To study the incidence of type 1 diabetes (T1D) in children <14 years in the island of Gran Canaria (Canary Islands, Spain) during the 2006–2018 period and to evaluate its temporal trend, seasonality, age and sex distribution.
Subjects and methodsWe studied children <14 years of age living in Gran Canaria. We calculated the annual and overall incidence using recorded data from the Pediatric Endocrinology Department as the primary source and the local Diabetes Association and the hospital's pharmacy as secondary sources. The primary source is the only paediatric endocrine unit in the island.
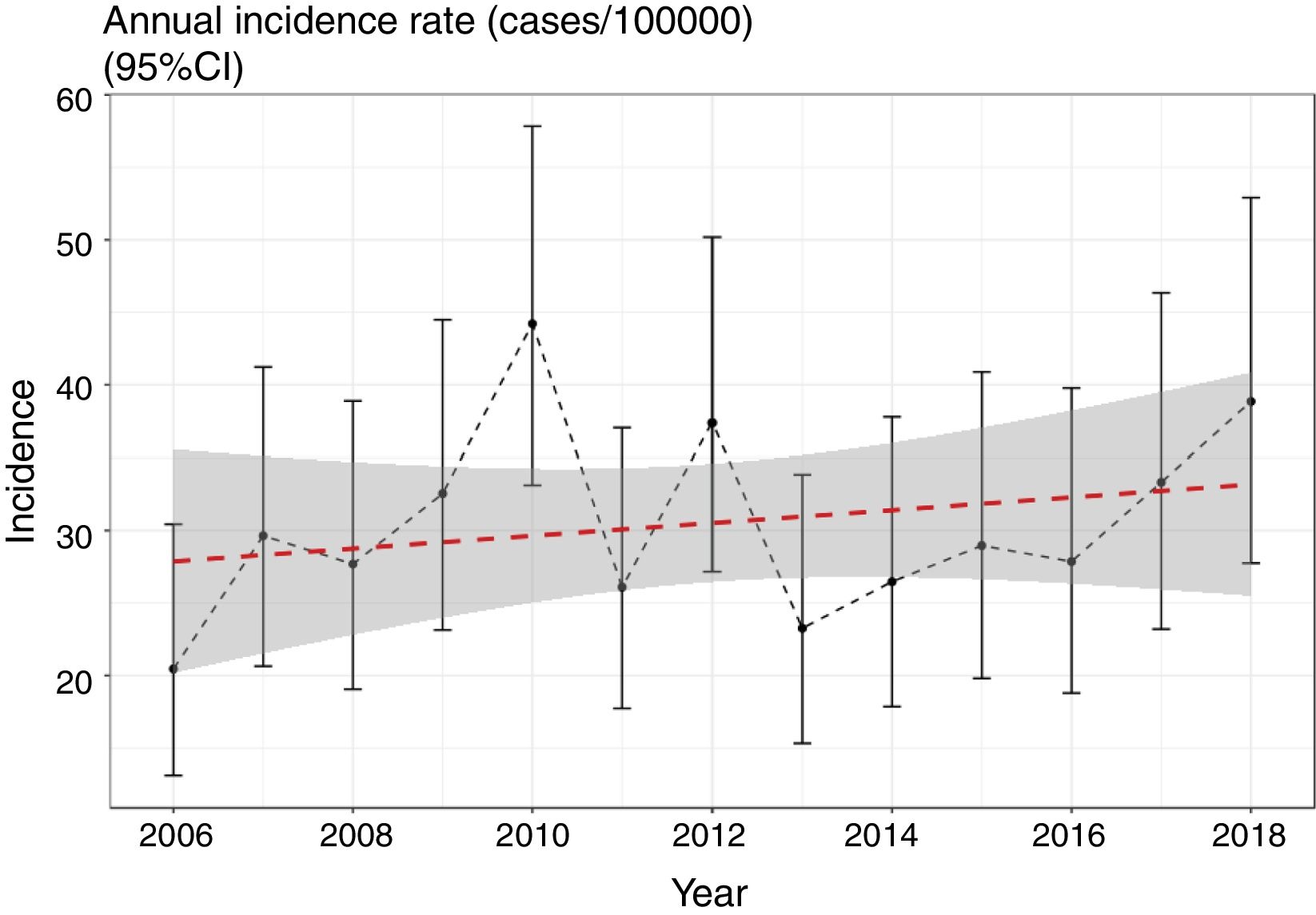
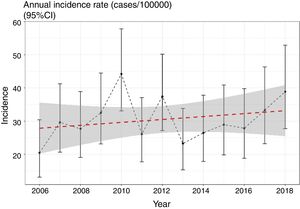
Results453 new T1D cases were observed during the 13-year period. The overall incidence of T1D between 2006 and 2018 was 30.48/100,000 (95% CI: 27.74–33.42). Distribution among age groups was 24.8%, 38.2% and 36.9% for children between 0–4, 5–9 and 10–13.9 years old respectively. No significant temporal trend, seasonality or sex differences were found.
