To examine the prescription pattern of the different dipeptidyl peptidase-4 inhibitors (DPP4is), depending on the estimated glomerular filtration rate (eGFR) under real-world clinical practice conditions.
MethodThis was a descriptive, observational study using a population database (SIDIAP Catalonia). Subjects diagnosed with T2DM with kidney function assessed and on active treatment with DPP4is were enrolled. Patients were included at the time of the measurement of eGFR (CKD-epi) and were monitored for 6 months after enrolment.
For each subject, the prescribed daily dose (PDD) of DPP4i, the theoretical dose according to the degree of renal failure established by the recommendations in the summary of product characteristics (DDD-adj), and the PDR ratio (PDD/DDD-adj) were estimated. A subject was considered overtreated if his/her RDR was greater than 1.2 (>20%).
ResultsThe study sample consisted of 72,135 subjects with a mean age of 69.7 (±11.6) years and 55.9% males. The proportion of patients overtreated varied depending on the type of DPP4i and the renal function stage. Overall, overdosage was recorded in 7.15% of all DPP4i treatments. In advanced stages (IIIb, IV, and V), overdosage was much higher (36.8% for all DPP4is, and 58.7% if linagliptin is excluded).
DiscussionUnder real-world clinical practice conditions, more than one third of T2DM patients with advanced renal failure were overdosed with DPP4is because the doses were not adequately adjusted to the glomerular filtration rate of each patient.
Examinar el patrón de prescripción de los distintos inhibidores de la dipeptidil- peptidasa 4 (iDPP4) en función del filtrado glomerular estimado (FGe) en condiciones de práctica clínica real.
MetodologíaEstudio descriptivo observacional con base de datos poblacional (SIDIAP Catalunya). Se incluyeron sujetos diagnosticados de diabetes mellitus tipo 2 (DM2) con evaluación de su función renal y tratamiento activo con iDPP4. Se incluyeron pacientes en el momento de la determinación del FGe (CKD-epi), con un periodo de observación de 6 meses posterior a su inclusión.
Para cada sujeto se estimó la dosis diaria prescrita (DDP) de iDPP4, su dosis teórica según su nivel de insuficiencia renal que establecen las recomendaciones de la Ficha Técnica (DDD-aj) y el ratio RDP (DDP/DDD-aj). Se consideró que un sujeto estaba sobretratado si su RDP era superior a 1.2 (>20%).
ResultadosLa muestra estudiada fue de 72.135 sujetos con una edad media fue de 69,7 (±11,6) años y un 55,9% de hombres. El porcentaje de pacientes con sobretratamiento varía según tipo de fármaco iDPP4 y estadio de la función renal. Globalmente la sobredosificación se registró en el 7,15% de todos los tratamientos con iDPP4. En estadios avanzados (IIIb, IV y V) la sobredosificación fue muy superior (del 36,8% para todos los iDPP4 y si excluimos la linagliptina del 58,7%).
DiscusiónEn condiciones de práctica clínica real, más de un tercio de los pacientes DM2 con insuficiencia renal avanzada presentaban una sobredosificación de los iDPP4 puesto que no se ajustan correctamente las dosis al filtrado glomerular de cada paciente.
Dipeptidyl-peptidase 4 (DPP-4) inhibitors are drugs widely used in the treatment of type two diabetes mellitus (T2DM).1–3 In our environment, they are prescribed to approximately 16% of patients with T2DM.
In Spain, the prescription and financing of a drug by the public health system is conditioned by the recommendations of the summary of product characteristics from the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) [Spanish Agency of Medicines and Medical Devices].4
DPP-4 inhibitors are antidiabetics which have a good safety profile1 and can be used in the presence of any degree of kidney failure.5 Of the DPP-4 inhibitors marketed in Spain, only linagliptin (which has predominantly biliary excretion) can be used at full doses regardless of the degree of kidney failure.6,7 In all other cases, the recommendations in the summaries of product characteristics are to adjust the dose to the value of the glomerular filtration rate (GFR).8 Thus, except for linagliptin, functionally in stage IIIb (GFR: 30−44 ml/min/1.73 m2) all DPP-4 inhibitors should be dosed at 50%; and in stages IV (GFR: 15−29 ml/min/1.73 m2) and V (GFR < 15 ml/min/1.73 m2) sitagliptin and alogliptin should be reduced to 25% of the usual dose.4
According to population data, chronic kidney failure is present in 28% of patients with T2DM. The prevalence of stages IIIb, IV and V are 8.6%, 3.3% and 0.7%, respectively.9
In general, the dangers of drug overdose can be very important,10,11 but in the case of DPP-4 inhibitors the data is limited and does not seem to be very serious. In a North American study, 98.3% of patients with DPP-4 inhibitor overdose were asymptomatic, and the main problem was hypoglycaemia among those who used them associated with another antidiabetic.12 There are doubts about a slight QTc prolongation13 with sitagliptin overdose, but overall the risk is low. However, in a retrospective Korean study14 of 82,332 patients, DPP-4 inhibitor overdose was associated with an increased risk of hospital emergency department admission (HR: 1.07), severe hypoglycaemia (HR: 1.19), and mortality (HR: 1.12) after multivariate adjustment.
On the other hand, in usual daily practice consultations, clinical practice guidelines or prescription according to the summary of product characteristics of the drugs are often not strictly followed.15–17
For this reason, we consider it important to examine the pattern of prescription of the different DPP-4 inhibitors based on glomerular filtration rate under real clinical practice conditions.
Material and methodsThis was a retrospective descriptive study based on an anonymised population database of clinical records (Sistema de Información para el Desarrollo de la Investigación en Atención Primaria [Information System for the Development of Research in Primary Care [SIDIAP]]).
Data sourceThe SIDIAP database includes clinical information from Institut Català de la Salut (ICS) users from a total of 288 primary care teams throughout Catalonia. SIDIAP contains information on demographic data, diagnoses, clinical variables, prescriptions, dispensing in pharmacies, laboratory tests and data on hospital discharges obtained from the Conjunto Mínimo de Datos Básicos (CMBD) [Minimum Basic Data Set], and has previously been used to conduct numerous useful clinical studies.18,19 On 31/12/2017 there were a total of 389,178 patients diagnosed with T2DM on SIDIAP.
