendocrine tumor.
The first patient, a woman of 68 years of age, had undergone surgery for an ileal neuroendocrine tumor in 2010 (Ki67 2%), including incomplete removal of mesenteric tumor implants, and treatment was started with somatostatin analogs. Progressive elevation of chromogranin A (357 ng/ml) and gastrin (143 pg/ml) was observed. The MRI and octreotide imaging studies proved inconclusive for persistent disease. The Ga68-DOTATOC PET/CT scan revealed a lesion in the pouch of Douglas, at retrouterine level, several peritoneal nodular lesions, and retroperitoneal lymph node involvement. Because of the extent of the disease, salvage or rescue surgery was ruled out, and the decision was made to increase the somatostatin analog therapy dose, with monitoring of the patient course over time.
The second patient corresponded to a 64-year-old male with a 7 kg loss of body weight in three months and elevated gastrin (143 pg/ml) and chromogranin levels (540 ng/ml). The studies performed (ultrasound, thoracoabdominal CT, octreotide and endoscopies) were inconclusive. The CT scan showed a sub-centimeter image in the third duodenal portion and a solid nodule measuring 11 mm in size in the lower lobe of the right lung, that proved difficult to characterize. The 68Ga-DOTATOC PET study confirmed the neuroendocrine nature of the duodenal lesion and ruled it out in the case of the lung lesion. Surgical resection of the duodenal lesion was performed. The histological study confirmed the presence of a neuroendocrine tumor in the duodenal mucosa, of sub-centimeter size, with Ki67 2%, corresponding to NET G1. Eighteen months later, the patient was free of disease and asymptomatic. The control CT scan showed that the lung nodule had disappeared.
The third patient was a 34-year-old woman with a suspicious left adrenal nodule identified during a study of arterial hypertension. Left adrenalectomy was performed, with a histopathological diagnosis of pheochromocytoma. Monitoring over time revealed catecholamine elevation and the presence of left sub-centimeter paraaortic retroperitoneal adenopathies. The study was completed with an 18F-DOPA PET scan that evidenced multiple millimeter-size supra- and infradiaphragmatic adenopathies with enhanced uptake. In view of the possibility of systemic treatment with Lu177-Dotatate, a Ga68-DOTATOC PET/CT scan was requested. The latter proved negative, thus contraindicating the aforementioned treatment. Therapy was provided with 150 mCi of MIBG, with improvement of the hypertensive symptoms, but no significant changes in the control 18F-DOPA PET study.
The fourth patient was a 67-year-old male consulting due to chronic diarrhea that had worsened over the last month, abdominal pain and vomiting. Chromogranin A elevation was observed (1600 ng/ml). The conventional imaging tests proved negative, in the same way as the Ga68-DOTATOC PET/CT study. A neuroendocrine tumor was discarded, and the condition was managed as diarrhea secondary to biliary salt malabsorption. After 6 months of drug treatment, the diarrhea had improved significantly. The subsequent CT scan remained negative.
The fifth patient was a 49-year-old male who since May 2017 presented a central mesenteric spiculate lesion and multiple liver metastases described in the abdominal CT and MRI scans, octreotide SPECT and 18F-FDG PET. Resection of the mesenteric lesion was performed in October, with a definitive histopathological diagnosis of peritoneal low-grade (G1) NET. Chromogranin A, synaptophysin, CD56, CDX2 exhibiting diffuse intense positivity, TTF1 negative and Ki67 1-2%. The control abdominal CT angiography, abdominal MRI and 18F-FDG PET/CT studies in May 2018 revealed stability of the disease, with persistence of the clinical suspicion of progression. A Ga68-DOTATOC PET/CT study was requested, revealing the presence of a nodular lesion in the root of the mesenterium, liver metastases and enhanced-uptake retroperitoneal adenopathies, suggestive of progression of the tumor process of neuroendocrine origin. Treatment with lanreotide 120 mg/28 days was continued, and the patient was referred for assessment of PRRT with Lu177-Dotatate.
The sixth patient was a 77-year-old male with a history of prostate cancer subjected to radical prostatectomy, and colon adenocarcinoma subjected to surgery and adjuvant chemotherapy. Both neoplastic conditions were in complete remission since 2013. During the extension study of the pancreatic neuroendocrine tumor using 18F-FDG PET, and in addition to the presence of the primary pancreatic lesion, multiple metastatic bone lesions were seen - the origin of which could not be specified (neuroendocrine versus prostate). The Ga68-DOTATOC PET/CT study identified uptake in the pancreatic lesion, in a left para-aortic adenopathy, and in multiple bone lesions - thus confirming their neuroendocrine origin. A percutaneous bone biopsy of the sacrum was initially inconclusive, though immunohistochemical studies subsequently confirmed the neuroendocrine origin of the bone lesions.