ConclusionsOur study shows that the Island of Gran Canaria has one of the highest childhood incidences of T1D reported worldwide: among the highest rates in Europe, and higher than the rates published for the neighbouring African countries.
Estudiar la incidencia de diabetes mellitus tipo 1 (DM1) en niños menores de 14 años en la isla de Gran Canaria (Islas Canarias, España) durante el período 2006-2018, así como evaluar su tendencia temporal, estacionalidad y distribución por sexo y edad.
Sujetos y métodosLos sujetos objeto de estudio fueron los niños menores de 14 años que habitan la isla de Gran Canaria. Calculamos la incidencia para todo el período, y la incidencia anual usando los datos recogidos en nuestra unidad de endocrinología pediátrica como fuente primaria y los datos de la asociación local de diabetes y la farmacia hospitalaria como fuentes secundarias. La fuente primaria es la única unidad de endocrinología pediátrica de la isla.
ResultadosObservamos un total de 453 nuevos casos de DM1 durante el período de estudio. La incidencia global para el período 2006-2018 fue de 30,48/100.000 (IC 95%: 27,74-33,42). La distribución por grupos de edad fue del 24,8, 38,2 y 36,9% para niños entre 0-4, 5-9 y 10-13,9 años de edad, respectivamente. No encontramos la aparición de ninguna tendencia temporal significativa. Tampoco encontramos la presencia de estacionalidad ni diferencias significativas en cuanto a la aparición de DM1 en base al sexo.
ConclusionesNuestro estudio muestra que la isla de Gran Canaria presenta una de las incidencias de DM1 más altas del mundo. Se encuentra entre las más altas de Europa, y es claramente superior a la publicada para los países vecinos africanos.
Type 1 diabetes (T1D) is the most frequent type of diabetes found in children. Its development is greatly influenced by individual genetic susceptibility1 and not fully understood environmental factors, including (but not limited to) feeding practices, vitamin D levels and infectious diseases.2 Reports from the last 20–30 years reflect wide geographical variation, as shown by international registries (mainly EURODIAB,3 the SEARCH study4 and DIAMOND5) as well as national, regional or locally based publications. Annual incidence rates range from 0.1/100,000 in China to over 60/100,000 in Finland.6 The highest incidences to date are reported in Finland, Sweden (44/100,000)7 and the Italian island of Sardinia (40/100,000).8 Temporal fluctuation in the incidence has also been reported, showing an annual increase in incidence during the last decades of 1.8% in the US4 and 3.4% in Europe,3 though this rate is not uniform. Some countries seem to show a stable incidence or even a decrease since 2000–2005.3,9 In addition, analysis of data from DIAMOND and EURODIAB revealed seasonal variation in incidence, with most cases appearing in autumn and winter.10
Regarding Spain, the country lacks a national registry, even though regions like Andalucía, Aragón, Cataluña, Madrid and Navarra have their own. The Catalan registry has been the Spanish reference in DIAMOND and EURODIAB, reporting an annual incidence of 12–13/100,000 over the last 24 years. Overall, child incidence in the country is reported to be 17.7cases/100,000,11 although wide geographical variation exists, with rates ranging from 7.8/100,000 in the Balearic islands to 32/100,000 in the island of La Palma (Canary Islands).12 Increasing incidence has been reported in some regions but, with current data, this cannot be considered a generalized trend.13
We present the incidence of childhood-onset T1D in the island of Gran Canaria during a 13-year period (2006–2018). We evaluated its distribution by sex and age groups, its temporal trend, the presence of seasonality and the influence of environmental factors such as flu.
