Gestational diabetes mellitus (GDM) increases the risk of adverse events in pregnancy and jeopardizes long-term health of the mother and offspring. There is currently no consensus as to what screening strategies improve the efficiency of GDM diagnosis. Which criteria should be used? Is the one-step or two-step procedure better? There is no agreement as to what the best dietary approach in the treatment of GDM is. In addition, different nutritional interventions have been studied in the prevention of GDM. The Mediterranean diet seems to be effective in preventing GDM and other maternofoetal outcomes. We review herein our experience using the one-step criteria for GDM screening; the treatment and prevention strategies used; and the overall impact of nutrition on maternofoetal health.
La diabetes gestacional (DG) incrementa el riesgo de tener eventos adversos durante el embarazo, y también afecta a la salud materna y de la descendencia a largo plazo. En la actualidad no existe un consenso sobre qué estrategia de cribado es más eficaz para el diagnóstico de la DG. ¿Qué criterios se deberían utilizar? ¿Es mejor hacerlo en un solo paso o en 2? Tampoco existe un acuerdo universal sobre cuál es el mejor tratamiento nutricional ni qué intervención nutricional es la más adecuada para su prevención. La dieta mediterránea parece ser las más efectiva en la prevención no solo de la DG, sino que también de otros eventos adversos materno-fetales. En este artículo revisamos la experiencia de nuestro grupo en la aplicación de los criterios diagnósticos de un solo paso para la DG; las estrategias empleadas en el tratamiento y prevención de la DG, y del impacto global que tiene la alimentación sobre la salud materno-fetal.
The American Diabetes Association defines gestational diabetes mellitus (GDM) as “diabetes first diagnosed in the second or third trimester of pregnancy that is not clearly either pre-existing type 1 or type 2 diabetes”.1 Its prevalence has been increasing on a worldwide scale due to older age of women at childbearing age and the ongoing epidemic of obesity. Prevalence varies widely due to differences in the screening criteria used.
In the short term, GDM entails complications such as pregnancy-induced hypertensive disorders, premature labour, shoulder dystocia, caesarean section, low and high birthweight for gestational age.2 In addition, women with a history of GDM and their offspring have a higher risk of developing glucose disorders, cardiovascular diseases and cancer in the long term.3–5
It is important to find strategies to reduce the impact of GDM by adequately screening, diagnosing and treating women afflicted by it. It is also ideal to develop programs to prevent the development of GDM.
Screening methodology and criteria for GDM have been a matter of debate for a long time, and no agreement has been found to date. There are also discrepancies as to what medical nutrition therapy is best in GDM management. It is not clear whether the best approach is a diet that restricts carbohydrates or one that permits a higher consumption. Since the Mediterranean Diet (MedDiet) has been proved beneficial in the prevention and treatment of type 2 diabetes, looking into using it in GDM treatment is worthy. Moreover, numerous studies have evaluated lifestyles and dietary patterns effective in preventing GDM and maternofoetal outcomes, finding no conclusive evidence.
Thus, we review herein our experience in the study of using the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) criteria for GDM screening; the treatment and prevention strategies used; and the overall impact of nutrition on maternofoetal health.
