Despite continuous glucose monitoring having been proven useful in patients with type 1 diabetes mellitus, A1C remains the gold standard for assessing disease management.
Material and methodsDescriptive, retrospective study which included 252 patients, 40.5% male, mean age 44.91±14.57 years, mean duration of diabetes 22.21±13.12 years, 88.1% on basal-bolus insulin therapy and 11.9% users of continuous subcutaneous insulin infusion. Glucose measurement, analytical and anthropometric data were obtained.
ResultsThe mean time in range was 60.18±15.60% and was associated with A1C after adjusting for age, gender, duration of diabetes, BMI, insulin regimen, %CV and time below range (ß: −0.548; p<0.01). The glucose management indicator (GMI) was 7.19±0.69% and was also associated with A1C (ß: 0.957; p<0.01) regardless of age, gender, duration of diabetes, BMI, insulin treatment, %CV and time in range. The average difference between A1C and GMI was 0.17±0.65% (−2.70-3.40%), being higher as A1C increased, in a linear and significant manner, without being influenced by the duration of diabetes or CV.
ConclusionsAlthough we found a positive correlation between continuous glucose monitoring glucose measurement parameters and A1C, there is still not enough evidence to replace one parameter with another.
La monitorización continua de glucosa (MCG) ha demostrado su utilidad en pacientes con diabetes mellitus tipo 1 (DM1). Sin embargo, la A1C sigue siendo el gold estándar para evaluar el manejo de la enfermedad.
Material y métodosEstudio descriptivo, retrospectivo que incluyó a 252 pacientes, 40,5% varones, media de edad 44,91±14,57 años, tiempo de evolución de enfermedad 22,21±13,12 años. El 88,1% en régimen de insulina bolo-basal y 11,9% eran usuarios de ISCI. Se obtuvieron datos glucométricos, analíticos y antropométricos para su relación.
ResultadosEl tiempo en rango (TIR) medio fue del 60,18±15,60% y se relación con la A1C tras ajustar por la edad, el sexo, la duración de la enfermedad, el IMC, el tratamiento insulínico, el %CV y el TBR (ß: −0,548; p<0,01). La GMI fue de 7,19±0,69% e igualmente asoció a la A1C (ß: 0,957; p<0,01) independientemente de la edad, el sexo, la duración de la enfermedad, el IMC, el tratamiento insulínico, el %CV y el TIR. La diferencia media entre A1C y GMI es de 0,17±0,65% (−2,70-3,40%) siendo mayor a medida que aumentaba la A1C de manera lineal y significativa sin verse influenciada por el tiempo de evolución de la enfermedad o del CV.
ConclusionesAunque observamos una buena correlación entre los parámetros glucométricos derivados de la MCG y la A1C, no existe evidencia suficiente para poder sustituir un parámetro por otro.
Glycosylated haemoglobin (A1c) is considered the gold standard for evaluating blood glucose control.1 It is also an important marker of risk of development of vascular complications2,3 and a predictor of mortality.4 Therefore, the main guidelines for diabetes management continue to use A1c as the indicator to be monitored when adjusting antidiabetic treatment.5 However, A1c is known to have a number of limitations. A1c determinations can be affected by anaemia, pregnancy, haemoglobinopathies and racial factors.6 In addition, A1c does not reflect fluctuations, such as episodes of hypoglycaemia or hyperglycaemic excursions, which can determine optimal blood glucose control in our patients.7
Flash glucose monitoring using the FreeStyle Libre (FSL) system (Abbott DiabetesCare, Witney, United Kingdom) enables reading of interstitial blood glucose levels, with no need for calibration, over a 14-day period. This system has proven useful in patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), reducing time in hypoglycaemia,8 hospital admissions due to hypoglycaemia9 and A1c levels,10,11 primarily in patients with poor control,12 as well as improving quality of life.13
In 2019, the International Consensus on Time in Range14 defined the glucose measurement parameters that all continuous monitoring systems must provide. The Average Glucose Profile (AGP) report must collect data such as: average glucose, variability measured by percentage coefficient of variation (%CV), time in range (TIR) (70–180mg/dl), time below range (TBR) (<70mg/dl), time above range (TAR) (>180mg/dl) and glucose management index (GMI). “GMI” has replaced the old term “estimated A1c”, since the latter can be misunderstood in the management of diabetic patients.15 GMI is calculated based on glucose levels obtained in the past 14 days of continuous glucose monitoring. Recent studies have shown, moreover, that glucose measurement data over a 14-day period offer a good estimate of longer periods of time — up to three months.16
Although few studies have determined the relationship between these indices and A1c, a better understanding of the association between GMI and A1c would enable more practical management of blood glucose control.