Patients and methodsGran Canaria has a population of 846,717 inhabitants, about 40% of the population of the archipelago. It is one of the 8 Canary Islands (Spain), located in the Atlantic Ocean (28°N, 15°35′W), about 100km off the African coast. Given its geographical location, the island has great climatic stability throughout the year.
Ours is the only centre with a Pediatric Endocrinology Unit in the island and all patients with suspected or diagnosed T1D were referred to us at the time of onset. Since 2014, a private hospital also admits new onsets, and has received 7 of the new cases during the last 5 years. To the best of our knowledge, we had the complete census of children diagnosed with T1D in the island during the given period. Nevertheless, we contrasted our data with information obtained from the hospital's pharmacy (new insulin prescriptions) and from the local Diabetes Association as described by the capture-recapture method.14
Inclusion criteriaWe included all patients who had been living on the island for at least 6 months at the time of diagnosis during the 2006–2018 period. Records of new T1D patients for each year are kept in our Unit since 2006. We included patients younger than 14 years of age because, given our health system, paediatric hospitals only receive children <14 years of age. Diagnosis was made according to American Diabetes Association (ADA) criteria.15 The date of the first insulin dose was considered as the date of onset. Data from the patients’ charts were collected retrospectively. Incidence was calculated as the number of new cases per year for every 100,000 inhabitants below 14 years of age. For some children whose onset was in 2006, we lacked access to the month of diagnosis. Thus, seasonality was assessed for 2007–2018 only.
Data sourcesCensus data were provided by the Canarian Institute of Statistics. Yearly flu incidence was obtained from the Office of Epidemiology and Prevention of the Department of Public Health of the Canary Islands. Geographical distribution was assessed by classifying cases by municipality and evaluating possible differences in incidence throughout the island.
Statistical analysisData at onset were described using basic descriptive tools (mean, standard deviation, percentages).
Yearly incidence was computed by dividing the number of cases by the total population for the age group and adjusting for 100,000 children at risk. Incidence for the 13-year period was computed by dividing the total number of cases by the total number of children at risk for the whole period and adjusting for 100,000 children. Age-standardized rates were computed using the standard European population distribution published by the WHO.16 95% confidence intervals (CI) were calculated for all incidence rates.
Temporal trend analysis was performed using Poisson regression. Chi-squared was used to assess the differences in cases across age groups. Seasonality was assed using Edward's test. Correlation analysis was performed to assess the potential associations between the number of new T1D cases and flu incidence. The correlation was only analyzed from 2006 to 2013 because we only had access to data related to the declared cases of flu for those years. A two-tailed p<0.05 was considered significant.
Chi-squared was used to assess differences in the distribution of cases in the different municipalities.
SPSS 22 (IBM Corp., Released 2013, IBM SPSS Statistics for Windows, Version 22.0, Armonk, NY: IBM Corp) and computing environment R v3.0.2 (R Foundation for Statistical Computing, Vienna, Austria) were used for the analysis.