Study periodThe period of recruitment and identification of subjects was between 1/1/2008 and 31/12/2017. The observation period was between the inclusion date, defined in the penultimate laboratory determination of the estimated glomerular filtration rate (eGFR) (CKD-EPI) and a maximum of six months later or death.
Inclusion criteriaSubjects older than 18 years included in the SIDIAP database, with a diagnosis of T2DM (International Classification of Diseases version 10: E11.0-E11.9, E14 or E14.0-E14.9) with two or more determinations of eGFR by CKD-EPI20 and any active prescribed treatment from the DPP-4 inhibitors group (Anatomical Therapeutic Chemical classification system [ATC] codes: A10BH), either as monotherapy or in combination. The DPP-4 inhibitors included in the study were: alogliptin (A10BH04), linagliptin (A10BH05), saxagliptin (A10BH03), sitagliptin (A10BH01) and vildagliptin (A10BH02).
Study variablesMain variablesKidney function: eGFR by CKD-EPI, albumin-to-creatinine ratio at the time of inclusion and staging classification for kidney function according to KDIGO.21 According to the summary of product characteristics, the doses of sitagliptin and vildagliptin should be adjusted to 50% with glomerular filtration values between 49 and 30 ml/min/1.73 m2, which does not correspond exactly to any KDIGO stage. By consensus of the researchers, it was homologated with stage IIIb.
PDD: For each subject, the prescribed daily dose (PDD) was calculated individually by their physician as a result of the daily average according to the dosage and frequency recorded in their clinical history during the observation period.
adj-DDD: The defined daily dose (DDD) of each drug was estimated, adjusted to the value of the estimated glomerular filtration rate of each patient according to the recommendations of the summary of product characteristics (adj-DDD).8 In cases where kidney function was good, the recommended DDDs22 were 100 mg/day for sitagliptin and vildagliptin, 25 mg/day for alogliptin and 5 mg/day for linagliptin and saxagliptin.
In cases of kidney failure, the following adj-DDDs were applied:
- -
Alogliptin: 12.5 mg/day (if eGFR was between 45−30) and 6.25 mg/day (if eFGR < 30)
- -
Linagliptin: no modifications
- -
Saxagliptin: 2.5 mg/day (if eFGR < 45)
- -
Sitagliptin: 50 mg/day (if eFGR was between 45−30) and 25 mg/day (if eGFR < 30)
- -
Vildagliptin: 50 mg/day (if eFGR < 45)
Ratio (RDP): Applying the equation proposed by the University of Manitoba, the PDD/adj-DDD ratio was calculated for each patient.23
Overtreatment: If the RDP ratio is equal to one, an adequate prescription would correspond to kidney function, since the dose prescribed by the doctor would correspond to the recommended theoretical dose; if the RDP is less than one, it would correspond to an undertreated patient and, consequently, if the RDP ratio is greater than one, it would correspond to an overtreated subject. By consensus of the researchers following the recommendations from Kale and Korenstein,24 and the ICS electronic prescription protocols, “overtreatment” was considered to be when the ratio was greater than 1.2 (more than 20% of the adequate dose).
Type of DPP-4 inhibitor: The pharmacological group of active antidiabetic DPP-4 inhibitor prescribed by the general practitioner. This also included drug and dose changes, and the addition of other pharmacological groups, so the global sum does not have to correspond to the sum of each family (in some cases the same patient will be counted in two different DPP-4 inhibitor families or a combination therapy will be started with the addition of another non-DPP-4-inhibitor drug).
Secondary variablesSociodemographic variables (age and gender), clinical picture related to T2DM, time of evolution of T2DM, HbA1c, coexistence of risk factors and medical history comorbidities at the time of inclusion. The clinical laboratory tests recorded at the time of inclusion or the most recent during the year prior to the date of inclusion.
Statistical analysisStatistical analysis was based on descriptively summarising the main indicators of interest for each DPP-4 inhibitor family and according to the stage of kidney function. Quantitative variables were summarised with the mean and standard deviation (±SD) and the qualitative variables were expressed with the frequency and its percentage. A comparison of the baseline characteristics by kidney function stages was made for each family of DPP-4 inhibitors, all families grouped except for linagliptin.
The data management process was carried out using the R statistical package, version 3.6.1, by the biostatistics team of the DAP.cat group.
The study was carried out in accordance with the indications of the protocol and was classified by the AEMPS as EPA-OD [Post-authorisation studies with a design other than prospective follow-up]. The treatment, communication and transfer of personal data belonging to all participating subjects is in accordance with the provisions of Organic Law 3/2018, of 5 December, on the protection of personal data. As it was an anonymised study, the informed consent of the patients was not required. The study was approved by the IDIAP [Institut Universitari d’Investigació en Atenció Primària] Jordi Gol Clinical Research Ethics Committee (code 19/029-P).
