Accurate measurement of sex steroids, particularly testosterone and estradiol, is relevant for the diagnosis and treatment of a wide range of conditions. Unfortunately, current chemiluminescent immunoassays have analytical limitations with important clinical consequences. This document reviews the current state of clinical assays for estradiol and testosterone measurements and their potential impact in different clinical situations. It also includes a series of recommendations and necessary steps to introduce steroid analysis by mass spectrometry into national health systems, a methodology recommended for more than a decade by international societies.
La correcta aproximación clínica a un amplio grupo de situaciones depende en gran medida de la disponibilidad de resultados analíticos de esteroides sexuales que sean exactos y reproducibles, obtenidos con métodos con la especificidad y sensibilidad analíticas adecuadas. En este sentido, los inmunoanálisis quimioluminiscentes actuales presentan limitaciones analíticas con repercusiones clínicas importantes. El documento de posicionamiento revisa el estado actual en la estandarización de los métodos de medida de estradiol y testosterona y su repercusión en distintas situaciones clínicas. Se incluye asimismo una serie de recomendaciones a seguir para introducir en los sistemas nacionales de salud los análisis de esteroides por espectrometría de masas, metodología recomendada desde hace más de una década por las sociedades internacionales.
Sex steroids are responsible for an individual’s development and maturation, in addition to intervening in many other functions.1,2 An imbalance is associated with disorders such as abnormalities in metabolism and bone and the development or progression of certain tumours.
Despite how important they are, our ability to accurately measure sex steroid concentrations varies greatly according to the method.
The specificity, sensitivity, accuracy, precision and standardisation of the measurement of these hormones are extremely important for the management of a broad range of clinical situations. Obtaining reliable results has a huge impact on decision-making, helping avoid inaccurate diagnoses and the wrong treatment, and unnecessary follow-up.
In this document we discuss issues related to the measuring of sex steroids in terms of the different measurement methods and their impact on clinical practice, and we provide some recommendations agreed on by the Spanish Societies of Laboratory Medicine (Sociedad Española de Medicina de Laboratorio [SEQCML]), Endocrinology and Nutrition (Sociedad Española de Endocrinología y Nutrición [SEEN]) and Paediatric Endocrinology (Sociedad Española de Endocrinología Pediátrica [SEEP]).
Pre-analytical aspectsThe pre-analytical conditions in any measurement are extremely important, regardless of the measurement method and they must therefore be followed meticulously.
Testosterone secretion follows a circadian cycle, with maximum values at 8 a.m. and minimum values at 8 p.m. The variations can have a range of 36%,3 so the blood sample has to be obtained in the morning. Testosterone circulates mainly bound to sex hormone-binding globulin (SHBG, high-affinity, low-capacity binding) and albumin (low-affinity, high-capacity binding), and only a small fraction circulates freely, reaching the target tissues. This free fraction is transformed in the target cells into dihydrotestosterone, which, after binding to its cytosolic receptor, travels to the cell nucleus and binds to specific androgen response elements in the DNA. The free fraction is therefore more directly related to the action of dihydrotestosterone, and has greater importance clinically.
However, the correct measurement of free testosterone concentration is a challenge for clinical laboratories. Although there are commercial immunoassays, they generally have significant limitations, so their use for healthcare purposes is not recommended.4–6 The reference methods for measuring free testosterone are the ultrafiltration and equilibrium dialysis methods, which separate free testosterone from protein-bound testosterone prior to analysis by radioimmunoassay or mass spectrometry. However, these methods are technically very time-consuming and are not available in most clinical laboratories. Alternatively, the recommendation for the measurement of free testosterone is to calculate levels from equations using the concentrations of total testosterone, SHBG and albumin.7
Some 95% of circulating oestradiol is bound to SHBG. The blood concentration of oestradiol does not have a circadian rhythm but, like testosterone, it changes during the different phases of the menstrual cycle, so in women of childbearing potential, the sample for both hormones has to be taken in the early follicular phase of the menstrual cycle.