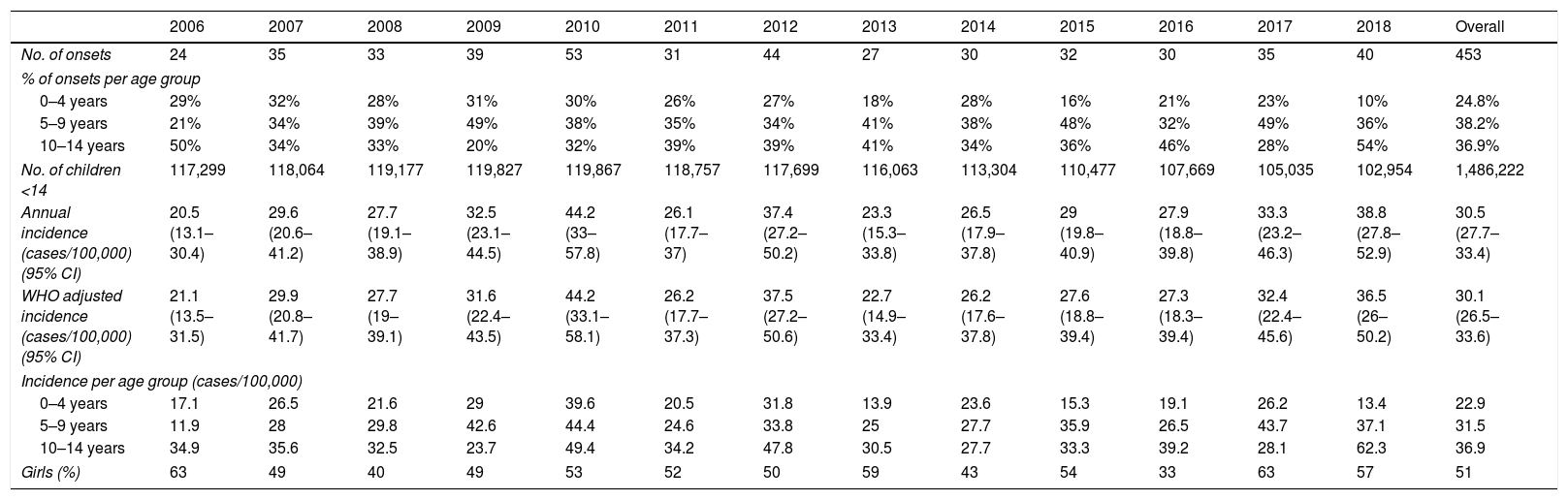
ResultsA total of 453 children presented with T1D in Gran Canaria during the 2006–2018 period. Annual and mean incidences (both crude and standardized), number of cases per year and number of children under 14 years of age in Gran Canaria are shown in Table 1. Secondary and tertiary sources did not provide any new cases: our Hospital's pharmacy list only had 69 new cases of diabetes (implying that their registry is incomplete) and the local diabetes association had less than half that number. All patients from the secondary and tertiary sources had already been included in our unit's registry.
Incidence of T1D in Gran Canaria (2006–2018).
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | Overall | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of onsets | 24 | 35 | 33 | 39 | 53 | 31 | 44 | 27 | 30 | 32 | 30 | 35 | 40 | 453 |
| % of onsets per age group | ||||||||||||||
| 0–4 years | 29% | 32% | 28% | 31% | 30% | 26% | 27% | 18% | 28% | 16% | 21% | 23% | 10% | 24.8% |
| 5–9 years | 21% | 34% | 39% | 49% | 38% | 35% | 34% | 41% | 38% | 48% | 32% | 49% | 36% | 38.2% |
| 10–14 years | 50% | 34% | 33% | 20% | 32% | 39% | 39% | 41% | 34% | 36% | 46% | 28% | 54% | 36.9% |
| No. of children <14 | 117,299 | 118,064 | 119,177 | 119,827 | 119,867 | 118,757 | 117,699 | 116,063 | 113,304 | 110,477 | 107,669 | 105,035 | 102,954 | 1,486,222 |
| Annual incidence (cases/100,000) (95% CI) | 20.5 (13.1–30.4) | 29.6 (20.6–41.2) | 27.7 (19.1–38.9) | 32.5 (23.1–44.5) | 44.2 (33–57.8) | 26.1 (17.7–37) | 37.4 (27.2–50.2) | 23.3 (15.3–33.8) | 26.5 (17.9–37.8) | 29 (19.8–40.9) | 27.9 (18.8–39.8) | 33.3 (23.2–46.3) | 38.8 (27.8–52.9) | 30.5 (27.7–33.4) |
| WHO adjusted incidence (cases/100,000) (95% CI) | 21.1 (13.5–31.5) | 29.9 (20.8–41.7) | 27.7 (19–39.1) | 31.6 (22.4–43.5) | 44.2 (33.1–58.1) | 26.2 (17.7–37.3) | 37.5 (27.2–50.6) | 22.7 (14.9–33.4) | 26.2 (17.6–37.8) | 27.6 (18.8–39.4) | 27.3 (18.3–39.4) | 32.4 (22.4–45.6) | 36.5 (26–50.2) | 30.1 (26.5–33.6) |