Material and methodsA retrospective descriptive study was designed that included patients in endocrinology and nutrition outpatient follow-up at Hospital Central de Asturias [Asturias Central Hospital] between December 2018 and January 2021, with a diagnosis of T1DM, being treated with insulin, using the FreeStyle Libre system to measure glucose and sharing their information through the LibreView platform. The platform data were collected from AGP reports containing a 70% or higher percentage of 14-day information.
Patients who were under 14 or over 85 years of age and patients who had secondary diabetes or another type of diabetes, acute metabolic complications (ketoacidosis or severe episodes of hypoglycaemia requiring medical care), intercurrent conditions causing hyperglycaemia, anaemia or other haemoglobinopathies that might affect their A1c levels were excluded.
From electronic medical records we extracted anthropometric data (weight and height in order to calculate body mass index [BMI]), as well as the latest laboratory results for A1c (measured using high-performance liquid chromatography [HPLC], Jokoh HS-10 analyser), standardised according to International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) criteria (JDS/JSCC A1c=0.927 [IFCC A1c]+1.73).
We also collected flash glucose monitoring data from the LibreView platform. The LibreView platform is an online application where data collected from continuous glucose monitoring are downloaded and where both users and healthcare personnel, after obtaining user consent to share data, are able to check interstitial glucose levels. There is a standardised format called AGP. This report, according to the recommendations of the latest consensus,14 should include the following variables: average blood glucose, %CV, TIR, TBR, TAR and GMI. For the data to be representative, according to this consensus, the percentage of valid information in a 14-day period must exceed 70%.
The download date must match the A1c laboratory testing date and the anthropometric data must be from the consultation date closest to the download date.
We arbitrarily divided patients into two groups based on the difference between A1c and GMI (less than or equal to 0.2% or greater than 0.5%). We also subdivided the sample based on blood glucose control by splitting it into three groups (A1c under 7%, 7%–8% or over 8%) and based on time since disease onset in terciles (less than 15 years since onset, 15–30 years and more than 30 years) as well as based on CV in accordance with the cut-off point recommended by the latest consensus14 (less than 36% or greater than 36%).
For statistical analysis, we used the SPSS software program, version 20 for Windows (SPSS, Inc., Chicago, IL, United States). Student's t test or analysis of variance (ANOVA) was used for quantitative variables, in addition to Bonferroni as a post hoc analysis after performing a normality test on the sample. For qualitative variables, we used the χ2 test, and correlations were performed with Pearson's test. In addition, linear regression studies were conducted taking age, sex, time since disease onset, BMI, insulin therapy, %CV, TBR and TIR as confounding variables. The level of significance was set at p<0.05 (two-tailed), and the data were expressed in terms of mean±standard deviation with range in brackets, except as otherwise indicated.
ResultsTwo hundred and fifty-two (252) patients with T1DM were selected, 40.5% men, with an age of 44±14 years (range: 15–79) and a time since disease onset of 22.21±13.12 years (1–65), on insulin therapy (88.1% with a basal-bolus regimen and 11.9% with continuous subcutaneous insulin infusion [CSII]). The mean BMI was 25.8±3.9kg/m2 (18.2–41.6). Table 1 shows all other characteristics. All patients had received diabetes education in our department and had been instructed in using and downloading from the monitoring system.
Sample characteristics.
| n=252 | |
|---|---|
| Sex (M/F) (%) | 40.5/59.5 |
| Age (years) | 44.91±14.58 (15–79) |
| Time since onset (years) | 22.21±13.12 (1–65) |
| % Less than 15 years since onset | 32.5% |
| % 15–30 years since onset | 38.6% |
| % More than 30 years since onset | 28.9% |
| BMI (kg/m2) | 25.81±3.96 (18.23–41.66) |
| A1c (%) | 7.36±0.94 (5.4–10.6) |
| % A1c <7% | 37.3 |
| % A1c 7%–8% | 40.9 |
| % A1c >8% | 21.8 |
| Tobacco use (%) | 21.2 |
| HTN (%) | 20.6 |
| Dyslipidaemia (%) | 31.3 |
| Retinopathy (%) | 33.7 |
| Nephropathy (%) | 8.8 |
| Basal-bolus therapy (%) | 88.1 |
| CSII (%) | 11.9 |
BMI: body mass index; CSII: continuous subcutaneous insulin infusion; HTN: hypertension; M/F: male/female.