GDM screening. How should we do it?All the lack of consensus and uniformity in screening methodology for GDM led to the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study.2 Its aim was to evaluate associations of risks of adverse outcomes with different degrees of maternal glucose intolerance less severe than that in overt diabetes mellitus. Degrees of glycaemia less than that diagnostic of diabetes were associated with increased risk of maternofoetal adverse outcomes. The HAPO study concluded that there was a linear association between maternal hyperglycaemia and adverse outcomes, which suggested that treating “mild” GDM could improve adverse outcomes. Based on the results of this study, the IADPSG consensus panel recommended new diagnostic criteria.6
While the hopes of the HAPO study was to provide a uniform strategy to screen and diagnose GDM, the global debate and controversies remain. Major concerns about using the IADPSG criteria as opposed to others are that using them significantly increases GDM prevalence (as high as 33%). Also, it became questionable whether their use would provide benefits for the mother and new-born. Inevitably, all this raised the concern of the implications on healthcare costs and cost-effectiveness. Currently, the Endocrine Society, World Health Organization, International Federation of Gynaecology and Obstetrics and American Diabetes Association endorse – not exclusively – these criteria.1,7–9 The American College of Obstetricians and Gynaecologists (ACOG) do not currently recommend using IADPSG criteria, concerned that using them increases the prevalence of GDM by about 18%.10 Moreover, there is a lack of clinical trials that justify the use of these criteria.9
How is it done at the Hospital Clínico San Carlos?The screening protocol for GDM at the Hospital Clínico San Carlos has been changed over the years. Before 2006, the process was markedly slower than it is currently. It required approximately five different appointments, leading to important delays in the diagnosis and treatment of GDM. The mean time delay between undergoing the O'Sullivan test and performing the OGTT was of 36.5 days. 88% of women underwent OGTT more than 7 days after having a positive O'Sullivan test. There were also time delays between being diagnosed with GDM and receiving GDM treatment. Only 2.5% of women had an appointment at the Diabetes and Pregnancy Unit in less than 10 days after diagnosis. From 2006 onwards, GDM screening has been universal and centralized. Since then, all these issues have been amended. The five appointments that were required previously have been reduced to one; only 12% of women undergo the OGTT more than 7 days after a positive O'Sullivan test; and 98% women have an appointment at the Diabetes and Pregnancy unit in less than 10 days after diagnosis and always before 28 gestational weeks.
Up until March 2012, the two-step Carpenter–Coustan criteria were used to diagnose GDM. Screening was performed between 24 and 28 gestational weeks, assessed by means of the O'Sullivan test. If plasma glucose levels 1-h post glucose load was ≥7.8mmol/L (≥140mg/dL), a 3-h OGTT of 100-g was performed. Diagnostic thresholds were fasting 95mg/dL, 1-h 180mg/dL, 2-h 155mg/dL and 3-h 140mg/dL, and GDM was diagnosed when two or more values were above the thresholds. These criteria were adopted following the recommendation of the Fourth and Fifth International Workshop-Conference on Gestational Diabetes Mellitus.11,12
Since April 2012, the screening protocol changed. The obstetrician provides women instructions and a scheduled visit to perform GDM screening at 24–28 gestational weeks (usually at 24 gestational weeks), where they undergo a 2-h OGTT of 75-g. IADPSG criteria have been used to diagnose GDM, establishing diagnosis when one or more cut-off values were met: fasting ≥92mg/dL, 1-h ≥180mg/dL and 2-h ≥155mg/dL. One week within diagnosis the patient is scheduled for follow-up at the Endocrinology Unit, where treatment is provided.
In 2014, our research team published results of a study that aimed to evaluate the effect of introducing IADPSG criteria on pregnancy outcomes and healthcare costs (with a cost-effectiveness analysis).13 Results showed increases in the prevalence of GDM from 10.5% using Carpenter–Coustan criteria to 35.5% when using IADPSG criteria. However, there were reductions in the rates of adverse outcomes. Cost-effectiveness analysis revealed that €14,358.06 could be saved per 100 women when using IADPSG criteria as compared to Carpenter–Coustan. This is in agreement with other studies.14–16 A study conducted in a high risk population of United Arab Emirates concluded that while using IADPSG criteria would increase the costs by 42% it would also reduce laboratory costs by 36%.14 In another study, authors concluded that using these criteria could be cost-effective if women received postdelivery counselling and lifestyle modifications in order to prevent onset of diabetes in the future.16 Contrary to this, other researchers have found that using these criteria increase the incidence of GDM and healthcare costs.17
Treatment of GDM. High-fat/low-carbs or low-fat/high carbs?GDM integrates three elements of risk. Perinatal morbidity and mortality throughout pregnancy and increased risk in the mother and her offspring of developing metabolic diseases in the future. A key to preventing all these complications once GDM has been diagnosed is to have appropriate glycaemic control. GDM treatment entails important reductions in the risk of developing maternofoetal complications.