| Incidence per age group (cases/100,000) | ||||||||||||||
| 0–4 years | 17.1 | 26.5 | 21.6 | 29 | 39.6 | 20.5 | 31.8 | 13.9 | 23.6 | 15.3 | 19.1 | 26.2 | 13.4 | 22.9 |
| 5–9 years | 11.9 | 28 | 29.8 | 42.6 | 44.4 | 24.6 | 33.8 | 25 | 27.7 | 35.9 | 26.5 | 43.7 | 37.1 | 31.5 |
| 10–14 years | 34.9 | 35.6 | 32.5 | 23.7 | 49.4 | 34.2 | 47.8 | 30.5 | 27.7 | 33.3 | 39.2 | 28.1 | 62.3 | 36.9 |
| Girls (%) | 63 | 49 | 40 | 49 | 53 | 52 | 50 | 59 | 43 | 54 | 33 | 63 | 57 | 51 |
We evaluated the existence of linear and nonlinear trends in the number of cases throughout the 13-year period. We found a non-significant trend towards a linear increase in the number of cases per year of 1.39% (95% CI: −1% to 3.8%) (p=0.423) (Fig. 1), still present after adjusting for overdispersion (z=0.7; p=0.22). We should mention, however, the low power of the model, at only 11.2%. We did not find any nonlinear trends (p=0.12) in the incidence rates throughout the period.
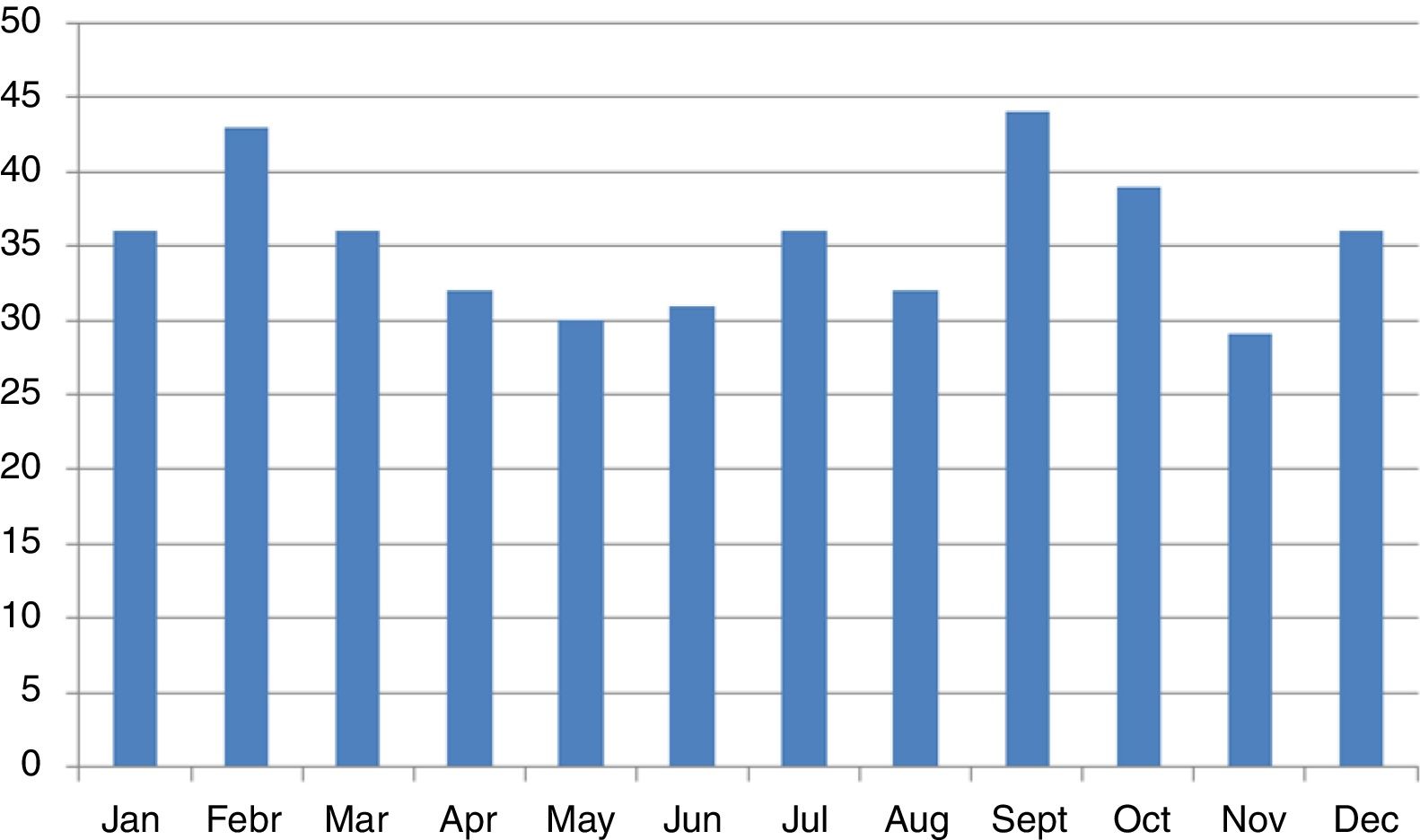
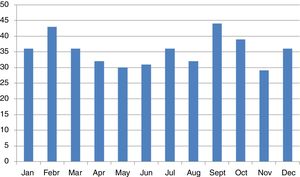
We did not find any significant differences in sex or age group distribution at onset either (Table 1). No significant seasonality was observed after applying Edward's test (Fig. 2) (Edward's test statistic=1.007; p=0.6).
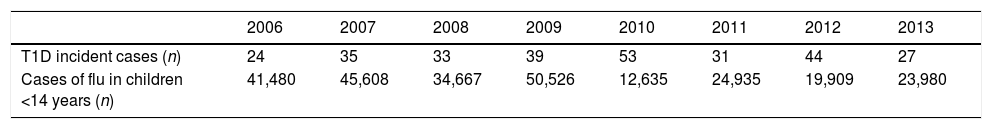
We found a positive, non-significant correlation between the new cases of T1D and the cases of flu the previous year, but not with the incidence of flu the same year (Tables 2 and 3).
Finally, no significant differences were found among the incidence rates in the different municipalities (X2=9.6; df=10; p=0.48).