Data are expressed in terms of mean±standard deviation and range in brackets or percentage in the cases indicated.
The mean A1c was 7.36%±0.94% (5.4%–10.6%), and just 37.3% of the patients achieved good metabolic control (A1c≤7%) (Table 1). In terms of the glucose measurement study, the mean percentage of valid information from downloads was 95.06%±7.21% of the time. The mean TIR was 60.18%±15.60%, and just 28.6% of the patients achieved a target TIR greater than 70%. With regards to time in hypoglycaemia, 57.9% of patients achieved a TBR less than 4%. GMI was 7.19%±0.69%, lower than A1c (Table 2).
Glucose measurement results for the sample.
| n=252 | |
|---|---|
| Information percentage (%) | 95.06±7.21 (70–100) |
| Average blood glucose (mg/dl) | 161.85±29.04 (102–276) |
| Coefficient of variation (%) | 37.35±6.23 (21.70–55.80) |
| % Coefficient of variation <36 | 44 |
| Time in range (%) 70–180mg/dl | 60.18±15.60 (13–97) |
| % Time in range >70% | 28.6 |
| Time below range (%) ≤70mg/dl | 5.00±4.90 (0–35) |
| % Time below range <4% | 57.9 |
| Time above range (%)>180mg/dl | 34.86±16.89 (0–85) |
| GMI (%) | 7.19±0.69 (5.70–9.90) |
GMI: glucose management index.
Data are expressed in terms of mean±standard deviation and range in brackets or percentage in the cases indicated.
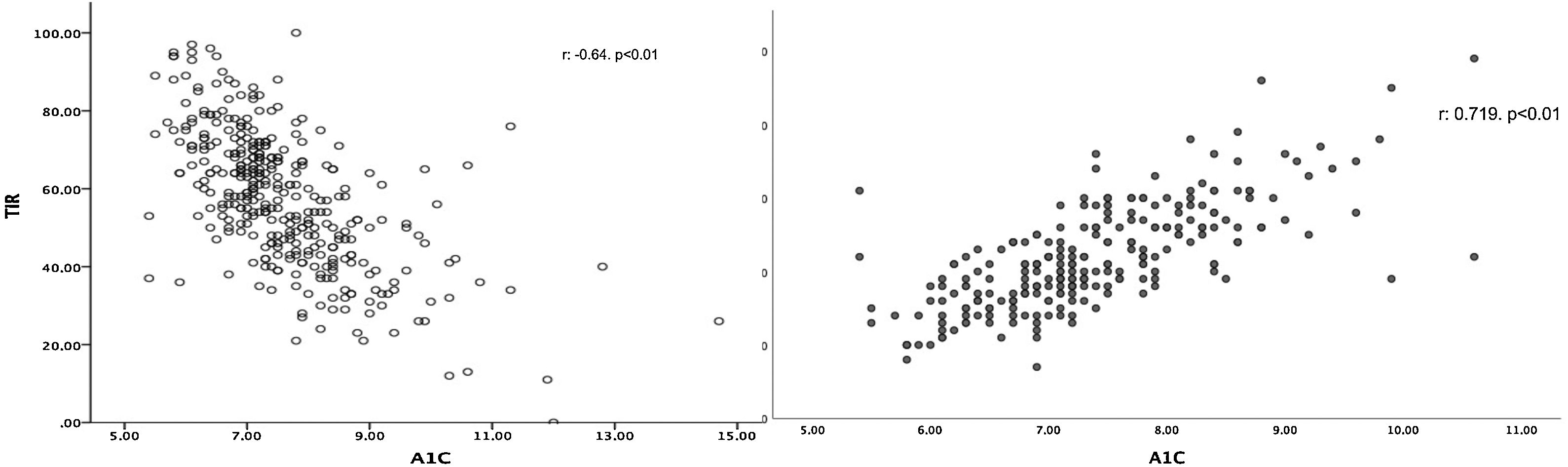
TIR was linked to A1c (r: −0.623; p<0.01) (Fig. 1a), and this association was maintained after adjusting for age, sex, time since disease onset, BMI, insulin therapy, %CV and TBR (β: −0.548; p<0.01). Similarly, we found a positive association between A1c and GMI (r: 0.719; p<0.01) (Fig. 1b). In the multivariate study, this association was maintained (β: 0.957; p<0.01) regardless of age, sex, time since disease onset, BMI, insulin therapy, %CV or TIR.