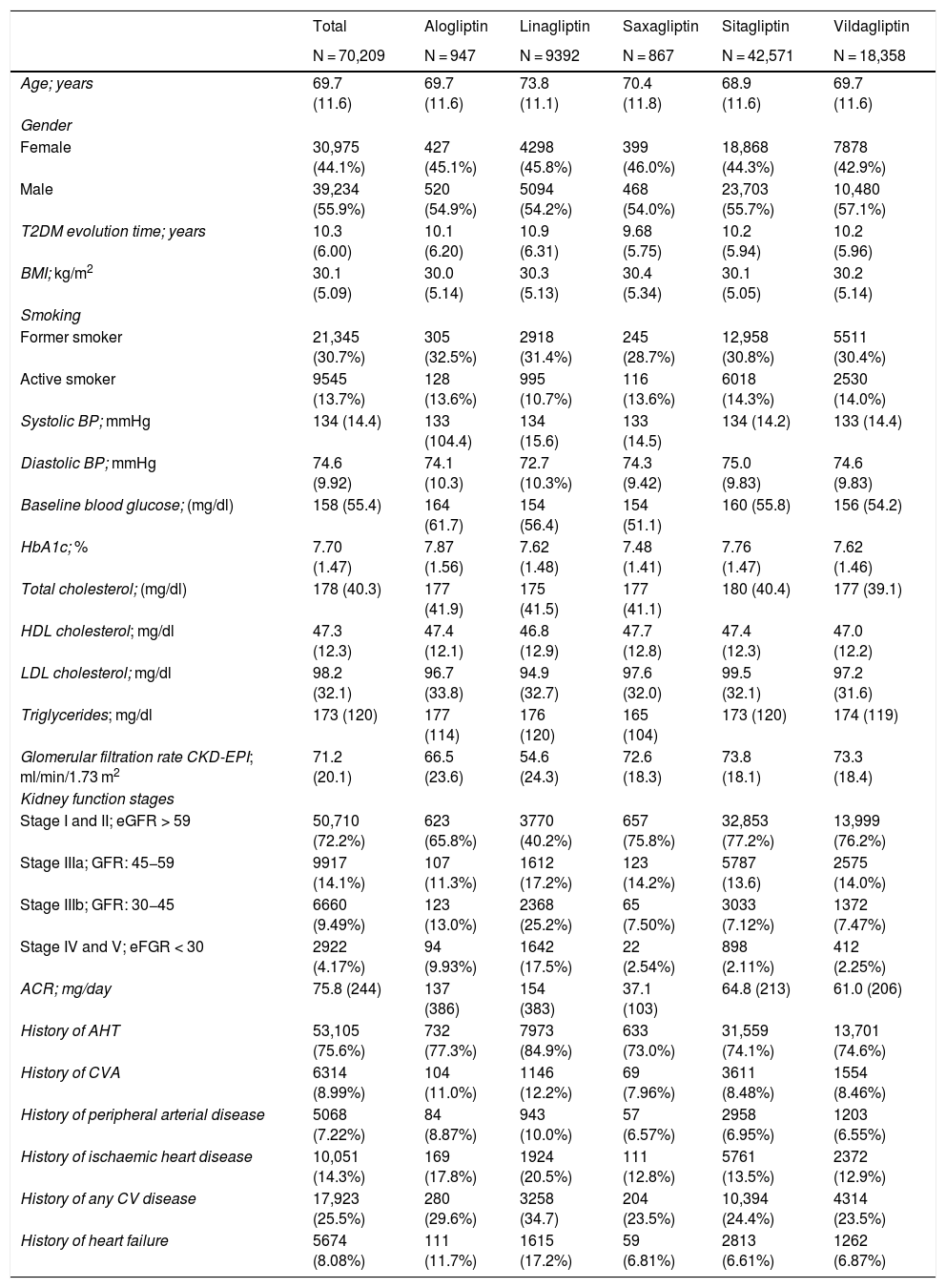
ResultsA total of 70,209 patients who met the inclusion criteria for this study were identified. A total of 1926 situations were recorded in which during follow-up a change in treatment was recorded with another DPP-4 inhibitor, or combined therapy with a non-DPP-4 inhibitor had been started. Table 1 shows the baseline characteristics of the patients included in the study according to the type of DPP-4 inhibitor used. The overall age at the start of treatment was 69.7 (±11.6) years with an average of 10.3 (±6.0) years of evolution of T2DM. Mean HbA1c at the start of treatment was 7.7% (±1.47).
Baseline characteristics of patients treated with any DPP-4 inhibitor.
| Total | Alogliptin | Linagliptin | Saxagliptin | Sitagliptin | Vildagliptin | |
|---|---|---|---|---|---|---|
| N = 70,209 | N = 947 | N = 9392 | N = 867 | N = 42,571 | N = 18,358 | |
| Age; years | 69.7 (11.6) | 69.7 (11.6) | 73.8 (11.1) | 70.4 (11.8) | 68.9 (11.6) | 69.7 (11.6) |
| Gender | ||||||
| Female | 30,975 (44.1%) | 427 (45.1%) | 4298 (45.8%) | 399 (46.0%) | 18,868 (44.3%) | 7878 (42.9%) |
| Male | 39,234 (55.9%) | 520 (54.9%) | 5094 (54.2%) | 468 (54.0%) | 23,703 (55.7%) | 10,480 (57.1%) |
| T2DM evolution time; years | 10.3 (6.00) | 10.1 (6.20) | 10.9 (6.31) | 9.68 (5.75) | 10.2 (5.94) | 10.2 (5.96) |
| BMI; kg/m2 | 30.1 (5.09) | 30.0 (5.14) | 30.3 (5.13) | 30.4 (5.34) | 30.1 (5.05) | 30.2 (5.14) |
| Smoking | ||||||
| Former smoker | 21,345 (30.7%) | 305 (32.5%) | 2918 (31.4%) | 245 (28.7%) | 12,958 (30.8%) | 5511 (30.4%) |
| Active smoker | 9545 (13.7%) | 128 (13.6%) | 995 (10.7%) | 116 (13.6%) | 6018 (14.3%) | 2530 (14.0%) |
| Systolic BP; mmHg | 134 (14.4) | 133 (104.4) | 134 (15.6) | 133 (14.5) | 134 (14.2) | 133 (14.4) |
| Diastolic BP; mmHg | 74.6 (9.92) | 74.1 (10.3) | 72.7 (10.3%) | 74.3 (9.42) | 75.0 (9.83) | 74.6 (9.83) |
| Baseline blood glucose; (mg/dl) | 158 (55.4) | 164 (61.7) | 154 (56.4) | 154 (51.1) | 160 (55.8) | 156 (54.2) |
| HbA1c; % | 7.70 (1.47) | 7.87 (1.56) | 7.62 (1.48) | 7.48 (1.41) | 7.76 (1.47) | 7.62 (1.46) |
| Total cholesterol; (mg/dl) | 178 (40.3) | 177 (41.9) | 175 (41.5) | 177 (41.1) | 180 (40.4) | 177 (39.1) |
| HDL cholesterol; mg/dl | 47.3 (12.3) | 47.4 (12.1) | 46.8 (12.9) | 47.7 (12.8) | 47.4 (12.3) | 47.0 (12.2) |
| LDL cholesterol; mg/dl | 98.2 (32.1) | 96.7 (33.8) | 94.9 (32.7) | 97.6 (32.0) | 99.5 (32.1) | 97.2 (31.6) |
| Triglycerides; mg/dl | 173 (120) | 177 (114) | 176 (120) | 165 (104) | 173 (120) | 174 (119) |