DiscussionOur data confirm the high incidence of T1D previously described in the Canary Islands.12,17 At 30 cases/100,000, it is much higher the mean (17.7/100,000) and highest (27.6/100,000) incidences reported for the rest of the country11 (even though there is some overlap in the CIs, it is worth mentioning that the regions with the highest reported incidence, only reported one year values, making it less likely that the value is representative of the true incidence in the region). Results from the Type 1 Diabetes Genetics Consortium suggest that HLA does not seem to explain this elevated incidence, since high-risk haplotypes were similarly present in Canarian families of children with diabetes and those from the rest of Spain.18
Our incidence values places us immediately behind Finland, Sweden and Sardinia. We report similar incidence rates to those described in Northern Ireland (2004–2013),3 Saudi Arabia19 and Norway,20 and higher rates than those described for the rest of European3 countries. The Canary Islands and regions from southern Spain21 seem to be another exception to the north-south gradient described for the incidence of T1D in Europe.22 However, given that we measured the incidence in children aged less than 14 years of age and that EURODIAB, previous data from the Canary Islands, and average data for the rest of Spain were computed for children aged less than 15 years of age, it cannot be ruled out (even though it is unlikely) that the differences are a result of differences in the studied age groups.
When comparing with African countr ies, we report an incidence greater than that found in the continent (6.4/100,000), according to data published by the International Diabetes Federation (IDF).23 Of course, poor health systems and high infant mortality due to infectious diseases and conflict make it difficult to effectively determine the real number of incident cases in the majority of countries in the continent.