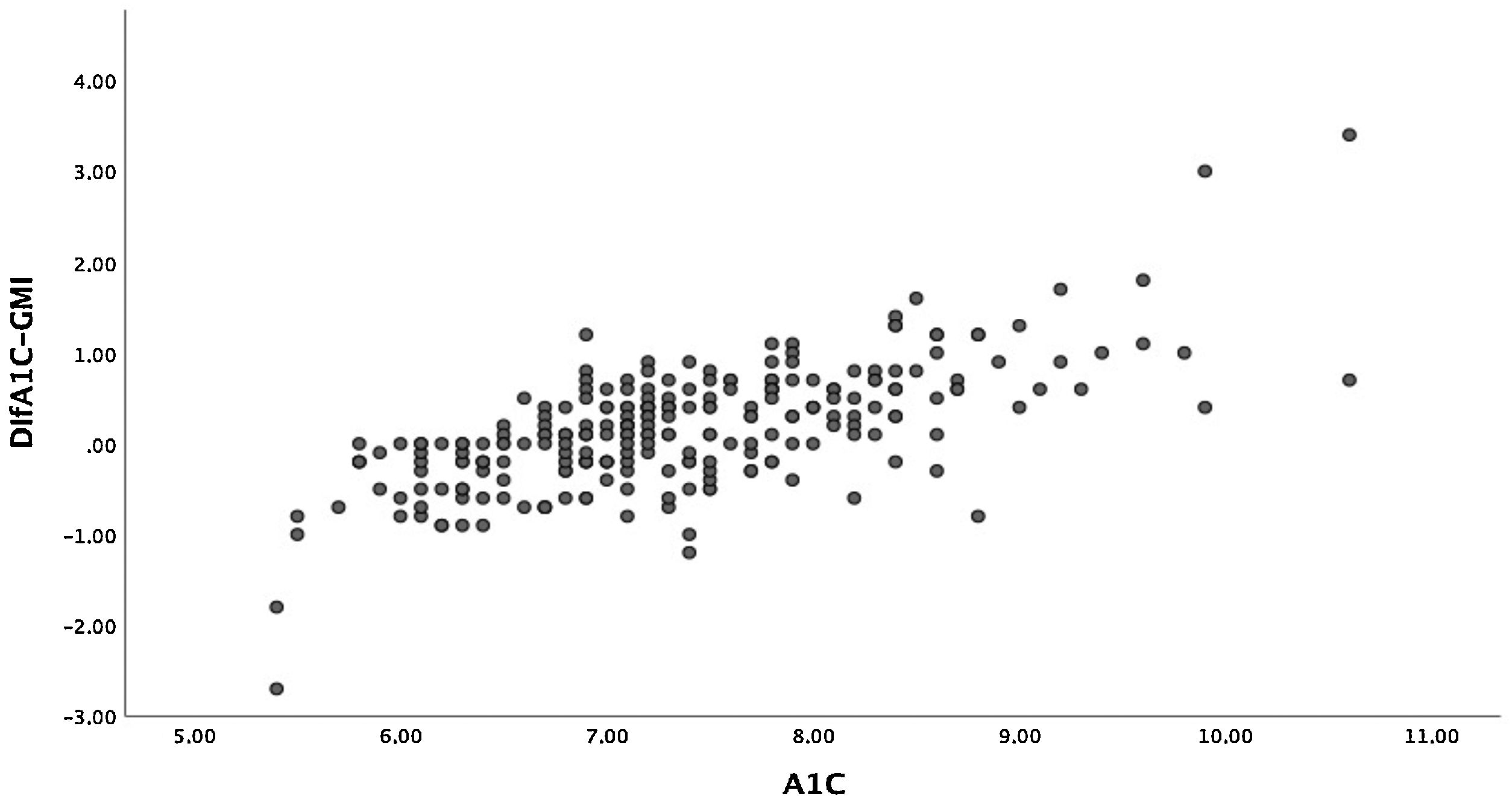
The mean difference between A1c and GMI was 0.17%±0.65% (−2.70-3.40). In 7.6% of patients, the difference between A1c and GMI was 0%, and in 57.4% of patients, A1c was higher than GMI. Given the wide range of this difference, we compared those with a difference between A1c and GMI less than or equal to 0.2% and those with a difference greater than 0.5%. We found that patients with greater differences were older, although time since disease onset was similar. Together they showed worse blood glucose control, higher rates of hyperlipidaemia and higher rates of microangiopathic complications in the form of diabetic retinopathy. No differences were seen in terms of treatment type. With regard to glucose measurement data, patients with a greater difference had a lower TIR and spent more time in hyperglycaemia. We found no differences in TBR. Table 3 describes all other characteristics.