Treatment of GDM starts with medical nutrition therapy (MNT). This includes changes in diet and physical activity, and control of gestational weight gain. When medical nutrition therapy fails, it is complemented with pharmacologic treatment (oral agents or insulin). At the Hospital Clínico San Carlos, only insulin therapy is used, not oral agents.
There is an ongoing debate as to what type of MNT is better for GDM management: diets with higher complex carbohydrate/lower fat distribution or the opposite? Consensus panels have not provided any specific diet recommendations due to inconclusive evidence. However, both the Endocrine Society and the ACOG support limiting carbohydrate intake in GDM treatment.
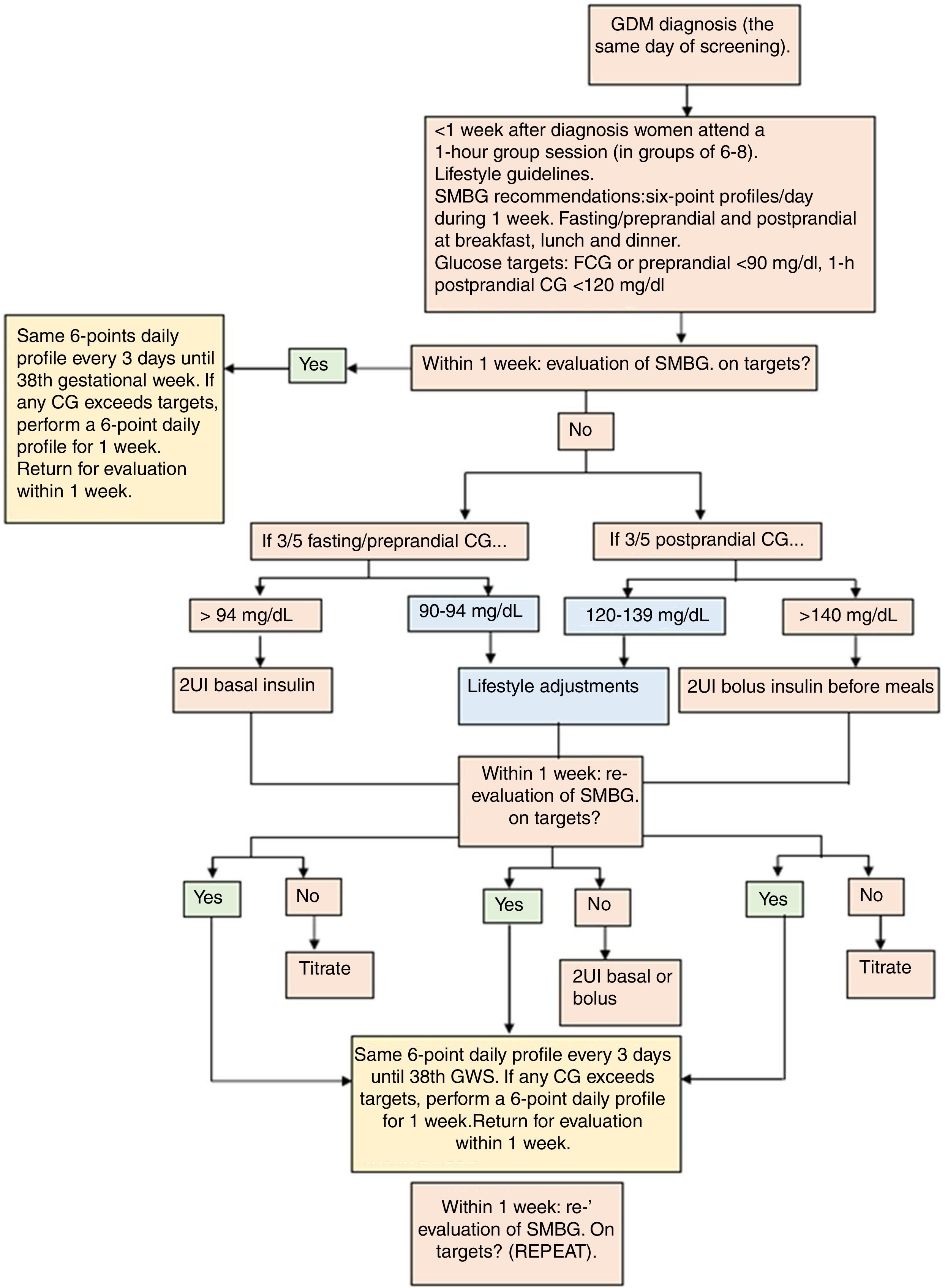
The protocol of GDM treatment applied at the Hospital Clínico San Carlos has been published in detail previously.18 In their first appointment at the Diabetes and Pregnancy Unit, women are instructed on how to perform self-monitored blood glucose. First line therapy is MNT based on a MedDiet in combination with an active lifestyle (Table 1). We establish therapeutic targets as fasting/preprandial <94mg/dL and 1-h postprandial glucose <140mg/dL. However, ideal glycaemic control is established at <90 and <120mg/dL, respectively. If women cannot achieve glycaemic goals with dietary and lifestyle modifications, insulin therapy is initiated. This is when >50% of fasting/preprandial and/or 1-hour postprandial glycaemia's exceed the glycaemic targets. A summarized description of GDM management is shown in Fig. 1.
Lifestyle and medical nutrition therapy guidelines used in GDM treatment.
| STAY ACTIVE: walking at least 15-min strolls and/or climbing stairs at least 5 times a week (4 floors, 4 times/day), if possible. |
| Using extra-virgin olive oil as the main fat source (daily); if possible, use it exclusively. |
| ≥40ml/day of extra-virgin olive oil. |
| One serving of vegetables with every main meal (lunch and dinner). |
| Three servings of fruit: fresh fruits instead of artificial and fresh juices. |
| One handful of nuts a day (mainly pistachios) instead of processed snacks. |
| Whole-wheat cereals instead of refined grains and potatoes. |
| Skimmed instead of full-fat dairy products. |
Sometimes, GDM diagnosis coexists with the mother being overweight or obese. High maternal body mass index can also implicate maternofoetal complications, independent to GDM. This raises the question of whether maternofoetal complications could be reduced in overweight/obese pregnant women who are also being treated for GDM.