Challenges in analysis: advantages and limitations of immunoassaysOnce the correct pre-analytical phase is ensured, the clinical value of the laboratory results depends to a great extent on the use of an appropriate method. Serum measurements of sex steroids for clinical and/or research purposes pose significant analytical challenges. They include the great diversity of metabolites structurally similar (molecules derived from cholesterol) endogenously or which can also be administered exogenously, the existence of a wide range of concentrations of clinical interest and the presence of free circulating forms and circulating forms bound to transport proteins. Immunoassay methods are currently the most widely used for measurement, despite often being limited due to their susceptibility to any of these confounding factors, to the point that a consortium of a large number of American scientific societies has positioned itself in favour of replacing immunoassay with far more accurate analysis methods based on mass spectrometry.8,9 The United States Endocrine Society already positioned itself in 2007,8 recommending the use of mass spectrometry as the reference method, and the Centers for Disease Control and Prevention (CDC) subsequently began a project to standardise the measurement of testosterone and oestradiol concentrations, called the Hormone Standardization Program (HoST).10 Results from monitoring in recent years consistently show that, although there are improvements in some immunoassay methods, techniques based on mass spectrometry provide the best results.
Automated immunoassays have the advantages of being practical and allowing a large number of samples to be measured quickly and at low cost. However, they have limitations, such as: the lack of specificity of the antibodies, which can overestimate the true concentrations when measuring molecules structurally similar to the analyte; a greater risk of differences caused by the different matrix of the serum samples and calibrators; greater difficulty with the complete release of testosterone or oestradiol from SHBG; and, in general, limited sensitivity to effectively measure low concentrations of steroids.
One of the endogenous constituents implicated as possibly interfering with testosterone immunoassays and overestimating concentrations is dehydroepiandrosterone sulfate (DHEA-S).11 This possible overestimation is particularly critical in situations where we need to measure relatively low concentrations, as occurs, for example, in women and children, or in men undergoing follow-up for prostate cancer, in whom the testosterone concentration is used as a criterion for surgical or chemical castration.12 There are also cross-reactions with other endogenous metabolites, such as 11-ketotestosterone or 11-hydroxytestosterone, or with synthetic steroids, such as nandrolone, danazol and norethisterone.13 Of particular importance, in the case of oestradiol, is cross-reaction in the immunoassays with the aromatase inhibitor drugs themselves, such as exemestane14 (which also causes false elevations of androstenedione, due to molecular similarity), the efficacy of which is monitored precisely by the analysis of oestradiol. Cross-reactions have also been described with oestrogen receptor antagonists such as fulvestrant.15 More recently, it has been reported that oral oestradiol treatment can result in falsely decreased oestradiol concentrations when measured by immunoassay.16 Specific interference and the degree of that interference in the analysis of sex steroids depends on the antibodies used in the particular immunoassay, with significant differences between manufacturers.
Benefits of mass spectrometryIdeally, analytical measurement of these compounds should be based on methods which are not susceptible to interference by other analytes (for example, new treatments with monoclonal antibodies, biological drugs) with a similar structure or which interfere with the measurement procedure, while at the same time have sufficient sensitivity to quantify low concentrations, such as those expected in certain physiological situations (as in paediatric samples and samples from women) or pathological situations.