| Glomerular filtration rate CKD-EPI; ml/min/1.73 m2 | 71.2 (20.1) | 66.5 (23.6) | 54.6 (24.3) | 72.6 (18.3) | 73.8 (18.1) | 73.3 (18.4) |
| Kidney function stages | ||||||
| Stage I and II; eGFR > 59 | 50,710 (72.2%) | 623 (65.8%) | 3770 (40.2%) | 657 (75.8%) | 32,853 (77.2%) | 13,999 (76.2%) |
| Stage IIIa; GFR: 45−59 | 9917 (14.1%) | 107 (11.3%) | 1612 (17.2%) | 123 (14.2%) | 5787 (13.6) | 2575 (14.0%) |
| Stage IIIb; GFR: 30−45 | 6660 (9.49%) | 123 (13.0%) | 2368 (25.2%) | 65 (7.50%) | 3033 (7.12%) | 1372 (7.47%) |
| Stage IV and V; eFGR < 30 | 2922 (4.17%) | 94 (9.93%) | 1642 (17.5%) | 22 (2.54%) | 898 (2.11%) | 412 (2.25%) |
| ACR; mg/day | 75.8 (244) | 137 (386) | 154 (383) | 37.1 (103) | 64.8 (213) | 61.0 (206) |
| History of AHT | 53,105 (75.6%) | 732 (77.3%) | 7973 (84.9%) | 633 (73.0%) | 31,559 (74.1%) | 13,701 (74.6%) |
| History of CVA | 6314 (8.99%) | 104 (11.0%) | 1146 (12.2%) | 69 (7.96%) | 3611 (8.48%) | 1554 (8.46%) |
| History of peripheral arterial disease | 5068 (7.22%) | 84 (8.87%) | 943 (10.0%) | 57 (6.57%) | 2958 (6.95%) | 1203 (6.55%) |
| History of ischaemic heart disease | 10,051 (14.3%) | 169 (17.8%) | 1924 (20.5%) | 111 (12.8%) | 5761 (13.5%) | 2372 (12.9%) |
| History of any CV disease | 17,923 (25.5%) | 280 (29.6%) | 3258 (34.7) | 204 (23.5%) | 10,394 (24.4%) | 4314 (23.5%) |
| History of heart failure | 5674 (8.08%) | 111 (11.7%) | 1615 (17.2%) | 59 (6.81%) | 2813 (6.61%) | 1262 (6.87%) |
The percentages refer to the total of the column (DPP-4 inhibitor family used).
The recommendation from the summaries of product characteristics from the AEMPS is to adjust the dose of this pharmacological family to the degree of glomerular filtration rate of each patient, but this recommendation is rarely followed in our environment.
Kidney function showed a mean eGFR of 71.2 (±20.1) ml/min/1.73 m2, with 27.8% of patients in a situation of kidney failure, and severe kidney failure was observed in 13.7% patients (GFR < 30 ml/min/1.73 m2). It should be noted that among those treated with linagliptin, the percentage of patients in stages IIIb, IV and V was clearly higher than that of the other DPP-4 inhibitors (42.7%).
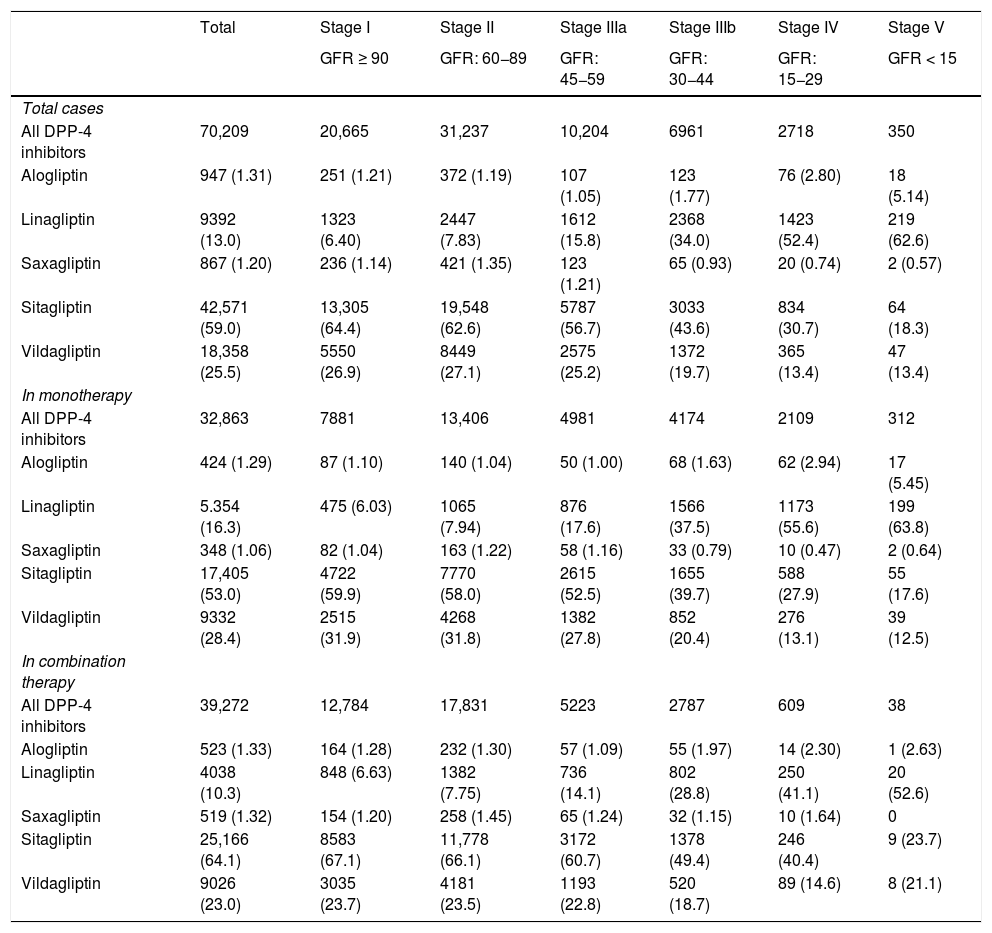
Table 2 shows the use of DPP-4 inhibitors in monotherapy (45.6%) or in combination therapy (54.4%) depending on the stage of kidney function. The most frequently used DPP-4 inhibitor was sitagliptin, both in monotherapy (53.0%) and in combined therapy (64.1%), followed by vildagliptin (28.4 and 23%, respectively). In the most advanced stages of kidney failure (stages IV and V), linagliptin was the most prescribed (in 53.5% of cases).