We conducted a study to determine whether women with excessive weight (overweight and obesity) diagnosed with GDM – subjected to a specific intervention and follow-up – had lower risk for maternal and neonatal complications compared to those with excessive weight who did not have GDM (who only received standard medical care). We hypothesized that the negative impact that excessive weight has on maternofoetal adverse events could be significantly lowered in those women who also have GDM, properly treated.
A total of 3312 pregnant women receiving prenatal medical care at the Hospital Clínico San Carlos, who were screened for GDM at 24–28 weeks of gestation between April 2011 and March 2013, were included in this study. Women were categorized into three groups according to prepregnancy body mass index: normal-weight women <25kg/m2 (2398/72.4%), overweight women 25–29.9kg/m2 (649/19.6%) and obese women ≥30kg/m2 (265/8%).19 Since there was a low incidence of overweight and obese women, both groups were clustered in a group named “excess weight women”. A multivariable logistic analysis was performed to determine the influence of the presence or absence of GDM coexistent with high prepregnancy body mass index in the development of adverse pregnancy outcomes. Odds ratio for excess weight women were significantly higher for prematurity (p<0.0001), admission to neonatal ICU (p<0.0001), caesarean delivery (p<0.001) and instrumental delivery (p<0.026). Surprisingly, this tendency was mainly observed in excess weight women with no GDM. In excess weight women with GDM adverse outcomes were similar to those found in the reference group (normal-weight women without GDM), finding no association with the risk of prematurity, caesarean section or instrumental delivery. Moreover, normal-weight women with GDM had a significantly lower risk of admission to neonatal ICU (p<0.03) and C-section (p<0.001), in comparison to normal-weight women without GDM. This indicates that treatment of GDM seems to mitigate the maternofoetal health disadvantages associated with excess weight. In addition, normal-weight women with treated GDM had significantly lower rates of admission to neonatal ICU and caesarean delivery in comparison to those who did not have GDM.