Outside Europe and Africa, somewhat lower estimates have been reported in non-Hispanic, white-population under 15 in the US (years 2002–2012)4 and in Australian paediatric population (years 2000–2011; 23.6/100,000).24
The large inter-annual variability found for Gran Canaria, with values ranging from 20.5 to 44.2/100,000, similar to that reported by Belinchón et al.12 for the island of La Palma (12.7 to 67.2/100,000 throughout the 15-year period) cast doubt on the reliability of reported incidences from isolated annual values, especially in small populations.
Our analysis shows no significant trend in the annual incidence of diabetes from 2006 to 2018. In the rest of Spain, reports are heterogeneous. Asturias, Salamanca, Valencia, País Vasco, Navarra, Malaga, Aragón and Cantabria report increasing trends (26.3% of the Spanish paediatric population lives in those regions), whereas data from the rest of the country does not. Overall, we cannot say that it is a generalized trend.
Similar heterogeneity is reported internationally. Patterson et al. report non-uniformity in rates of increase over Europe in a recent review of data from EURODIAB.3 Norway,20 Finland6 and Sweden7 report plateaus in their previously increasing trends after 2005. Similar findings have been reported in Australia, with the plateau occurring after 2003.24
We found no significant difference in incidence by sex, in line with data published from Australia9 or other international reviews.25 Some studies seem to show a slightly higher incidence in boys: in Massachusetts26 with children under 6 and in other reports from Italy8 and other parts of Spain.27
Regarding age distribution at onset, we found no statistically significant differences across the three age groups (Table 1). We chose those age groups to ensure comparability with international reports and to evaluate differences among them in our population.
Belinchón et al.12 reported similar findings for the island of La Palma. Studies with larger sample sizes, such as national registries from Finland, report greater incidences in children over 5 years of age.
We found no significant seasonality in the appearance of T1D, with small, non-significant peaks in September and February, in agreement with previous reports from the Canary Islands.12 International reports describe a non-generalized seasonal pattern in the distribution of cases,10 especially in areas of high incidence. The small number of cases reported in our study may have accounted for the lack of significance, although an effect of climatic stability allowing for less variability cannot be ruled out.
Viral infections have been previously described as a potential trigger of T1D2 and some reports mention increased numbers of T1D in children after H1N1 epidemics.28 We found an interesting, albeit non-significant, correlation between the number of new T1D cases and the cases of flu the previous year, which might be worth exploring further. Once again, we are limited by the low number of years analyzed. Kondrashova et al.,29 from the DIPP study group in Finland, concluded that in children with increased genetic susceptibility for T1D, influenza A infections were not associated with the development of islet autoimmunity. Studies in children with a different genetic predisposition to T1D are needed before any definitive conclusions can be reached. Like Fourlanos et al. concluded in their paper,30 “perhaps it is in children without high genetic risk where the environmental factors have a greater impact”.
The main strengths of our study are the high degree of ascertainment achieved and the inclusion of data from 13 consecutive years. On the other hand, the low number of cases, resulting from the small population size, limits the conclusions that can be drawn about age distribution, temporality, seasonality and association with flu. Another limitation is the low number of cases reported by the secondary and tertiary sources, which may result in us missing some cases and underestimating the true incidence in our population (even though we think it is not very likely). Trend analysis is also limited by the sample size, resulting in a small capacity to detect changes (in order to reach 70% power, we would need an interannual variation of almost 66%).
Further studies to investigate the role of genetic and environmental factors influencing the appearance of T1D in our region are currently under way.
FundingYNM was supported by research grants from “Colegio Oficial de Médicos de Las Palmas” and “Spanish Pediatric Endocrine Society” and AMW received research funding from Instituto de Salud Carlos III [PI 10/02310, PI 11/01441 and PI16/00587] during the performance of the study. Sandoz Farmacéutica S.A supported data collection.
Conflict of interestThe authors declare no conflict of interests. The funders did not participate in the conception, design, performance, reporting or publication of the study.
We would like to thank Lucas Fernando González, from the Office of Epidemiology and Prevention of the Department of Public Health of the Canary Islands, for providing the data regarding flu in Gran Canaria.