Patients treated with DPP-4 inhibitors according to the stage of kidney function.
| Total | Stage I | Stage II | Stage IIIa | Stage IIIb | Stage IV | Stage V | |
|---|---|---|---|---|---|---|---|
| GFR ≥ 90 | GFR: 60−89 | GFR: 45−59 | GFR: 30−44 | GFR: 15−29 | GFR < 15 | ||
| Total cases | |||||||
| All DPP-4 inhibitors | 70,209 | 20,665 | 31,237 | 10,204 | 6961 | 2718 | 350 |
| Alogliptin | 947 (1.31) | 251 (1.21) | 372 (1.19) | 107 (1.05) | 123 (1.77) | 76 (2.80) | 18 (5.14) |
| Linagliptin | 9392 (13.0) | 1323 (6.40) | 2447 (7.83) | 1612 (15.8) | 2368 (34.0) | 1423 (52.4) | 219 (62.6) |
| Saxagliptin | 867 (1.20) | 236 (1.14) | 421 (1.35) | 123 (1.21) | 65 (0.93) | 20 (0.74) | 2 (0.57) |
| Sitagliptin | 42,571 (59.0) | 13,305 (64.4) | 19,548 (62.6) | 5787 (56.7) | 3033 (43.6) | 834 (30.7) | 64 (18.3) |
| Vildagliptin | 18,358 (25.5) | 5550 (26.9) | 8449 (27.1) | 2575 (25.2) | 1372 (19.7) | 365 (13.4) | 47 (13.4) |
| In monotherapy | |||||||
| All DPP-4 inhibitors | 32,863 | 7881 | 13,406 | 4981 | 4174 | 2109 | 312 |
| Alogliptin | 424 (1.29) | 87 (1.10) | 140 (1.04) | 50 (1.00) | 68 (1.63) | 62 (2.94) | 17 (5.45) |
| Linagliptin | 5.354 (16.3) | 475 (6.03) | 1065 (7.94) | 876 (17.6) | 1566 (37.5) | 1173 (55.6) | 199 (63.8) |
| Saxagliptin | 348 (1.06) | 82 (1.04) | 163 (1.22) | 58 (1.16) | 33 (0.79) | 10 (0.47) | 2 (0.64) |
| Sitagliptin | 17,405 (53.0) | 4722 (59.9) | 7770 (58.0) | 2615 (52.5) | 1655 (39.7) | 588 (27.9) | 55 (17.6) |
| Vildagliptin | 9332 (28.4) | 2515 (31.9) | 4268 (31.8) | 1382 (27.8) | 852 (20.4) | 276 (13.1) | 39 (12.5) |
| In combination therapy | |||||||
| All DPP-4 inhibitors | 39,272 | 12,784 | 17,831 | 5223 | 2787 | 609 | 38 |
| Alogliptin | 523 (1.33) | 164 (1.28) | 232 (1.30) | 57 (1.09) | 55 (1.97) | 14 (2.30) | 1 (2.63) |
| Linagliptin | 4038 (10.3) | 848 (6.63) | 1382 (7.75) | 736 (14.1) | 802 (28.8) | 250 (41.1) | 20 (52.6) |
| Saxagliptin | 519 (1.32) | 154 (1.20) | 258 (1.45) | 65 (1.24) | 32 (1.15) | 10 (1.64) | 0 |
| Sitagliptin | 25,166 (64.1) | 8583 (67.1) | 11,778 (66.1) | 3172 (60.7) | 1378 (49.4) | 246 (40.4) | 9 (23.7) |
| Vildagliptin | 9026 (23.0) | 3035 (23.7) | 4181 (23.5) | 1193 (22.8) | 520 (18.7) | 89 (14.6) | 8 (21.1) |
In parentheses is the percentage with respect to the total of the column (kidney function stage).
GFR: glomerular filtration rate; DPP-4 inhibitors: dipeptidyl peptidase 4 inhibitors.
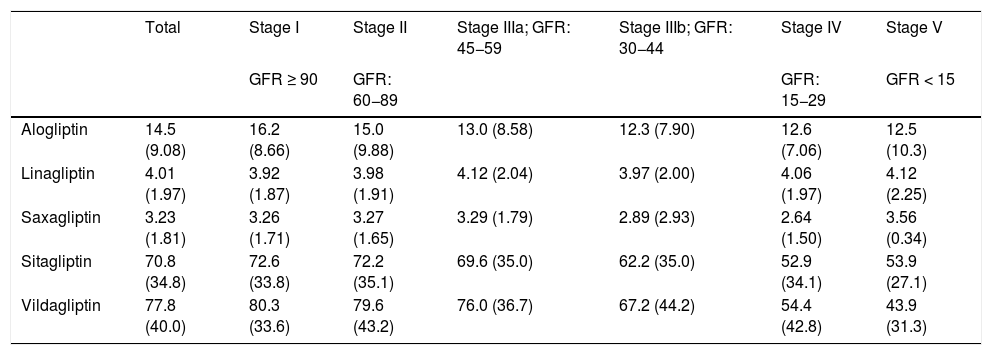
Regarding the prescription by general practitioner, it should be noted that the mean prescribed daily doses (PDD) were lower than the theoretical defined daily doses (DDD) for each type of DPP-4 inhibitor according to the AEMPS in the stages of kidney function that do not require dose adjustment (Table 3). Overall, the mean PDD for alogliptin was 14.5 (DDD: 25), for linagliptin it was 4.01 (DDD: 5), for saxagliptin, 3.23 (DDD: 5), for sitagliptin, 70.8 (DDD: 100) and for vildagliptin, 77.8 (DDD: 100). In the more advanced stages (IV and V) the means were: alogliptin PDD: 12.6 (adj-DDD: 6.25); saxagliptin PDD: 2.82 (adj-DDD: 2.5); sitagliptin PDD: 53.0 (adj-DDD: 25); vildagliptin PDD: 53.2 (adj-DDD: 50).