MedDiet to treat GDM. Does it work?After observing these results, we sought to analyze the benefits of using a MedDiet-based MNT. Therefore, we aimed to compare maternofoetal health of women with GDM treated with a MedDiet -based MNT versus women with normal glucose tolerance.18 Results revealed that at 36–38 gestational weeks, roughly 3 months after treatment, mean values of HbA1c and HOMA-IR were similar between women with and without GDM. Adjusted odds ratio analysis of maternal and neonatal outcomes showed that women with GDM compared with normal glucose tolerance had an increased risk for insufficient weight gain, urinary tract infections, small-for-gestational-age new-borns and neonatal ICU admissions. There were no differences in other adverse events such as c-section, prematurity, urinary tract infections and large-for-gestational-age new-borns.
A recent systematic review and meta-analysis analyzed different diet interventions used in GDM treatment as compared to standard diets on maternal glycaemic control and adverse events.20 The Dietary Approaches to Stop Hypertension (DASH) and low glycaemic index diets were associated with decreases in fasting and postprandial glucose and lower weight gain. The DASH diet was also associated with significantly lower need of medication, birth weight and macrosomia. No significant results were observed in terms of preeclampsia, neonatal hypoglycaemia, prematurity, admission to neonatal ICU and small-for-.gestational-age new-borns for any of the diet interventions included. Analysis of the Mediterranean diet was not included in this analysis because no study groups have used this type of diet as their medical nutrition therapy in GDM treatment.
Results from our study indicate a high fat/low carbohydrate diet – like the MedDiet – is adequate to treat GDM. However, more research needs to be done in this area to confirm our findings.
What can be done to prevent GDM onset?In finding strategies to prevent GDM, researchers have focused their attention on managing modifiable risk factors such as maternal weight, lifestyle and dietary patterns. Numerous studies have evaluated the effect of specific dietary patterns and lifestyles in the risk of developing GDM.
Prevention of GDM with a Mediterranean diet interventionTo evaluate dietary patterns associated with GDM we conducted a retrospective study from April 2011 to March 2013.21 This study included a cohort of women identified Carpenter–Coustan criteria and IADPSG criteria. A reduced risk of GDM was associated with the following dietary patterns: intake of nuts >3 times (p=0.015), refined cereals ≤1 serving (p=0.003), juices <4 servings (p=0.017), cookies and pastries <4 servings (p=0.003), as compared to opposite habits. This was observed in women diagnosed by IADPSG criteria; however, in those identified by Carpenter–Coustan criteria, there were no significant results. This reinforces the importance of using the former rather than the latter criteria.
These results led us to conduct a randomized controlled trial to evaluate whether following a MedDiet during early pregnancy could prevent GDM. Therefore, the “St. Carlos GDM prevention study” was performed.22 The primary outcome was to compare the effect of a standard diet versus a MedDiet – supplemented with extra virgin olive oil (EVOO) and pistachios – on GDM incidence at 24–28 gestational weeks. This was performed in pregnant women with normal fasting glucose (<92mg/dL) at the first gestational visit (8–12 gestational weeks).
Women in the control and intervention groups received the following dietary guidelines: ≥2 servings/day of vegetables, ≥3 servings/day of fruit (avoiding juices), 3 servings/day of skimmed dairy products, wholegrain cereals, 2–3 servings of legumes/week, moderate to high consumption of fish; a low consumption of red and processed meat, avoidance of refined grains, processed baked goods, pre-sliced bread, soft drinks and fresh juices, fast foods and precooked meals. The intervention group received recommendations by a dietitian in a 1-h group session, one week after inclusion (12–14 gestational weeks). The key recommendation was to consume daily≥40ml of EVOO and a handful (25–30g) of nuts. To ensure compliance, participants were give 10L of EVOO and 2kg of roasted pistachios at the beginning of the first and second trimester. Women in the control group followed recommendations given in regular clinical practice by midwifes, were consumption of dietary fat, including EVOO and nuts, is restricted.