The seventh patient was a 55-year-old male with a history of ileal neuroendocrine neoplasm, having undergone surgery in November 2015. Since then, the patient was receiving first-line treatment with lanreotide 90 mg/28 days. He currently had normal chromogranin levels (basal 89 ng/ml), with serotonin elevation to 293 µg/l (lower than basal [660 µg/l]). The control CT scan of November 2018 evidenced radiological stability of the retroperitoneal lymph nodes, and an octreotide scan showed supra- and infradiaphragmatic adenopathies, with no new lesions compared with previous studies. A Ga68-DOTATOC PET study was indicated to secure correct current staging of the neuroendocrine tumor process, to assess maintenance of lanreotide therapy or indicate a change in treatment (possible PRRT). The Ga68-DOTATOC PET/CT scan evidenced progression, with very extensive supra- and infradiaphragmatic adenopathic involvement suggesting tumor progression. The patient was referred to the reference center to examine the possibility of inclusion in a clinical trial.
On globally considering the results of the different cases, the following can be summarized:
Of the 7 Ga68-DOTATOC PET scans performed, 5 were true positive (cases 1, 2, 5, 6 and 7) - with histopathological confirmation in all of them. No false positive results were obtained. Of the two PET scans with negative results, one was a true negative scan (case 4), while the result of the other patient was considered to be a false negative Ga68-DOTATOC PET/CT scan (case 3) - though it proved positive with 18F-DOPA PET. The sensitivity and specificity levels were 86% and 100%, respectively.
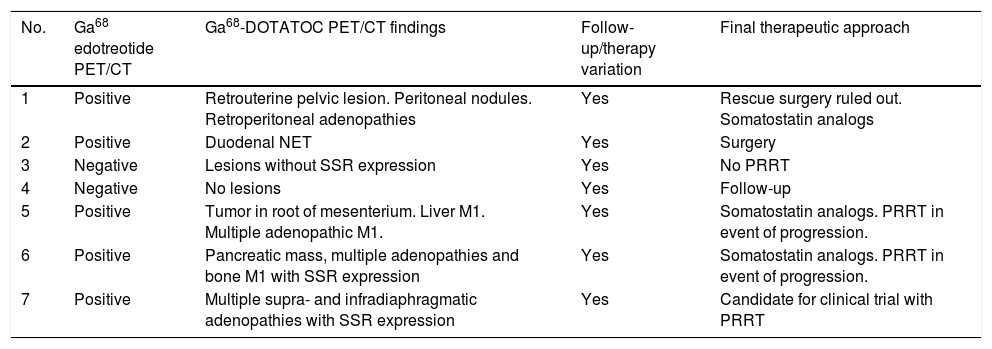
In all patients, the Ga68-DOTATOC PET/CT findings entailed a significant change in the diagnosis of the ongoing disorder, with a direct impact upon patient follow-up and/or treatment (Table 2).
Ga68-DOTATOC PET/CT findings. Clinical impact. Changes in therapy
| No. | Ga68 edotreotide PET/CT | Ga68-DOTATOC PET/CT findings | Follow-up/therapy variation | Final therapeutic approach |
|---|---|---|---|---|
| 1 | Positive | Retrouterine pelvic lesion. Peritoneal nodules. Retroperitoneal adenopathies | Yes | Rescue surgery ruled out. Somatostatin analogs |
| 2 | Positive | Duodenal NET | Yes | Surgery |
| 3 | Negative | Lesions without SSR expression | Yes | No PRRT |
| 4 | Negative | No lesions | Yes | Follow-up |
| 5 | Positive | Tumor in root of mesenterium. Liver M1. Multiple adenopathic M1. | Yes | Somatostatin analogs. PRRT in event of progression. |
| 6 | Positive | Pancreatic mass, multiple adenopathies and bone M1 with SSR expression | Yes | Somatostatin analogs. PRRT in event of progression. |
| 7 | Positive | Multiple supra- and infradiaphragmatic adenopathies with SSR expression | Yes | Candidate for clinical trial with PRRT |
M1: metastasis; PRRT: peptide receptor radionuclide therapy; SSR: somatostatin receptors; NET: neuroendocrine tumor.
The present study describes the first experience with the use of the Ga68-DOTATOC PET/CT in Spain. Specifically, we describe the first 7 cases in which the use of this new radiopharmaceutical was indicated. All the patients were clinically evaluated by physicians experienced in this type of tumor, with reasonable doubts regarding the presence of lesions of neuroendocrine origin, the extent of the disease, and/or therapeutic planning.
The Ga68-DOTATOC PET/CT scan provided relevant information in each of the patients, though it should be underscored that case selection was very careful, with strict indications agreed upon by the different medical committees.
Five of our 7 patients with previous inconclusive imaging findings yielded a positive Ga68-DOTATOC PET/CT scan - all of which were true positive results. This consequently confirms greater sensitivity in the detection of small neuroendocrine tumors and/or lesions in atypical locations that may go unnoticed by other diagnostic tests both in the diagnosis of primary neuroendocrine tumors and in the event of suspected relapse. Likewise, more comprehensive staging of the tumor process may be made in the same study13,14.
The absence of false positive results implied 100% specificity. This outcome is partly due to careful patient selection, which increases the specificity. Despite the lack of adequate statistical power because of the small sample size, we believe that the results highlight the need for the decision to perform Ga68-DOTATOC PET/CT to be made by multidisciplinary committees.