General characteristics and glucose measurement data for patients based on the difference between A1c and GMI.
| Difference between A1c and GMI≤0.2% (n=87) | Difference between A1c and GMI>0.5% (n=95) | p | |
|---|---|---|---|
| Sex (M/F) (%) | 42.5/57.5 | 34.7/62.3 | 0.28 |
| Age (years) | 42.51±12.59 | 48.06±15.40 | 0.09 |
| Time since onset (years) | 21.56±12.03 | 24.68±13.52 | 0.10 |
| BMI (kg/m2) | 25.46±3.65 | 26.32±4.11 | 0.16 |
| A1c (%) | 6.95±0.63 | 7.7±1.15 | <0.01 |
| Tobacco use (%) | 18.6 | 21.7 | 0.64 |
| HTN (%) | 16.1 | 25.3 | 0.13 |
| Dyslipidaemia (%) | 20.7 | 38 | 0.011 |
| Retinopathy (%) | 33.1 | 41 | 0.082 |
| Nephropathy (%) | 7 | 8.4 | 0.72 |
| Basal-bolus therapy (%) | 86.2 | 85.3 | 0.86 |
| CSII (%) | 13.8 | 14.7 | 0.86 |
| Information percentage (%) | 94.74±7.09 | 95.31±7.11 | 0.59 |
| Average blood glucose (mg/dl) | 152.93±25.77 | 167.58±31.20 | 0.01 |
| CV (%) | 36.18±5.85 | 37.77±6.59 | 0.87 |
| % CV <36 | 50.6 | 45.3 | 0.75 |
| TIR (%) | 65.41±15.02 | 56.44±15.66 | <0.01 |
| % TIR >70% | 41.4 | 22.1 | <0.01 |
| TBR (%) | 5.17±4.74 | 5.24±5.57 | 0.93 |
| % TBR <4% | 52.9 | 61.1 | 0.26 |
| TAR (%) >180mg/dl | 29.34±16.28 | 38.51±17.17 | <0.01 |
| GMI (%) | 6.97±0.62 | 7.33±0.74 | <0.01 |
BMI: body mass index; CSII: continuous subcutaneous insulin infusion; CV: coefficient of variation; GMI: glucose management index; HTN: hypertension; M/F: male/female; TAR: time above range; TBR: time below range; TIR: time in range.
Data are expressed in terms of mean±standard deviation and range in brackets or percentage in the cases indicated. Student’s t test was used to compare quantitative variables after performing a normality test on the sample. The χ2 test was used for qualitative variables.