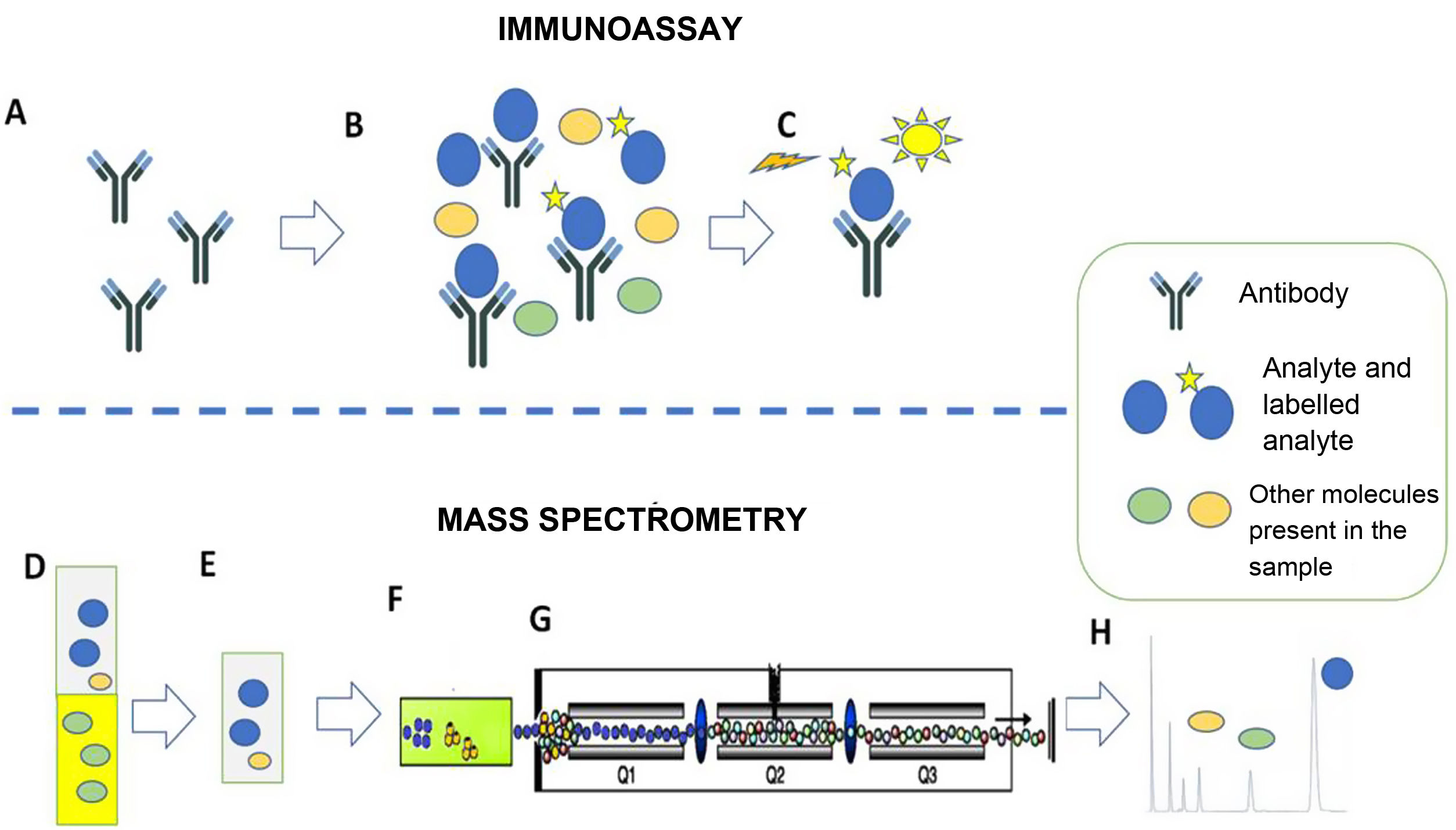
In this context, the emergence of mass spectrometry makes it possible to overcome the limitations of immunoassays in a large majority of clinical scenarios. The fundamentals of the two methods (immunoassay and mass spectrometry) are different. Effectively, although there is a great diversity of laboratory methods using immunoassays and mass spectrometry, the differences can be summarised as the fact that immunoassays are based on antigen-antibody reactions, while mass spectrometry does not use antigen-antibody reactions and is directly related to the structural characteristics (mass and mass spectra) of the analytes (Fig. 1).