Description of the prescribed daily dose of DPP-4 inhibitors according to the stage of kidney failure.
| Total | Stage I | Stage II | Stage IIIa; GFR: 45−59 | Stage IIIb; GFR: 30−44 | Stage IV | Stage V | |
|---|---|---|---|---|---|---|---|
| GFR ≥ 90 | GFR: 60−89 | GFR: 15−29 | GFR < 15 | ||||
| Alogliptin | 14.5 (9.08) | 16.2 (8.66) | 15.0 (9.88) | 13.0 (8.58) | 12.3 (7.90) | 12.6 (7.06) | 12.5 (10.3) |
| Linagliptin | 4.01 (1.97) | 3.92 (1.87) | 3.98 (1.91) | 4.12 (2.04) | 3.97 (2.00) | 4.06 (1.97) | 4.12 (2.25) |
| Saxagliptin | 3.23 (1.81) | 3.26 (1.71) | 3.27 (1.65) | 3.29 (1.79) | 2.89 (2.93) | 2.64 (1.50) | 3.56 (0.34) |
| Sitagliptin | 70.8 (34.8) | 72.6 (33.8) | 72.2 (35.1) | 69.6 (35.0) | 62.2 (35.0) | 52.9 (34.1) | 53.9 (27.1) |
| Vildagliptin | 77.8 (40.0) | 80.3 (33.6) | 79.6 (43.2) | 76.0 (36.7) | 67.2 (44.2) | 54.4 (42.8) | 43.9 (31.3) |
In each cell is the mean of the PDD and in parentheses is the standard deviation.
PDD: prescribed daily dose; GFR: glomerular filtration rate; DPP-4 inhibitors: dipeptidyl peptidase 4 inhibitors.
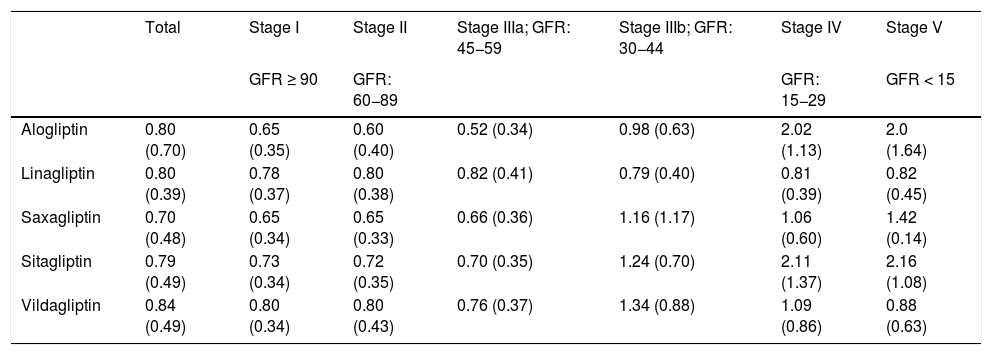
When the PDD/adj-DDD ratio of the DPP-4 inhibitors was calculated at the individual level to measure the adequacy of the dosage according to the stage of kidney failure, the data in Table 4 were obtained. In stages I, II and IIIa, the ratio is less than one, demonstrating the tendency towards underdosing. In stage IIIb (eGFR: 44−30), overdosing is observed with saxagliptin, sitagliptin, and vildagliptin (ratios of 1.16, 1.24, and 1.34, respectively). And in stages IV and V the average ratio of overdosage rises to 2.16.
PDD/adj-DDD ratio of DPP-4 inhibitors according to the stage of kidney failure.
| Total | Stage I | Stage II | Stage IIIa; GFR: 45−59 | Stage IIIb; GFR: 30−44 | Stage IV | Stage V | |
|---|---|---|---|---|---|---|---|
| GFR ≥ 90 | GFR: 60−89 | GFR: 15−29 | GFR < 15 | ||||
| Alogliptin | 0.80 (0.70) | 0.65 (0.35) | 0.60 (0.40) | 0.52 (0.34) | 0.98 (0.63) | 2.02 (1.13) | 2.0 (1.64) |
| Linagliptin | 0.80 (0.39) | 0.78 (0.37) | 0.80 (0.38) | 0.82 (0.41) | 0.79 (0.40) | 0.81 (0.39) | 0.82 (0.45) |
| Saxagliptin | 0.70 (0.48) | 0.65 (0.34) | 0.65 (0.33) | 0.66 (0.36) | 1.16 (1.17) | 1.06 (0.60) | 1.42 (0.14) |
| Sitagliptin | 0.79 (0.49) | 0.73 (0.34) | 0.72 (0.35) | 0.70 (0.35) | 1.24 (0.70) | 2.11 (1.37) | 2.16 (1.08) |
| Vildagliptin | 0.84 (0.49) | 0.80 (0.34) | 0.80 (0.43) | 0.76 (0.37) | 1.34 (0.88) | 1.09 (0.86) | 0.88 (0.63) |
In each cell is the mean of the PDD/adj-DDD ration and in parentheses is the standard deviation.
Adj-DDD: defined daily dose adjusted for kidney function; PDD: prescribed daily dose; GFR: glomerular filtration rate; DPP-4 inhibitors: dipeptidyl peptidase 4 inhibitors.