The technique also has a superior negative predictive value, affording greater confidence by ruling out the presence of well-differentiated tumors of neuroendocrine origin. High-grade neuroendocrine tumors may have elevated glycolytic metabolism, and F18-FDG PET is therefore the most sensitive test in this scenario. Both PET radiopharmaceuticals, Ga68-DOTATOC and F18-FDG, are of importance in the study and follow-up of neuroendocrine tumors, depending on the degree of tumor dedifferentiation, and both techniques may be required depending on tumor heterogeneity. Both tests are complementary and not mutually excluding13–15.
Ga68-DOTATOC PET detects significantly more lesions than SPECT/CT with octreotide and the sum of CT and MRI, whether located in the intestine or pancreas, liver, lung or supra- and infradiaphragmatic adenopathies16–19. In our study, staging was raised in 5 of the 7 cases, compared to the sum of the rest of diagnostic tests.
The greater sensitivity of the technique also makes it possible to detect tumor relapses early, thus allowing a prompter start of adequate therapy and with a theoretically lower tumor volume17.
Our second patient was under study due to long-standing chronic diarrhea with high chromogranin A levels. A previous thoracoabdominal CT scan described a nodular image in the left lung base and inconclusive nodularity of infiltrating characteristics in the duodenum. The duodenal wall location, small size, and physiological intestinal uptake of FDG complicated early detection by CT and 18F-FDG16 PET/CT. However, Ga68-DOTATOC PET was able to confirm the initially doubtful neuroendocrine nature of the duodenal lesion. The absence of uptake on the part of the nodular lung lesion discarded its neuroendocrine nature (high negative predictive value). These findings allowed adequate treatment decision - in this case surgery with healing intent - with histopathological confirmation of the neuroendocrine process, and evidenced the high positive predictive value of Ga68-DOTATOC PET. Eighteen months later, the patient was free of disease and asymptomatic. The control CT scan showed that the lung nodule had disappeared.
A Ga68-DOTATOC PET/CT scan could be recommended for initial neuroendocrine tumor staging, since the additional information afforded may lead to changes in the surgical plans in up to 47% of all cases7,15,17.
In our study, in all cases, the results of the Ga68-DOTATOC PET/CT study modified the initially planned treatment approach. These changes were reflected in the indication of surgery for a tumor lesion not unequivocally detected by other diagnostic tests, while rescue surgery was ruled out in one case, somatostatin analog therapy was indicated in two patients, the presence of neuroendocrine tumor was firmly ruled out in one case, PRRT was proposed in one patient, and finally PRRT was ruled out in another case.
Peptide receptor radionuclide therapy (PRRT) with Lu177-Dotatate is currently available, with very promising results in advanced neuroendocrine tumors20. The basic condition for the indication of such therapy is the confirmation of somatostatin receptor expression in the tumor cell membrane. Both SPECT/CT with octreotide and Ga68-DOTATOC PET/CT allow us to evidence these receptors and indicate PRRT, though PET sensitivity is significantly better7,18 - thus increasing the number of patients with PRRT indication.
In our study, three of the patients with advanced neuroendocrine tumors were evaluated for immediate or deferred treatment with Lu177-Dotatate, in view of the high somatostatin receptor expression detected by Ga68-DOTATOC, and despite the fact that two of them had shown no significant octreotide uptake.
Likewise, the negative predictive value is significantly better with Ga68-edotreotide, excluding only those patients without somatostatin receptors. One of our patients, with metastatic pheochromocytoma, was ruled out for PRRT in view of Ga68-DOTATOC negativity. Although the usefulness of Ga68-DOTATOC PET in the diagnosis and extension study of pheochromocytomas and paragangliomas has been validated21,22, in our case immunohistochemical re-evaluation after PET negativity confirmed the absence of active somatostatin receptors, SDHB; SDHD; SDHC and SDHA (active). The absence of Ga68-DOTATOC uptake has been reported in these cases23, and Lu177-Dotatate therapy is therefore contraindicated.
The present study has several limitations. In effect, it is a descriptive study involving a limited number of patients. The cases moreover were highly selected, which could bias the results obtained. We are aware that implementation of the technique in our country is proving to be laborious, and this is why at least in this initial stage, the patients should be highly selected in order to secure a higher diagnostic yield and to have an effect upon the management of these patients.
We know that there is still a long way to go and many questions to be answered. However, we believe that multidisciplinary work and the availability of Ga68-DOTATOC PET/CT will have a very positive impact upon the management of these patients.
ConclusionsThe Ga68-DOTATOC PET/CT technique has great potential in the study of neuroendocrine tumors, opening the door to a new approach in diagnosis and staging, treatment and patient follow-up. The results of this study are still very preliminary, though they are in line with the international literature to date. We consider that multidisciplinary work and the availability of PET with new tracers will have a positive impact upon the management of these patients.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Plaza López PJ, Suarez Pinera M, Mestre Fusco A, Domenech Brasero B, Pifarré Muntané P, Rivera Codias E. Impacto clínico del PET/TC Ga68-DOTATOC en tumores de origen neuroendocrino. Experiencia preliminar. Endocrinol Diabetes Nutr. 2020;67:636–642.