The incidence of GDM was significantly lower in the intervention group as compared to the control group (17.1% versus 23.4%, p=0.012). They also had a significantly lower incidence of episodes of urinary tract infections, emergency C-sections, perineal trauma as well as a significant reduction in the rates of prematurity new-borns large-for-gestational-age and small-for-gestational-age. Multivariable logistic regression analysis was used to evaluate the effect of the intervention on GDM. The crude relative risk for GDM was of 0.73 (95% CI: 0.56–0.95) and of 0.75 (95% CI: 0.57–0.98) adjusted for all confounding variables. This shows that an early dietary intervention based on a MedDiet, enriched with EVOO and pistachios, seems to reduce the incidence of GDM and improve several maternofoetal outcomes.
Various randomized controlled trials have been conducted, setting as a primary outcome the prevention of GDM. Lifestyle and dietary changes, as well as weight gain control have been the focus of these interventions. Most of these randomized controlled trials have been conducted in high risk women. Some researchers have found the intervention to be effective while other have not.19,23,24 A recent metanalysis analysing the effectiveness of lifestyle interventions in the prevention of GDM has concluded that for the intervention to be successful, high-risk women should be targeted.25 However, results from the St. Carlos GDM Prevention study showed that prevention of GDM is possible even in low risk women. This heterogeneity is due to differences in interventions used, characteristics of the studied sample and moment of implementation of the intervention. Most studies agree that early intervention should be provided to improve maternofoetal health.25,26 Moreover, while there is inconclusive evidence as to what dietary interventions are more appropriate in preventing GDM, it seems that the MedDiet pattern is the most protective against it.
A very recent study27 showed that an early, easy-to-apply, universal nutritional intervention (also based on a Mediterranean diet rich in EVOO and nuts) applied in a real-world clinical setting attained similar rates of GDM from those previously reported in the St. Carlos GDM Prevention study,22 without having to provide the EVOO and nuts. Moreover, perinatal adverse outcomes associated to GDM were similar between women with and without GDM.
Importance of the Mediterranean diet in low-risk pregnanciesA healthy nutrition during pregnancy is important for both women of high and low risk. It is unknown which dietary pattern is more appropriate in pregnant normoglycemic women – those who do no develop GDM at any point. Therefore, we conducted a sub analysis to compare maternofoetal outcomes of normoglycemic pregnant women who followed a MedDiet supplemented with EVOO and pistachios versus women who followed a standard diet.28 Standard guidelines provided in regular clinical practice are based on a MedDiet but limit total fat consumption. Outcomes like GDM, urinary tract infections, prematurity, hypertensive disorders (pregnancy induced hypertension and preeclampsia), emergency c-sections, perineal trauma, large-for-gestational-age and small-for-gestational-age new-borns were evaluated. A composite of maternofoetal outcomes was also evaluated. This was defined as having at least event of emergency caesarean section (C-section), perineal trauma, pregnancy-induced hypertension and preeclampsia, prematurity, large-for-gestational-age, and small-for-gestational-age. Crude relative risk analysis of maternal and neonatal outcomes showed that the intervention was associated with a significant reduction of the risk of having a composite of maternofoetal outcomes (0.47 (0.35–0.64)). The number-needed-to-treat (NNT) was five. The intervention was also associated with a significantly lower risk of having urinary tract infections (0.37 (0.20–0.66); NNT=14), emergency C-sections (0.28 (0.13–0.64); NNT=20), perineal trauma (0.22 (0.12–0.41); 10), large-for-gestational-age (0.25 (0.07–0.90); NNT=40) and small-for-gestational-age new-borns (0.26 (0.08–0.80); NNT=33)
From this study we concluded that even women at low risk can benefit from adhering to nutritional recommendations based on a MedDiet enhanced with EVOO and nuts in pregnancy. This was associated with a reduction in over 50% the risk of developing at least one event of a composite of maternofoetal outcomes in normoglycaemic women. Current recommendations limiting fat consumption during pregnancy should be reconsidered. Recommendations based on a MedDiet that liberalizes EVOO and nut consumption (mainly pistachios) should be considered an adequate dietary pattern to follow during pregnancy.