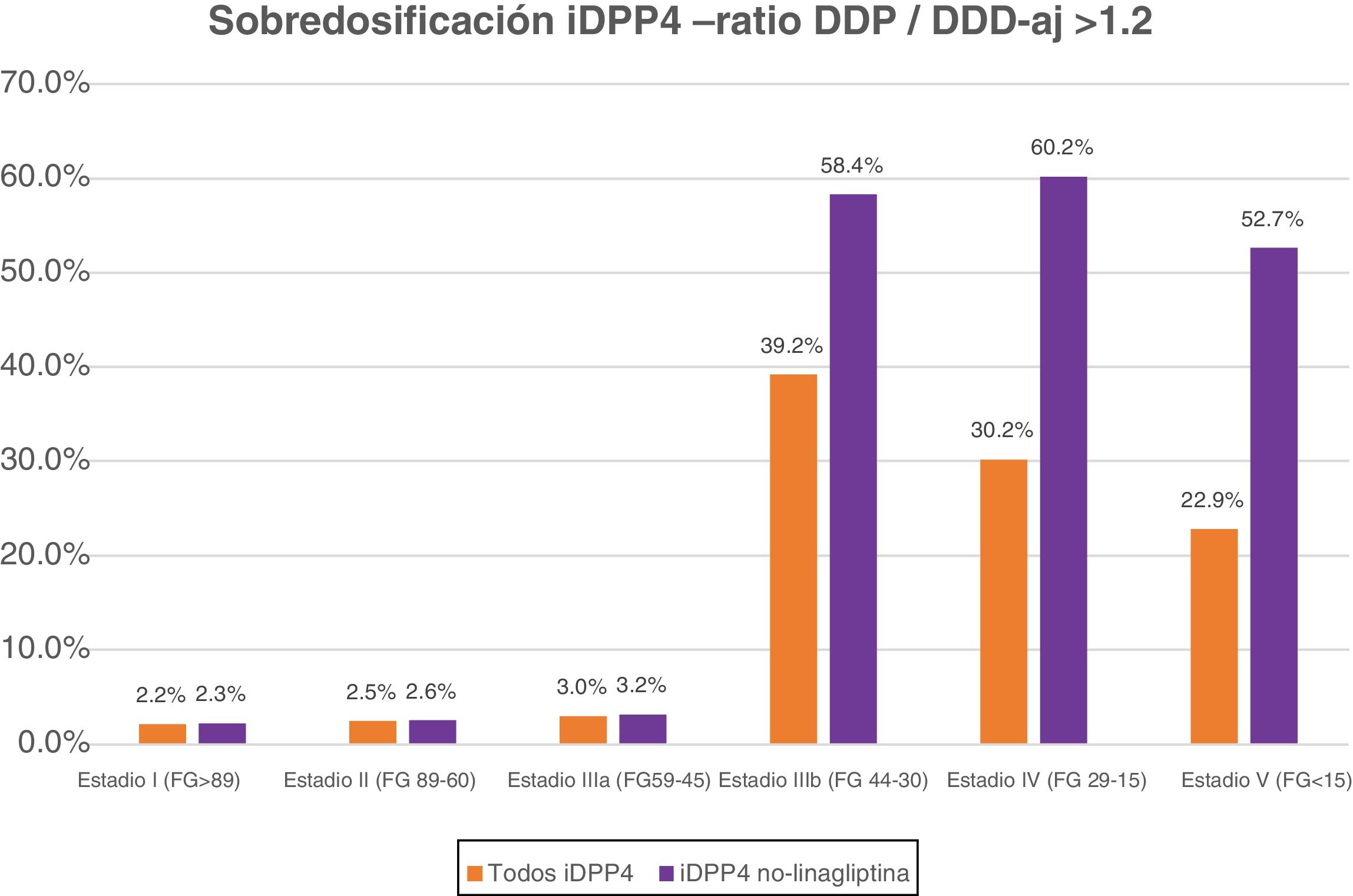
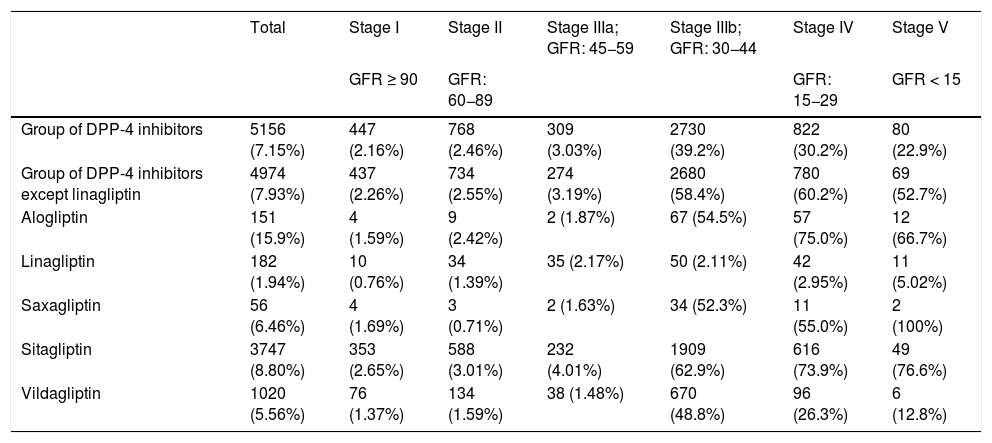
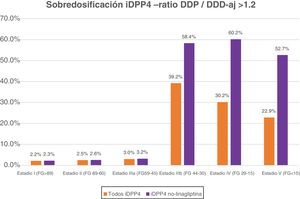
Table 5 shows the percentage of patients with overdose of the different DPP-4 inhibitors in the different stages of kidney failure. It should be noted that globally, overdose of DPP-4 inhibitors occurs in 7.15% of treatments and increases in the most advanced stages of kidney disease (36.8% of patients with GFR < 45) (stages IIIb, IV and V).
Frequency and percentage (%) of overdosea of DPP-4 inhibitors according to stage of kidney failure.
| Total | Stage I | Stage II | Stage IIIa; GFR: 45−59 | Stage IIIb; GFR: 30−44 | Stage IV | Stage V | |
|---|---|---|---|---|---|---|---|
| GFR ≥ 90 | GFR: 60−89 | GFR: 15−29 | GFR < 15 | ||||
| Group of DPP-4 inhibitors | 5156 (7.15%) | 447 (2.16%) | 768 (2.46%) | 309 (3.03%) | 2730 (39.2%) | 822 (30.2%) | 80 (22.9%) |
| Group of DPP-4 inhibitors except linagliptin | 4974 (7.93%) | 437 (2.26%) | 734 (2.55%) | 274 (3.19%) | 2680 (58.4%) | 780 (60.2%) | 69 (52.7%) |
| Alogliptin | 151 (15.9%) | 4 (1.59%) | 9 (2.42%) | 2 (1.87%) | 67 (54.5%) | 57 (75.0%) | 12 (66.7%) |
| Linagliptin | 182 (1.94%) | 10 (0.76%) | 34 (1.39%) | 35 (2.17%) | 50 (2.11%) | 42 (2.95%) | 11 (5.02%) |
| Saxagliptin | 56 (6.46%) | 4 (1.69%) | 3 (0.71%) | 2 (1.63%) | 34 (52.3%) | 11 (55.0%) | 2 (100%) |
| Sitagliptin | 3747 (8.80%) | 353 (2.65%) | 588 (3.01%) | 232 (4.01%) | 1909 (62.9%) | 616 (73.9%) | 49 (76.6%) |
| Vildagliptin | 1020 (5.56%) | 76 (1.37%) | 134 (1.59%) | 38 (1.48%) | 670 (48.8%) | 96 (26.3%) | 6 (12.8%) |
Adj-DDD: defined daily dose adjusted for kidney function; DPP: dipeptidyl peptidase; DPP-4 inhibitors: dipeptidyl peptidase 4 inhibitors.