Immunoassay (A–C). Example of competitive chemiluminescent immunoassay. A) The method is based on a specific antibody that recognises the analyte of interest. B) The antibody is incubated with the sample (containing the analyte of interest and other molecules) and with the labelled analyte. Analyte and labelled analyte compete for binding to the antibody. C) The chemiluminescent signal from the labelled analyte bound to the antibody is recorded. In the example, the recorded signal will be inversely proportional to the amount of analyte originally present in the sample.
Mass spectrometry (D–H). Example of liquid chromatography with tandem mass spectrometry (LC-MS/MS). D,E) Extraction of the serum sample with organic solvent. This step cuts out possible interferers. F) Separation of sample components by liquid chromatography. G) Selection of the specific ions of the analytes. H) Representation of the results. The area of the chromatographic peak is directly proportional to the amount of analyte originally present in the sample.
The importance of the need to use methods with adequate analytical behaviour for hormone measurements is also reflected in the growing consensus in the high-impact scientific journals on their requirements to accept publications. The Journal of Clinical Endocrinology and Metabolism published an editorial in which it demanded, from 2015 onwards, the use of mass spectrometry in studies in which the sex steroid results were an important endpoint.17 Shortly after, the same journal published a Letter of Concern addressing the complexity of the issue18 and set out instructions for authors on the requirements for steroid hormone measurements.19