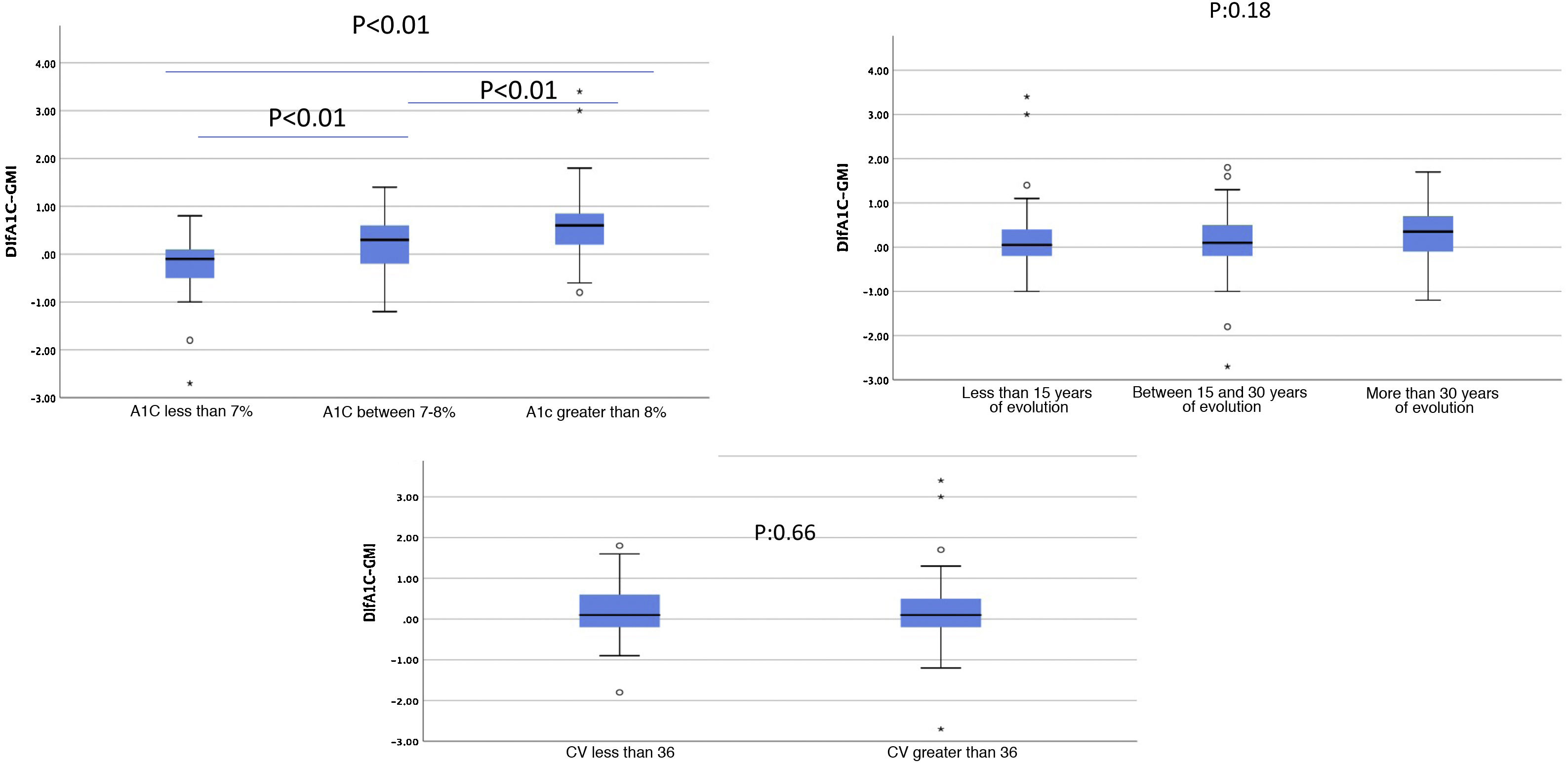
Moreover, there was a wide range of differences obtained between these two variables. Patients with A1c between 7% and 8%, who in our sample accounted for the majority, had smaller differences than those with A1c over 8% (Fig. 2). The differences between A1c and GMI became greater as A1c increased, in a linear and significant manner (Fig. 3a), indicating that in patients with poor control GMI underestimates time in hyperglycaemia. However, when we established groups based on time since onset (Fig. 3b) and CV (Fig. 3c), no significant differences were found.
Differences between A1c and GMI based on: (a) HbA1c; (b) years since disease onset; c) coefficient of variation.
(a) and (b) ANOVA to compare means; (c) Student’s t test to compare independent samples.
A1c: glycosylated haemoglobin (%); CV: coefficient of variation; GMI: glucose management index (%).
The results of this study showed the relationship between glucose measurement data derived from the FSL system and A1c levels. The Exchange T1 study17 identified a gradual increase in the use of technology in T1DM in recent years. However, this same report warned of this increase in technological development, although percentages of patients with T1DM and good control were limited. In Spain, the SED1 study18 demonstrated that just 33% achieved a target of good control. These data were consistent with those for our sample, which consisted entirely of FSL continuous monitoring technology users, where scarcely 37% achieved A1c <7%.