If we exclude the linagliptin DPP-4 inhibitors from this analysis, the values of overdose rise: globally it is observed in 7.93% and in advanced stages in 58.7% of cases (58.4% in IIIb, 60.2% in IV and 52.7% in V) (Fig. 1).
DiscussionThe data from this study reveal that, under real clinical practice conditions, a very significant percentage of patients with T2DM and advanced kidney failure (stages IIIb, IV and V) present with an overdose of non-linagliptin DPP-4 inhibitors. The recommendation from the summaries of product charcteristics from the AEMPS is to adjust the dose of this pharmacological family to the degree of glomerular filtration rate of each patient, but this recommendation is rarely followed in our environment. In patients with advanced kidney failure (eGFR<45 ml/min/1.73 m2) DPP-4 inhibitor overdose occurs in 36.8% of treatments (in 58.7% if we exclude linagliptin).
Regarding the general characteristics of our study, and as has been observed by other authors,25 overall, there do not seem to be any major differences in the clinical profile of patients or in adherence to treatment between the different families of DPP-4 inhibitors.
This study analysed the prescription by the doctor and not the attitude of the patient as far as pharmacy dispensing and therefore its compliance. What we fundamentally wanted to find out was the intention-to-treat of health professionals before each new prescription of a DPP-4 inhibitor according to the degree of kidney function.
When trying to compare the data obtained with previous studies, we were surprised by the scarcity of studies that look at this issue. We have not found any similar studies in Spain. Recently, Spanopoulos D et al.26,27 in a study, also in a real clinical practice situation in the United Kingdom, analysed the overdosing of DPP-4 inhibitors based on several characteristics such as kidney function. Their results indicate that with GFR < 50 ml/min/1.73 m2 there is an overdose in 16.0% of treatments with any of the DPP-4 inhibitors (a figure that rises to 35.2% if linagliptin is excluded). By different DPP-4 inhibitors, this is observed in 38.9% of cases treated with alogliptin, 28.1% with saxagliptin, 32.7% with sitagliptin and 18.8% with vildagliptin.
In 2017 in the USA, Huang H et al.,28 studied overdose only in patients with stages IIIb or higher (GFR < 45) of kidney failure and estimated that there is overtreatment with DPP-4 inhibitors in 36.2% of cases (57.9% with saxagliptin and 44.9% with sitagliptin), which are practically identical data to those of our study (36.8%). They also analysed the total cost derived from T2DM, observing that it is higher in overdosed patients ($304 per year).
In Korea,14 a large retrospective population study among patients with T2DM and stages IIIb and IV found an overdose of DPP-4 inhibitors of close to 40% in 2011, which was reduced to 24.4% four years later.
Regarding limitations, the following should be noted: this was a retrospective descriptive study with clinical records with implicit limitations such as the risk of under-reporting and missing values. However, electronic prescriptions have been used for many years in our system and in this study only patients with complete values for the main variables were analysed.
There was the possibility that during the observation period a subject would experience changes in the prescription, either in the dose, in the starting of combined treatment with another antidiabetic, or even in the change of DPP-4 inhibitor class. All possible situations have been analysed together since the objective of the study was to carry out an analysis of the real situation and to study the prescription pattern of the primary care physician.
Temporary changes in the treatment pattern and the clinical repercussions that DPP-4 inhibitor overdosage may have not been analysed. This may represent a line for future studies in real practice, especially regarding possible adverse effects that may occur.
A recently published literature review has concluded that the adverse effect profile of DPP-4 inhibitors in kidney failure does not seem to be different from that of people with normal kidney function; but taking into account the pharmacokinetic data, dose adjustments are recommended for those DPP-4 inhibitors with significant kidney excretion to avoid drug accumulation.29 Plasma accumulation of DPP-4 inhibitors is considered a potential risk due to the possibility of an unknown adverse event appearing30 or a rare but potentially serious adverse event, such as heart failure or pancreatitis.29 The risk of inducing hypoglycaemia is less likely. The large sample and long study period were the main strengths of this study. It has been possible to describe the prescription habits of the primary care physician prescribing DPP-4 inhibitors in a real medical practice setting.
Dose reduction, in addition to the implications this may have on pharmacological safety, has sometimes been an argument put forward to assert a lower cost of treatment in these patients.28
Our data and those from the literature show that, in the presence of kidney failure, the dose of DPP-4 inhibitors is not adjusted for the most part. Approximately one third of patients with T2DM and advanced kidney failure (GFR < 45 ml/min/1.73 m2) treated with DPP-4 inhibitors are overdosed.
The main recommendation that can be established from the analysis of the data must necessarily be oriented towards the need to comply with the recommendations of the AEMPS and adjust the dose of the different DPP-4 inhibitors according to the glomerular filtration rate.
Data availabilityData will be made available on request.
FundingThis work has not received any external funding.
AuthorshipJ.F-N, M.M-C, J.R, D.M, E.O, J.A.V and B.V, participated in the study design. J.R carried out the statistical analysis J.F.-N wrote the initial draft of the manuscript, which was edited by M.M-C, J.R, D.M, E.O, J.A.V and B.V. All the authors approved the final manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest in relation to this study.
Please cite this article as: Franch-Nadal J, Gatius JR, Mata-Cases M, Ortega E, Valles JA, Vlacho B, et al. Cumplimiento de las recomendaciones de ajuste de la dosis de inhibidores DPP4 según la función renal en una base de datos poblacional, Endocrinol Diabetes Nutr. 2022;69:83–91.