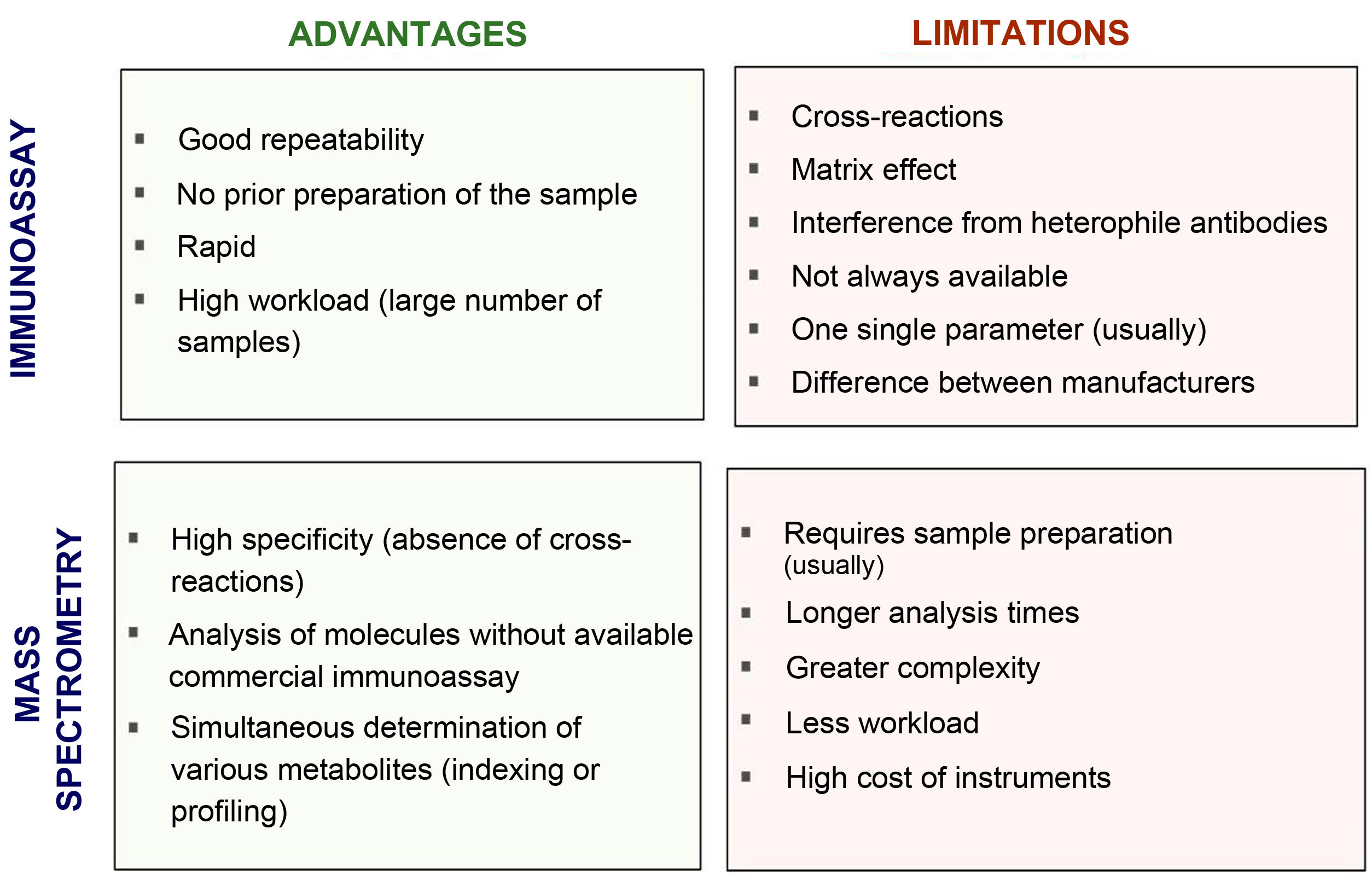
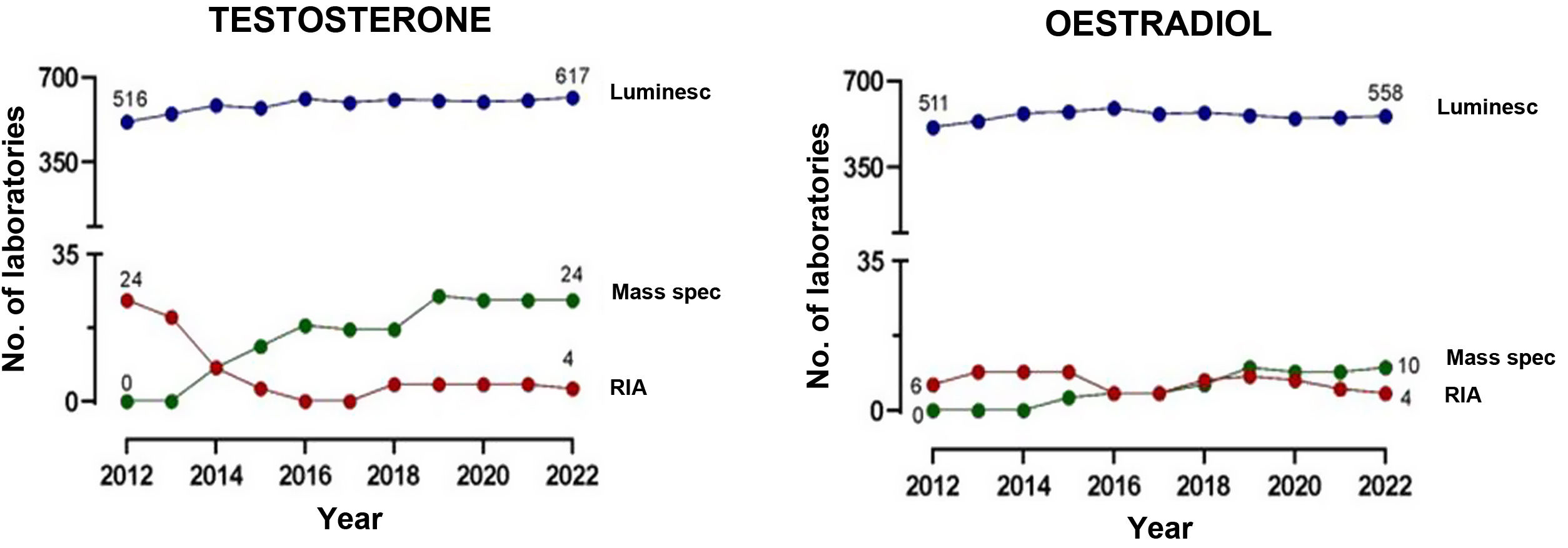
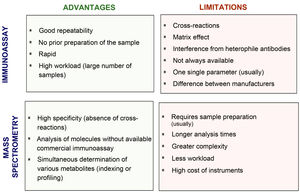
Fig. 2 compares the advantages and disadvantages of commonly used chemiluminescence immunoassays with methods based on mass spectrometry. Although immunoassays have good repeatability and speed, which allows high workloads to be handled adequately, mass spectrometry has a high degree of analytical specificity (absence of cross-reactions by endogenous or exogenous metabolites; for example, drugs13–15), less matrix effect and no interference from heterophile antibodies. Other advantages of mass spectrometry include its versatility (for example, almost any metabolite can be measured) and the ease of simultaneous measurement of several metabolites (profiles or panels can be created from the same sample). In addition, given its high specificity, inter-laboratory variability is minimised, facilitating the development of common reference intervals or cut-off points for medical decisions between laboratories. However, mass spectrometry is relatively expensive instrumentation and generally requires prior preparation of the sample, which usually makes it more complex (requires staff with good technical training) and means a longer response time. This probably limits large-scale introduction in clinical laboratories. From analysis of external quality control programmes at a European level, we can see that they have gradually been incorporated into clinical reference laboratories in Europe in recent years, with most of these laboratories continuing to use the reference methods using radioimmunoassay with extraction (Fig. 3). Here in Spain, mainly in the last three to four years, we have started to see mass spectrometry methods being introduced for measurement of hormones other than sex steroids in clinical practice, with a number of laboratories managing to acquire instrumentation dedicated to hormones.
Number of laboratories providing testosterone and oestradiol results with chemiluminescence immunoassay (Luminesc), mass spectrometry (Mass Spec) and radioimmunoassay (RIA) in the European external quality control programme organised by the Referenzinstitut für Bioanalytk (Germany).
As we have highlighted above, the analysis methods for sex steroids have to be sensitive, specific, accurate and precise at a wide range of concentrations.8,9,20,21 There are some particularly important situations in clinical practice deserving of special mention.
Sensitivity: measurement of steroids at low concentrations- a)
Measurement of oestradiol at low concentrations.
In monitoring certain special situations, for example, breast cancer patients treated with aromatase inhibitors (where endogenous oestradiol concentrations need to be suppressed), methods need to be capable of distinguishing between suppressed concentrations of less than 1 pg/mL22 and pretreatment concentrations, which in menopausal women are typically 10–15 pg/mL. Similarly, women with other disorders, such as endometriosis or leiomyomas, who are on block-and-replace regimens (GnRH agonists to induce medical castration, along with a low dose of oestrogen), cannot be adequately controlled as there is no specific, sensitive and effective system for measuring low concentrations of circulating oestrogens.