Six food targets to prevent GDM.Lastly, we sought to evaluate the effect of late first-trimester degree of adherence to a MedDiet pattern – using six specific food items – on maternofoetal complications. This was a posthoc analysis of the “St. Carlos GDM Prevention Study” cohort.29 The six food targets chosen were intake of EVOO >6 times/week and ≥40ml/day, >3 servings/week of nuts, >12 servings/week of vegetables (raw or cooked), >12 servings/week of whole fruits (excluding fresh juice), <2 servings/week of juice (fresh or bottled). The first three items are tools used to ensure an appropriate compliance to the MedDiet. The last three items are elements that were scored with slightly different criteria to those used in the Mediterranean Diet Adherence Screener (MEDAS) questionnaire. In this questionnaire, in the “vegetable servings” item, 1 point is scored if ≥2 servings/day are consumed, of which ≥1 should be raw or in the form of salads. For “fruit units”, 1 point is scored if ≥3 units are consumed per day, including natural fruit juice. However, in our study we considered vegetable consumption independent of being raw or cooked, fruit consumption did not include natural fruit juice and consumption of juices (bottled or fresh) were scored negatively. The MEDAS and Nutrition Scores were also evaluated per group of adherence.
The sample was stratified into three groups according to degree of adherence to these six food items in late first trimester (from 12–14 to 24–28 gestational weeks). A high adherence was set for achieving 5–6 targets; a moderate adherence for achieving 2–4 targets; and a low adherence for achieving 0–1 targets.
The primary endpoint for this post hoc analysis was to analyze the associations of late first-trimester degrees of adherence to the six food targets with the risk of GDM and a composite of maternofoetal outcomes (CMFO) defined as at least having one event of emergency C-section, perineal trauma, pregnancy-induced hypertension and preeclampsia, prematurity, large-for gestational-age and small-for gestational-age. Secondary outcomes were to analyze maternofoetal complications individually.
In the high adherence group, there was a total of 115 (13.1%) women, 623 (71.3%) in the moderate adherence group and 136 (15.6%) in the low adherence group. Results indicated that there was a linear association between the degrees of adherence and the MEDAS (r=0.760; p<0.001) and Nutrition scores (r=0.707; p<0.001). A crude logistic regression analysis was performed to analyze the risk of having maternofoetal complications according to the degree of adherence. The higher the adherence, the lower the incidence and risk of GDM, urinary tract infections, prematurity, small-for-gestational-age new-borns, and composite of maternofoetal outcomes (p-trend all<0.01).
This study suggests that the higher the adherence to six food targets of the MedDiet, the lower the risk of developing GDM, composite of maternofoetal outcomes, urinary tract infections, prematurity and small-for-gestational-age new-borns. Moreover, the six food targets used to evaluate healthy eating patterns seem appropriate to evaluate adherence to a MedDiet.
The results reinforce the importance of providing early nutritional education to pregnant women. Moreover, adhering to a MedDiet – liberalizing the consumption of EVOO and nuts – improves maternofoetal outcomes in women of high and low risk.
Future. What next?Nutrition during pregnancy may have important implications on the long-term health of the mother and their infant. It is unknown whether there is a beneficial impact on the overall health status and future risk of type 2 diabetes mellitus, metabolic syndrome, and cardiovascular diseases in both women and their offspring.