A study by Gómez-Peralta et al.19 conducted in FSL users in Spain found that the percentage of patients with target TIR ranged from 47.8% to 65.5%. This study did not distinguish by type of diabetes, and this could explain the differences with our study, where less than 30% of patients achieved TIR more than 70% of the time.
With respect to the correlation between A1c and glucose measurement parameters in T1DM, several indices have been proposed, primarily TIR and GMI (previously called estimated A1c). With respect to TIR, a study by Petersson et al.,20 conducted in 133 children and adolescents with T1DM in Switzerland, found a linear and significant relationship between TIR and A1c. In this vein, in the REAL-Life glucoSe Monitoring in Type 1 Diabetes (REALISM-T1D) study,21 conducted in adults with T1DM, this same positive relationship was observed in the same way. Finally, Vigersky and McMahon22 recently published a meta-analysis with 18 different studies confirming this linear correlation between TIR and A1c. These data prompted them to consider the true usefulness of TIR as a replacement for venous determination of A1c. However, a recently published study by Díaz-Soto et al.23 found that this relationship between TIR and A1c was dependent on blood glucose variability. Patients with a higher CV had a worse correlation between the two variables.
Our study found a significant, linear relationship between TIR and A1c not seen to be affected by type of treatment or time since disease onset, among other factors. However, this relationship was weak and consistent with the data published by Beck et al.,24 who, despite finding a weak relationship between TIR and A1c, put forward that, for a single TIR, the A1c levels found were highly disparate. Unlike in the Díaz-Soto et al. study,23 the relationship we found was independent of CV. The fact that our study did not include paediatric patients and the fact that our sample had a higher percentage of patients with a CV≤36% could account for these differences.
For this very reason, we decided to use GMI. GMI is a calculated index that uses average blood glucose. This involves the use of all blood glucose values, not just those bounded by a range. In our sample, we found a stronger correlation between GMI and A1c than between GMI and TIR, and similarly this effect was independent of age, sex, time since disease onset, BMI, insulin therapy, %CV and TIR. In this vein, Hu et al.25 conducted a study in T2DM comparing the relationship between A1c and GMI, and also observed a positive relationship. Similarly, Yamada et al.26 found a positive correlation between A1c and average blood glucose also in patients with T1DM.
However, when we looked at the differences between the A1c measurement and GMI, we saw a wide range. When we examined the factors that might explain these discrepancies, we only found differences in A1c figures, such that the greater the A1c, the greater the difference with GMI, with this difference being smaller when A1c was 7%–8%. In our study, around 50% of patients were in this range, and this could explain the good correlation obtained. Mean GMI was lower than A1c, and this underestimation was greater in patients in whom A1c was higher than 8%, which might underestimate vascular risk in these poorly controlled patients. The data extracted from the Diabetes Patienten Verlaufsdokumentation (DPV) registry (Germany, Austria, Switzerland and Luxembourg) also showed the differences between these two variables based on A1c, with GMI being lower in 75% of patients with A1c ≥7.5%.27 For this reason, some authors25 have warned against using GMI as a control parameter, since its tendency to show lower values compared to A1c in poorly controlled patients may underestimate the chronic effects of hyperglycaemia on the development of complications.
Classically, most studies in which a correlation between blood glucose control and the development of vascular complications was observed have used A1c as a reference parameter.2 However, other glucose measurement parameters are increasingly being sought that enable stratification of cardiovascular risk in patients, synergistically with A1c or as a reference method in patients in whom A1c values cannot be assessed (anaemia, infection, acute disease, etc.). Some studies have attempted to link some glucose measurement parameters to the development of complications.28 Lu et al.29 found a direct relationship between TIR and carotid atheromatosis, as well as the development of retinopathy in patients with T2DM. However, more studies are needed to determine whether the parameters derived from the AGP report may be able to replace A1c in predicting the development of complications.
In conclusion, our study showed the relationship between glucose measurement data derived from the FSL system and A1c in patients with T1DM, revealing a positive correlation between GMI and A1c. In the future, a closer approximation of glucose measurement laboratory parameters to A1c could be a useful tool in patients with diabetes and could eliminate the need for some laboratory determinations.
FundingThis study received no sources of funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.