Older men and women, moreover, have very low oestradiol concentrations, in the range of 5–30 pg/mL. In some cases, monitoring these low concentrations could be useful. For example, in men with prostate cancer on androgen deprivation therapy, oestrogen measurements could be useful in evaluating the effects of the therapy on targets such as the bones and the heart, and on metabolic status.20
Along the same lines, to assess pubertal development in children, we need to accurately measure low levels of sex steroids, particularly in prepubertal children and in patients with precocious puberty, whether central, peripheral or mixed, as well as defining normal concentration ranges throughout childhood. At this stage in life, although rare, some gonadal tumours secrete human chorionic gonadotropin, leading to sex hormone production which is often below the detection limit of the method.20 Additionally, pubertal gynaecomastia in boys, which involves an imbalance of testosterone and oestrogen, cannot be detected with routine immunoassays.23
- b)
Measuring testosterone
The majority of patients suffering from polycystic ovary syndrome (PCOS) and other forms of androgen excess are not being adequately assessed due to the lack of sensitivity and/or specificity of most current immunoassays. Some studies have shown that free testosterone correlates better with the clinical presentation of PCOS than total testosterone,24,25 although, as explained above, testing for free testosterone is not routinely available in clinical laboratories. We should stress that the formulas used for calculating free testosterone require total testosterone to have been measured with a precision method; only then do they provide accuracy similar to the calculation by ultrafiltration or equilibrium dialysis. Since 2009, the Androgen Excess & PCOS Society has recommended the use of liquid chromatography/tandem mass spectrometry as the reference method for the measurement of total testosterone, and the calculation of free testosterone using SHBG and albumin, as the ideal method to estimate biochemical hyperandrogenism in these patients.26
Testosterone testing in women with reduced sexual desire could be informative and is an unmet clinical need. There is some, albeit disputed, evidence suggesting an improvement in sexual desire with testosterone replacement in women with hypopituitarism27 or in premenopausal women after oophorectomy.28 However, current measurement methods do not provide sufficient confidence in the testosterone concentration results after replacement therapy, so it is not recommended.
Other situations in which highly sensitive methods are required are: follow-up of prostate cancer patients undergoing chemical castration to suppress endogenous testosterone concentrations; in adolescence, for the assessment of early or late puberty; and after birth, during the assessment of minipuberty in male infants.
SpecificityPatients may have circulating oestrogens/androgens deriving from exogenous sources, for example, sex steroid hormones from food or nutritional supplements.29 Some of these compounds can cross-react with the antibody in the immunoassay and lead to misdiagnosis, which leads to a significant increase in healthcare costs for unnecessary tests, as well as inconvenience to the patient.
AccuracyLastly, the results should be comparable between different laboratories; reproducible data are essential for analysis and control of patients whose tests are performed by several different laboratories using different methods. The introduction of correctly standardised methods in most hospitals would avoid patients followed up in referral centres from having to travel for a simple blood test, just because of the lack of accuracy and inter-assay reproducibility between centres.
Working group recommendationsIn the measurement of sex steroid concentrations, there are situations in which mass spectrometry has demonstrated methodological characteristics far superior to those of immunoassays, especially when high sensitivity and specificity are required.
As long as we do not have standardisation of all the measurement procedures, it is recommended that the procedures used in clinical laboratories be validated and meet the methodological characteristics of analytical reproducibility, sensitivity and accuracy required to measure concentrations, according to the indications of the CLSI guide30 for the population served, and that each laboratory define its own reference intervals.31–33 In this context, the CDC HoST Program offers its services to laboratories around the world at a price which is perfectly affordable. We would therefore highly recommend the use of this program by the laboratories of the Spanish national health system to assess the analysis methods currently used and whether or not they should be replaced by others already standardised.
Even though immunoassays may retain certain utility in general medicine for measuring total testosterone in males and oestradiol in females, recommendations from the last ten years indicate the need to use mass spectrometry-based methods for accurate measurement of sex steroids, and very particularly in:
- 1)
Testosterone: measurements of serum testosterone concentration in paediatric patients, women and patients with hormone-dependent cancers must be performed by highly specific and analytically sensitive mass spectrometry-based methods. If this is not possible, immunoassays which show good analytical performance compared to a validated mass spectrometry method should be considered, such as the one already evaluated by the HoST program.34
- 2)
Oestradiol: measurements of serum oestradiol concentrations in paediatric patients and in women with breast cancer undergoing treatment with aromatase inhibitors should be performed with highly sensitive and specific mass spectrometry-based methods in the initial assessment and during treatment monitoring.
While understanding that it is not currently feasible to use mass spectrometry-based measurement methods in all hospitals in Spain, the working group recommends introducing these methods in at least one centre for each regional health system, in order to provide adequate support for the clinical situations we have described above and acquire the necessary experience to then gradually expand them to the rest of the hospital network. These moves will help adapt the Spanish national health system to current international recommendations.