We are currently studying postpartum health of the sample of 874 women included in the “St Carlos GDM Prevention Study”. Our next steps are to evaluate whether the mechanisms underlying the protective effect of the MedDiet pattern include factors like adipokines and miRNAs, that have been associated with GDM development. The analysis of these parameters could provide new biomarkers that can have a certain involvement and application in the diagnosis and prognosis of GDM.
Cytokines such as TNF-α e IL-6 and adipokines such as leptin and adiponectin have been associated with the expression of GDM.30–33 Moreover, specific miRNAS have been found to be present in significantly higher concentrations in women with GDM versus women without GDM.34
Therefore, we will analyze the expression of cytokines (TNF-α and IL-6), adipokines (leptin and adiponectin), miRNAS in the first trimester of gestation, at 24–28 gestational weeks and at three years postpartum. We will also study associations between FTO-rs9939609, MC4Rrs17782313, TCF7L2-rs7903146, CLOCK-rs4580704, GIPR rs10423928, GCKR rs780094, MTNR1B rs10830963 and ADIPOQ rs266729 polymorphisms with GDM and their potential modulation through a MedDiet. There is evidence of gene-lifestyle interaction and there could be a possible modulation of GDM with the MedDiet. These data will be presented at next ADA meeting. Moreover, we will evaluate the impact of maternal diet on maternal health at 3 months postpartum and at 2–3 years postpartum. The health of their offspring will also be evaluated.
ConclusionsOur research experience has lead us to conclude that using IADPSG criteria to diagnose GDM is beneficial to improve maternofoetal health in pregnancy. These criteria are not only useful in identifying women at risk but also enable the application of successful preventive strategies. Using a MedDiet to treat and prevent GDM is an appropriate approach. Using a medical nutrition therapy improves maternal glycaemic control and GDM-related complications. Moreover, providing an early nutritional intervention based on MedDiet principles have shown to reduce GDM risk in 30%. Other randomized controlled trials are needed to support these results. However, our findings are encouraging.
We are hopeful to attain novel findings in the analysis of the potential modulation of the MedDiet on GDM-related polymorphisms and the effect of maternal diet on offspring's health at 2–5 years.
FundingThis work was supported by the PI14/01563 project, integrated in the Plan Nacional of I+D+D, AES 2013_2016 (funded by the Instituto de Salud Carlos III (ISCIII) of Spain)) and co-funded by Fondo Europeo de Desarrollo Regional (FEDER) and Sociedad de Endocrinología Nutrición y Diabetes de la Comunidad de Madrid (SENDIMAD) (IPI/2017/NR2) available at http://www.sendimad.org/nuevasendimad/ayuda-a-la-investigacion-historico-premiados. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. These were responsibilities of the authors alone and independent of the funders.
Conflicts of interestThe authors declare no conflict of interest.
We wish to acknowledge the kind collaboration of: IRIAF—Instituto Regional de Investigación Agroalimentaria y Forestal de Castilla La Mancha (C.I.A. El Chaparrillo, Ciudad Real), Olive Oils from Spain (www.oliveoilsfromspain.org – Organización Interprofesional del Aceite de Oliva Español).
Endocrinology (Alfonso L Calle-Pascual, Nuria Garcia de la Torre, Alejandra Durán, Inés Jiménez, Miguel Ángel Rubio); Obstetrics Unit (Miguel Ángel Herraíz, Nuria Izquierdo, Noelia Pérez); Nurses (Amparo Sabaté Garcia y Georgina Cutillas Dominguez); Laboratory Unit (María José Torrejón, María Ángeles Cuadrado); Dietiticians (Carla Assaf-Balut, Laura del Valle); Analysys of samples (Elena Bordiú, Johanna Valerio, Ana Barabash); and administrative staff, nurses (Marisol Sánchez Orta, María Victoria Sáez de Parayuelo, Luzdivina Fernandez Muñoz, and Félix Calzada). All members of St Carlos Study Group have read and agreed with the content of the last version of manuscript. Each member named has participated actively and sufficiently in the case reported and fulfilled all conditions as authors.